Abstract
Free full text

Spontaneous Myocarditis mimicking human disease occurs in the presence of an appropriate MHC and non-MHC background in transgenic mice
Abstract
Most individuals have viral infections at some point in their life, however, only few develop autoreactivity to cardiac myosin following infection resulting in myocarditis suggesting a genetic predisposition. Most mouse models of myocarditis are induced by viral infection or by immunization with cardiac myosin. We generated HLA-DR3.Aβo and HLA-DQ8.Aβo transgenic mice in NOD and HLA-DQ8.Aβo in B10 background to study spontaneous autoimmunity. A high mortality was observed in NOD.DQ8 female mice 16 weeks or older. Echocardiography showed marked systolic dysfunction. Histopathology of various organs revealed an enlarged heart with mononuclear infiltrate consisting of CD4 and Mac-1+ cells and myocyte necrosis. The autoimmunity was associated with the presence of spontaneous autoreactive T cells and antibodies to cardiac myosin. Serologically, mice were negative for all known mouse viruses. NOD.DR3.Aβo, the transgene negative littermates, NOD, and B10.DQ8 Aβo mice had no gross or microscopic cardiac pathology. Spontaneous cellular and humoral response to cardiac myosin suggest that NOD.DQ8 may harbor autoreative cells that can lead to spontaneous myocarditis and dilated cardiomyopathy. HLA-DQ8 is required for predisposition to the spontaneous autoreactivity while NOD background influences onset and progression of disease. This model of myocarditis occurs predominantly in female mice and may provide insight into the pathogenesis of heart disease in women.
Introduction
Myocarditis is an inflammatory heart disease defined pathologically as mononuclear or mixed cellular infiltration associated with myocyte necrosis and degeneration which can progress to dilated cardiomyopathy (DCM) [1]. Several studies have indicated an aberrant immune response following viral-mediated damage to myocytes [2,3]. Familial aggregation, presence of antibodies to cardiac antigens, association of HLA with DCM and antibodies, and abnormal expression of HLA class II on cardiac endothelium underscore the influence of genetic factors in development of autoimmune processes [4-6]. Several recent studies demonstrated an immunological basis of other systemic autoimmune disorder, celiac disease and anti-tissue transglutaminase antibodies, in patients with myocarditis [7,8].
Infection of susceptible mouse strains with coxsackie B3 virus or immunization with cardiac myosin and its derived-peptides leads to experimental myocarditis, reviewed in several papers [9-13]. All models of induced autoimmune myocarditis are strain-dependent, suggesting MHC class II as an important genetic factor for susceptibility. A strong T cell response accompanied by autoantibodies to cardiac α myosin heavy chain has been shown in experimental models of autoimmune myocarditis.
Inbred non-obese diabetic (NOD) mice develop spontaneous autoimmune diabetes. Although multiple genes contribute to the susceptibility, the major susceptibility factor is the class II gene H-2Ag7. Ag7 has been shown to be structurally similar to HLA-DQ8 [14], an allele known to be associated with susceptibility to human autoimmune diseases like rheumatoid arthritis and diabetes.
MHC genes are the main restricting elements for specific response to foreign molecules by T lymphocytes and can provide important information concerning the immunopathogenesis and susceptibility to certain diseases. However, It is difficult to study the role of individual genes in humans. Humanized mice provide a valuable tool to study the unanswered questions. The background genes are controlled in the transgenic mice and all mice share a common environment. These conditions provide a means to study the role of MHC genes and non-MHC genes in disease pathogenesis. Transgenic mice expressing HLA-DQ8 develop diabetes and collagen-induced arthritis [15,16]. We introduced HLA-DQ8 transgene into NOD and B10 mice lacking endogenous class II molecules to generate NOD.DQ8.Aβo and B10.DQ8.Aβo transgenic mice. In addition, we generated NOD.DR3.Aβo mice to understand the role of MHC and non-MHC background in pathogenesis. Since DQ8 is known to be associated with autoimmunity, our hypothesis was that NOD.DQ8 mice should develop spontaneous autoimmunity. Predominantly female NOD.DQ8 mice developed spontaneous autoimmune myocarditis that was evident by mononuclear infiltration of the myocardium accompanied by autoantibodies to cardiac myosin heavy chain. This is the first model of spontaneous myocarditis leading to dilated cardiomyopathy predominantly in female mice.
Material and Methods
Mice
The DQ8 transgene (gift from J. Strominger, Harvard Medical School, Boston, MA) was microinjected into (CBA/JX B10.M) F2 and backcrossed onto a B10.M (H-2f/f) background. The B10.M.DQ8 mice were mated with class-II deficient mice (Aβo) to generate Aβo.DQ8 mice, which were then mated with NOD mice. F1 generation was intercrossed and transgene positive mice were backcrossed to NOD for 10 generations to generate DQ8 (Ag7-/-) mice with congenic NOD background as published before [17]. Similarly DR3.Aβo mice were mated with NOD mice to generate congenic NOD.DR3.Aβo mice. Also, DQ8 (Aβo) mice with congenic B10 background were generated as published before [17]. However, the NOD.DQ8. Aβo mice used in this study were backcrossed for 5 more generations, N15 F15 and B10.DQ8 were N12, F15. Cell surface expression of DQ8 and DR3 was analyzed by FACS after staining with IVD12 (anti-DQ antibody) and L227 (anti-DR antibody).
All mice were bred and maintained in the pathogen-free facility in the Immunogenetics Mouse Colony in accordance with National Institute of Health guidelines and approved by the Institutional Animal Care and Use Committee. B10.DQ8 mice were used as controls for non-MHC genes and NOD.DR3 mice were used as control for HLA allele. Transgene negative littermates of NOD.DQ8 mice and NOD mice were also used as controls.
Histology and Immunochemistry
Various organs including heart, lungs, kidney, pancreas and salivary glands were removed surgically at different time points, embedded in paraffin, sectioned and assessed for histopathologic changes after staining with hematoxylin and eosin (H&E). The extent of infiltration of myocardium was quantified using light microscopy and KS400 Image Analysis Software (Carl Zeiss, Inc. Oberkochen, Germany) and is reported as percentages (mean ±SE).
Hearts free of fat tissue were fixed in OTC embedding medium (Sakura Finetek, Torrance, CA). Cryostat sections were prepared, fixed and stained with FITC conjugated or biotinylated anti-mouse antibodies specific for CD4, CD8, CD19 and Mac-1 (BD Pharmingen, CA). DQ8 staining was performed on frozen sections using biotinylated IVD12 (anti-DQ) antibody and peroxidase-conjugated streptavidin (BD-Pharmingen, CA) and AEC (Sigma Chemicals, Missouri). Trichrome staining was also performed on heart sections. Staining for eosinophils was performed on paraffin sections.
Delayed type hypersensitivity (DTH)
Mice were immunized subcutaneously with 10μl of 2mg/ml of porcine cardiac myosin (PCM) (Sigma-Aldrich) in PBS into the right footpad. As a control, left footpad received 10μl of PBS alone. To determine DTH, footpad thickness was measured with dial gauge caliper calibrated with 0.01 mm graduations before and after 48 hrs of challenge with antigen. The degree of swelling was calculated as percent increase = (right footpad thickness after challenge-left footpad thickness) × 100.
Immune complex
Deposition of IgG immune complex was studied in heart tissue. Briefly, charged slides with sections from heart were stained with goat anti-mouse IgG conjugated with FITC (Accurate Chemicals, NY) for IgG immune complex.
Antibodies
The expression of various cell surface markers was analyzed by Flow cytometry using FACSR IV (Beckton Dickinson) using specific antibodies. Antibodies used for the expression of T-cell receptor Vβ chains molecules were mAb MR9-4 (anti Vβ5.1), MR9-8 (anti Vβ5.1.2), 44-22-1(anti Vβ6), F23.1 (anti Vβ8.1.2.3), KJ-16 (anti Vβ8.1.2), F23.2 (anti Vβ8.2), KT11 (antiVβ11). Anti-CD3, CD4, CD5, CD8, CD25, CD44, CD69 and B220 conjugated with FITC or PE (BD Pharmingen) were used on PBLs or splenic cells of naive mice. Other antibodies used were IVD12 (anti-DQ), K24-199 (anti-Ag7) and HB163 (anti-Ab).
Peptides
Two peptides of cardiac myosin α heavy chain were synthesized and purified at Mayo peptide Core Facility. The peptide SLKLMATLFSTYASAD (614-629) does not have the binding motif for DQ8 while peptide GQFIDSGKAGAEKL (735-747) has a binding motif for DQ8 according to the prediction algorithm done by software RANKPEP .
In vitro T cell proliferation
T cell proliferation using lymph node cells from transgenic mice and controls was carried out as described previously [16]. Ten days postimmunization, draining lymph nodes/ spleen were removed and cultured in vitro. LNCs (1×106) were cultured in HEPES-buffered RPMI 1640 containing 5% heat inactivated horse serum and antibiotics streptomycin and penicillin in 96-well flat bottom tissue culture plates. Cells were challenged by adding 100μl of RPMI medium (negative control), Con A (20 μg/ml, positive control) and 2.5μg/ml PCM (Sigma-Aldrich). The cells were incubated for 48 h at 37° C. During the last 18 h the cells were pulsed with 3H-thymidine (1μCi/well). At the end of the assay, the cells were harvested using a plate harvester and incorporated radioactivity was determined using an automated counter ( Micrebeta, Perkin Elmer Wallac, Germany). For inhibition experiments, culture supernatant containing 5μg of IVD12 (anti-DQ), L227 (anti-DR) or MKD6 (anti Aq) was added to the cells challenged in vitro with myosin at 2.5 μg/ml. The results are reported as stimulation index (S.I.). Stimulation index of 2 was taken as cut off for positive response.
T cell response to peptides was carried out by immunizing transgenic and control mice with 200μg of peptide emulsified in complete Freunds’ adjuvant (1:1). LNCs isolated from primed and naïve mice were challenged in vitro in the presence or absence of specific peptides and proliferation assay carried out as described [18].
Autoantibodies
Antibodies to porcine cardiac myosin in sera (Sigma-Aldrich) were measured by standard ELISA and absorbance read at 450nm. Plates were coated with 100μl/well of cardiac myosin at 5μg/ml in bicarbonate buffer. Anti-cardiac myosin antibodies were further categorized in to subclasses IgG1, IgG2b, IgM and IgE . ELISA assays were performed as described previously with some modifications [19]. Briefly, ELISA plates were coated with .25μg cardiac myosin in 100 μl 0.15 M carbonate-bicarbonate buffer. Plates were incubated overnight at 4° C, washed with Phosphate buffered saline with o.1% Tween 20 (PBST), and blocked with PBST containing 2% BSA for 1 hr at 37oC. Diluted sera (1:100) were added to the washed plates and incubated for overnight at 4°C . After washing, peroxidase conjugated goat anti mouse IgG1, IgG2b (BD Pharmingen, San Diego, CA) and IgM (Cappel laboratories, Cochranville, PA) were added according to manufacturer’s instructions. Alkaline phosphate conjugated goat anti-mouse IgE (BD Pharmingen, San Diego, CA) was also used. Plates were incubated for I hr at room temperature, washed and 100 μl of pNPP (p-Nitrophenyl phosphate) substrate solution (Southern Biotech, Birmingham, AL) was added. Reaction was stopped after 20 mintues and O.D measured at 405nm.
Antibodies to skeletal myosin was also tested in sera. Plates were coated with striational antigen and ELISA was performed as described previously [20]. Known positive and negative control sera were included.
IgG and IgA anti-tissue transglutaminase antibodies were measured by ELISA. Plates were coated with 100μl/well guinea pig tissue transglutaminase (Sigma-Aldrich) overnight at 4C at a concentration of 1μg/ml. After 24 h, plates were washed with TBS plus 0.1% tween 20 (TTBS) and 100μl of diluted sera (1:400) were added to each well and incubated for one hr at room temp. Plates were washed with TTBS and secondary antibody HRP-conjugated with goat anti-mouse IgG or IgA (Accurate Chemicals, NY) was added according to manufacturer’s instructions. Plates were incubated for one hr and antibodies detected by adding substrate TMB (Sigma-Aldrich). Reaction was stopped with 1M H2SO4 and absorbance read at 450nm.
Cytokines
Cytokines were measured in supernatants collected after 48 h from cultured splenic cells in presence of media or PCM. Capture ELISA was done for measuring cytokines IFNγ, IL-2, IL-4, IL-6, TGFβ, IL-10, IL-18 and TNF-α using kits (BD Pharmingen, CA).
Echocardiographic assessment
Transthoracic echocardiography was performed on lightly anesthetized (1.5% isoflurane) B10.DQ8 (n=5) and NOD.DQ8 (n=5) mice with a 15-MHz transducer (15L8, Acuson, Mountain View, CA). A lead II electrocardiogram was simultaneously recorded. Left ventricular fractional shortening (%FS) was defined as %FS = [(LVDd-LVDs)/LVDd]×100, where LVDd represents left ventricular end-diastolic dimension, and LVDs left ventricular end-systolic dimension. For functional analysis both circumferential shortening velocity that is a preload independent parameter of ventricular function, and fractional shortening that is dependent on loading and heart rate were determined. Myocardial velocity of left ventricular circumferential shortening (Vcf expressed in circumferences per second) was calculated as Vcf = [(LVDd-LVDs)/LVDd]/(ejection time) as described [21].
Statistical Analysis
Comparison between groups for antibodies, cytokines and DTH response was carried out using student’s t test with a significance level of p<0.05.
Results
Both strains of DQ8 transgenic mice showed similar expression of DQ8 in splenic cells by FACS using IVD12 antibody. The Vβ TCR profile suggested representation of all the Vβs in both transgenics (data not shown). DQ8 in both backgrounds, NOD and B10, could present previously known DQ8-restricted peptides [data not shown, 18]. Both DQ8 transgenic strains showed restoration of CD4 compartment as published before. NOD.DR3 mice expressed DR3 as analyzed by FACS using L227 antibody and had restoration of CD4 compartment (data not shown).
NOD.DQ8 mice develop spontaneous myocarditis
A high mortality was observed among female mice after 16 weeks of age. Morbidity was observed in 90% (36/40, mean age 18±3 weeks) female mice and 20% male mice (3/15, mean age of 25±3 weeks). The control mice, B10.DQ8, NOD.DR3, transgene negative littermates and NOD mice survived with no signs of disease till about 50 weeks. Hearts devoid of adjoining fat and muscle were removed surgically (n=15, 10F, 5M) and weighed. Only NOD.DQ8 affected mice had enlarged hearts compared to control B10.DQ8 mice (Figure 1) and NOD, NOD.DR3 and negative littermates (data not shown). Heart/body weight ratio for the NOD.DQ8 mice was higher (heart weight 0.2-0.25 gms and body weight 22-25 gms) than B10.DQ8, NOD.DR3 and control mice (heart weight 0.12-0.15gms and body weight 25-30 gms), (heart/body weight ratio, 0.01±0.002 in affected and .004±0.0006 in controls, p<0.05).
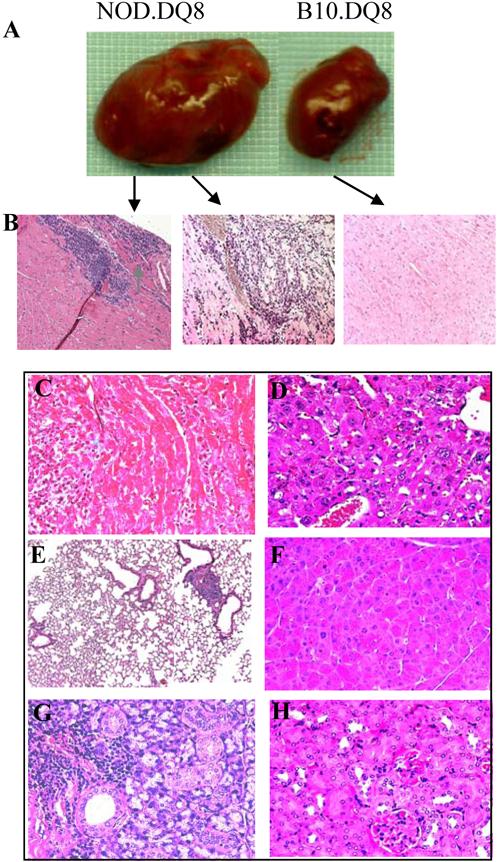
NOD.DQ8 mice develop myocarditis progressing to dilated cardiomyopathy. A) 16 week old NOD.DQ8 mice with severe edema and enlarged heart compared to healthy age and sex matched B10.DQ8 mice. B) Mononuclear infiltration observed in heart of NOD.DQ8 mouse first appeared as focal (left) which progressed to diffused with myocyte necrosis (middle) while B10.DQ8 (right) did not show any infiltration. Micrographs are at X100 magnification. C) trichrome stain of heart section shows mononuclear infiltration but no fibrosis, X400 magnification. Haematoxylin and Eosin stained sections of D) Liver E) Lungs F) Pancreas G) Salivary glands and H) Kidney. Micrographs are X400 magnification except E is at X100 magnification..
Histologically NOD.DQ8 hearts revealed a widespread, mixed inflammatory infiltrate consisting predominantly of lymphocytes and histiocytes with lesser numbers of neutrophils and eosinophils. The inflammatory lesions were associated with myocyte destruction but no granuloma, giant cells or fibrosis (Figure 1C). Focal infiltration was observed between 10-14 weeks (n=5) occupying 21.1±13.8 percent of the myocardium (Figure 1b left). At 14-20 weeks (n=5), the lesions were identical in composition, but more widespread, comprising up to 71.5±28.2 percent of the myocardium (Figure 1b middle). At the latter timepoint, both ventricles and the atria were affected with lesions distributed from endomyocardium to epicardium. Histologic examination of B10.DQ8, NOD.DR3 and other control mice hearts revealed normal cardiac pathology (Figure 1b right). Transthoracic echocardiography on 18-22 weeks old, female NOD.DQ8 mice (Figure 2) showed cardiomegaly (Figures 2A and 2C) with systolic dysfunction, as indicated by reduced circumferential shortening velocity (NOD.DQ8: 6.4±0.9 circumferences/s, n = 5; B10.DQ8: 9.8±1.2 circumferences/s, n = 5, P < 0.05, Figure 2E), compared with age- and sex-matched B10.DQ8 mice. Hearts from NOD, NOD.DR3 and negative littermates were normal (not shown). To investigate if organs other than heart were also affected in NOD.DQ8 mice, histology of liver, lungs, pancreas, salivary glands and kidney was done. Histopathology of these organs did not show any pathology except few perivascular infiltration of mononuclear cells in lungs (Figure 1D-H). Salivary glands also showed scattered infiltration of cells, which is known to be a feature of sialadenitis in NOD mice.
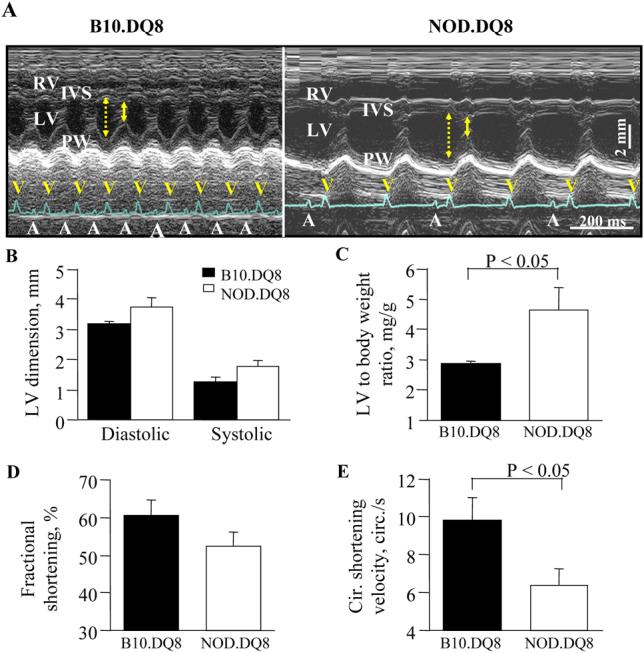
Echocardiographic assessment of hearts of NOD.DQ8 (n=5) and B10.DQ8 (n=5) mice. A) M-mode echocardiograms showed dilatation of both right venrticle (RV) and left ventricle (LV) with atrio-ventricular conduction block in NOD.DQ8 mice, in contrast to normal echo- and electro-cardiograms in B10.DQ8 mice. Arrows indicate diastolic (dotted) and systolic (continuous) dimensions. A and V in electrocardiograms represent P and QRS waves, respectively. B) Left ventricular (LV) dimensions (diastolic dimension; NOD.DQ8: 3.7±0.3 mm, B10.DQ8: 3.2±0.1 mm, systolic dimension; NOD.DQ8: 1.8±0.2 mm, B10.DQ8: 1.3±0.2 mm, P = 0.08). C) NOD.DQ8 hearts were significantly heavier and demonstrated reduced systolic function as demonstrated by fractional shortening (D) and, Circumferential (Cir.) shortening velocity E), compared with B10.DQ8 hearts. IVS - intra ventricular septum, PW - posterior wall.
Immunostaining of heart revealed that most of the cells in myocardium of NOD.DQ8 mice comprised of CD4 and Mac-1 positive cells with some eosinophils (Figure 3a). Hearts from most of the control mice had few cells stained positive in some mice with conjugated anti-CD4, CD8, and Mac-1 antibodies. An increased expression of DQ8 was observed in NOD.DQ8 mice with disease compared to healthy NOD.DQ8 and B10.DQ8 mice (Figure 3b). Immunofluorescence staining with major basic protein (MBP) showed infiltration of eosinophils into the cardiac tissues of NOD.DQ8 mice (Figure 3c) but not control NOD or NOD.DQ8 mice (data not shown). Heart sections of NOD.DQ8 mice showed deposition of IgG immune complexes that were not observed in B10.DQ8 (Figure 3d). The other controls, NOD.DR3, healthy NOD.DQ8 and NOD mice were similar to B10.DQ8 mice (not shown).
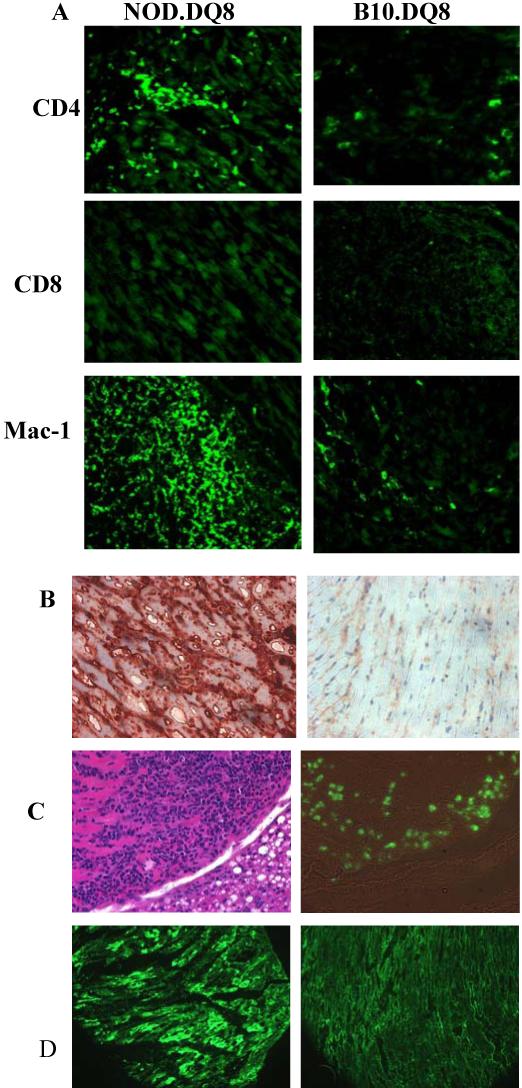
Characterization of inflammatory cells in heart. A) Heart sections stained with various makers, CD4, CD8, and Mac-1 in DQ8 transgenic mice. B) Heart sections stained with anti-DQ antibody (IVD12) exhibit increased expression of class II in NOD.DQ8 mice. C) Haematoxylin & eosin (left) and eosinophil staining of consecutive section (right, bright field) of a heart from affected NOD.DQ8 mice. D) Deposition of IgG immune complex in heart sections of NOD.DQ8 (left) and B10.DQ8 transgenic mice shows deposition only in the former mice. Micrographs are at X100 magnification.
Cell mediated and humoral response to self-antigen leads to autoimmunity
Both transgenic and control mice were negative serologically for all the known mouse viruses (Table 1). LNCs of NOD.DQ8 mice responded spontaneously to porcine cardiac myosin when challenged in vitro. A lower T cell response was observed in B10.DQ8 and NOD mice with negligible response in NOD.DR3 mice (Figure 4A). None of the mice responded spontaneously to peptides, however, NOD.DQ8 mice primed with specific peptides mounted a strong response to the peptide 735-747 and not to the other peptide (Figure 4B). All mice responded to PCM after priming.
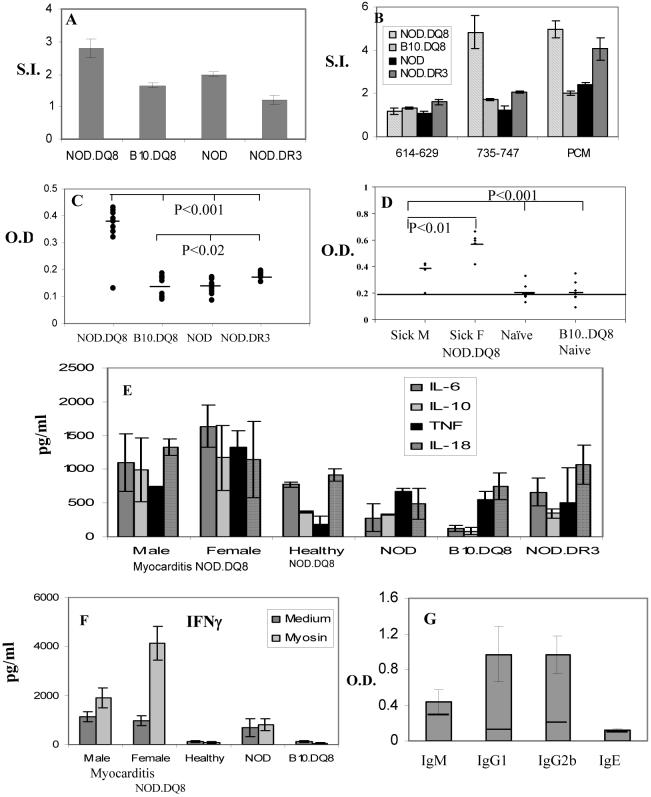
A) Spontaneous response to porcine cardiac myosin was observed in NOD.DQ8 (n=6) and NOD (n=4), and NOD.DR3 (n=6) mice but not in B10.DQ8 (n=6) mice. B) Only NOD.DQ8 mice respond to cardiac-myosin derived peptide, 735-747. C) Antibodies to porcine cardiac myosin in naïve DQ8 (n=10) and NOD (n=12) mice suggests high levels of antibodies in NOD.DQ8 mice (n=10), p<0.001 and NOD.DR3 mice (n=8), p<0.02. D) Anti tissue transglutaminase antibodies in male and female mice with myocarditis and controls, healthy NOD.DQ8 and B10.DQ8 mice, n=6/gp). Female NOD.DQ8 mice produce higher amounts than male mice (p<0.01). E) Spontaneous production of cytokines produced by spleen cells of NOD.DQ8 mice in response to myosin challenge in vitro (n=4/gp). TNF, myocarditis mice vs healthy NOD.DQ8 mice, p<0.02; TNF and IL-6 female myocarditis vs B10.DQ8 mice, p<0.05; IL-18 male myocarditis vs healthy NOD.DQ8 mice, p<0.04, B10.DQ8 vs healthy NOD.DQ8, IL-10, IL-6 p<0.01 . F) IFN-γ production by spleen cells from myocarditis and control NOD.DQ8 mice in the presence and absence of cardiac myosin. Myocarditis female vs male, p<0.05, myocarditis mice vs all healthy and B10.DQ8 , p<0.02 G) Subclass of anti-cardiac myosin antibodies, IgM, IgG1, IgG2b and IgE, in DQ8.NOD mice (n=10) shows O.D. ± S.D. The line in the bars depicts negative control.
Table 1
Serological profile for DQ8 and DR3 transgenic mice.
| Mouse hepatitis virus | Mycoplasma Pulmonis |
| Sendai virus | Mouse parvovirus |
| Pneumonia virus | Mouse cytomegalovirus |
| Reovirus 3 | Rotavirus, Epizootic diarrhea of infant mice |
| Theiler’s meningoencephalitis virus | Lymphocytic choriomeningitis |
| GDVII | Encephalitis cuniculi |
| Ectromelia virus | Cilia associated respiratory bacillus |
| Mouse adenovirus 1 and 2 | Tyzzer’s Clostridium piliforme |
| Polyoma virus | Minute virus of mice |
NOD.DQ8, B10.DQ8 and NOD.DR3 transgenics were tested serologically for the mentioned viruses. All strains were found negative for the above viruses.
NOD.DQ8 mice with myocarditis produced significantly high amounts of cardiac anti-myosin antibodies; no antibodies were observed in NOD and B10.DQ8 mice, p<0.001 (Figure 4C). NOD.DR3 mice produced very low amounts of anti-myosin antibodies but higher than B10.DQ8 and NOD mice, p<0.02. Most of the antibodies produced were of IgG1 and IgG2b subclass ( Figure 4G). None of the transgenic mice produced antibodies to skeletal myosin confirming the specificity of antibodies to be cardiac myosin (O.D. for NOD.DQ8= 0.30, positive control =0.69 and negative control= 0.42). Anti-tissue transglutaminase (Ttg) antibodies were produced in higher amounts in female mice with myocarditis compared to male mice, p<0.01and controls, naïve NOD.DQ8 male and B10.DQ8 mice, p<0.001 (Figure 4D).
DTH response with cardiac myosin in naïve NOD.DQ8 mice showed a thickness of 35% over that observed with PBS alone (Figure 5). B10.DQ8 and NOD mice had some thickening (15% and 10% respectively) over the PBS control although it was significantly less than NOD.DQ8 mice (p<0.05). DTH response 5 days after priming with PCM showed considerable paw swelling in both NOD and NOD.DQ8 mice while B10.DQ8 had a mild increase only.
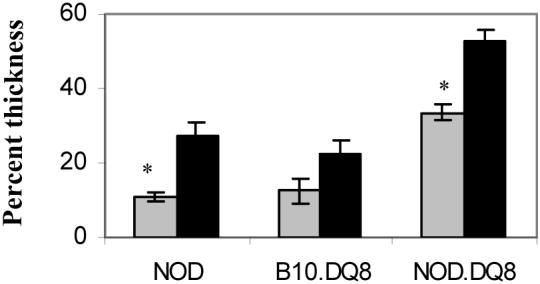
DTH response to porcine cardiac myosin in naïve (n=4) (gray bars) and 5 days primed (black bars) (n=4) mice. *P<0.05
Cytokine profile showed that mice with spontaneous myocarditis produced high amounts of IFN-γ, IL-6 and TNF-α than healthy mice (Figure 4E). There was no significant difference between male and female mice with myocarditis although TNFα levels were higher in female mice and reached near significance, p<0.06. TNF-α was produced significantly in higher levels by myocarditis mice than healthy NOD.DQ8 mice, p<0.02. Among affected mice, female mice produced higher amounts of TNF-α and IL-6 than healthy controls and HLA gene controls, B10.DQ8, mice, p<0.05. Male mice with myocarditis produced higher amounts of IL-18 compared to NOD, p<0.04 and healthy controls, p<0.06. NOD and NOD.DR3 mice produced similar amounts of all the cytokines tested. B10.DQ8 mice produced very low amounts of all the cytokines except TNF α and IL-18, B10.DQ8 vs myocarditis and healthy NOD.DQ8 mice IL-6 and IL-10, p<0.05. None of the mice produced IL-4 while all of them produced low levels of IL-2. Among affected mice, female mice produced higher levels of IFN-γ than male mice, p<0.05 After challenge with cardiac myosin, myocarditis NOD.DQ8 mice produced higher amounts of IFN-γ compared to healthy NOD.DQ8 and B10.DQ8 mice, p<0.02. (Figure 4F) while rest of the cytokine profile remained similar (data not shown).
To determine the cells involved in immune response, we studied cell surface markers (Figure 6). We utilized male NOD.DQ8 mice and B10.DQ8 mice as control. An increased number of CD3 cells and CD4 cells with activation markers CD25, CD69 were observed in sick mice compared to both controls, p<0.05. CD4+CD44+ cells were higher in frequency in sick mice compared to B10.DQ8 mice, p<0.01 and NOD.DQ8 healthy mice although it did not reach statistical significance when compared with latter, p<0.06. There was an increase in B220+ in mice with myocarditis compared to healthy male NOD.DQ8 and B10.DQ8 mice (not shown). CD5+ were more in number in NOD.DQ8 sick mice compared to B10.DQ8 and NOD.DQ8 healthy mice, p<0.05. An increase of CD5+ cells was also observed in affected mice, which was reflected in B220+ cells in NOD.DQ8 mice compared to B10.DQ8 mice (17% and 9% respectively).
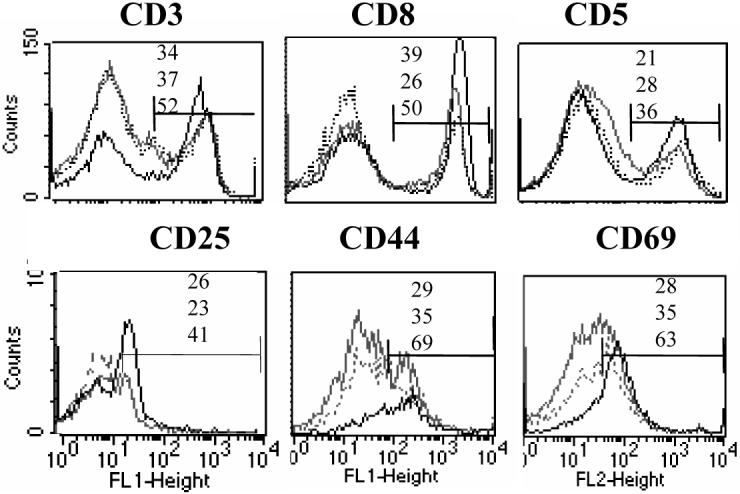
Analysis of splenic cells by FACS in control mice, B10.DQ8 (grey) and healthy NOD.DQ8 (dotted) and NOD.DQ8 mice with myocarditis (black). Upper panel shows a) expression of CD3 cells (left), CD8 cells gated on CD3+ cells (middle) and CD5+ cells (right). Lower panel shows expression of CD25 (left), CD44 (middle) and CD69 (right) gated on CD4 cell. Numbers indicate percent positive in B10.DQ8 (upper), healthy NOD.DQ8 (middle) and NOD.DQ8 myocarditis (lower). Difference in cell numbers between healthy NOD.DQ8 and sick mice was significant for CD3, CD5 and activated cells CD4+CD25 and CD69+, p<0.05.
Discussion
We had generated NOD.DQ8.Aβo mice with the hypothesis that DQ8 could replace Ag7 of NOD mice and develop spontaneous autoimmunity. These mice did not develop spontaneous diabetes. Backcrossing with NOD mice for up to 15 generations led to high mortality in female transgenic mice. Histopathology of several organs revealed that mice developed spontaneous autoimmune myocarditis and shared many of the clinical, immunological and histological features consistent with myocarditis in human. The echocardiography showed biventricular dialation with reduced systolic function in affected mice, which is similar to patients (1). Autoimmunity in the affected mice was evident by infiltrating mononuclear cells comprising of CD4 cells and macrophages in myocardium. In addition, mice produced cardiac myosin-specific IgG antibodies and spontaneous response to cardiac myosin. Anti cardiac-myosin antibodies to cardiac-myosin have been reported in myocarditis patients [22,23]. Thus the present data suggest a role of T and B cell in pathogenesis of myocarditis, which is in agreement with previous findings [12, 24,25]. T cell response to myosin peptide 735-747 suggests that autoreactive T cells restricted by DQ8 mediate the response to cardiac myosin in our model although the present data does not exclude the role of other peptides. This peptide is homologous in human and mouse and has been used to induce myocarditis in mice [26]. DR3 mice do not develop myocarditis and also do not respond to this peptide. Present data together with previous data suggest that this peptide may contribute to pathogenesis in context of MHC polymorphism. In contrast, peptide 614-629, which did not result in proliferation, has two amino acid differences between mouse and human. In vitro spontaneous T cell response to cardiac myosin and derived-peptide could be blocked by anti-DQ antibody thus suggesting it to be DQ-specific. Cardiac myosin MHC class II complexes have been shown to be constitutively expressed on cardiac APCs [27]. There was a marked increase in the expression of DQ8 in these mice similar to what is known from biopsies of myocarditis patients [28]. Thus, DQ8 might be able to positively select T cells with specificity to cardiac antigens that are able to present self-peptides in heart of NOD.DQ8 mice. In human myocarditis, cardiac myosin heavy chain is the major target of autoantibodies, however few patients with myocarditis also produce anti-tissue transglutaminase (Ttg) antibodies [7,8]. We also observed an increased level of anti-Ttg antibodies in mice with myocarditis suggesting similarity with human disease.
The present data suggest polymorphism in class II is important in predisposition to myocarditis as expression of DR3 transgene in NOD background does not lead to any spontaneous autoimmunity. This is consistent with previous studies suggesting that susceptible MHC alleles are responsible for selection of autoreactive T cells (18, 29). NOD.DR3 mice do not respond spontaneously to myosin and produce very low levels of anti-cardiac myosin antibodies. However, Class II of NOD mice, IAg7, shares structural homology with DQ8 [14], but NOD mice did not develop this pathology. This is in confirmation with the observations that DQ8 and Ag7 are capable of presenting different epitopes [30]. Lack of disease in B10.DQ8 mice demonstrates that NOD background influences the pathology. However, DQ8 is required since transgene negative littermates, NOD.DR3 and NOD mice do not develop this disease. The observations suggest that like other autoimmune disorders, disease expression in these mice requires both MHC and non-MHC genes.
To define the mechanism and role of autoantibodies in disease, we studied DTH and immune complexes. The present data suggest that both T cell-mediated response to cardiac myosin and deposition of IgG immune complexes in the heart might lead to antibody-dependent cell-mediated cytotoxicity and cardiomyocyte destruction observed in mice with myocarditis. Furthermore, extracellular release of MBP and its deposition was observed in lesions. These cardiac pathologies of NOD.DQ8 mice show a striking resemblance to those patients with acute necrotizing endomyocarditis [31]. Although acute myocarditis in humans is associated with infiltrate composed of lymphocytes and histiocytes, the most severe form of human disease are associated with eosinophils, including giant cell myocarditis and necrotizing eosinophilic myocarditis (32). One can speculate that, in NOD.DQ8 mice, eosinophils were recruited to the cardiac tissue and activated locally in response to IgG antibodies deposited on cardiac myocytes which caused damage to them by releasing cytotoxic granule proteins. However, no anti-IgE antibodies to cardiac myosin were observed in sera suggesting that major response by eosinophils is probably locally in cardiac tissue.
Since CD4 cells infiltrated the heart, we characterized CD4 splenocytes to understand if the response is local. Mice with myocarditis had increased number of CD4+ cells with activation markers even though there was no significant increase in splenic CD4+ cells compared to both controls, healthy NOD.DQ8 and B10.DQ8 mice. This suggests that a subset of CD4+ cells is activated/autoreactive in mice with myocarditis. An increase in CD5+ B cells in mice with myocarditis suggests role of autoreactive B cells in pathogenesis. CD5+ B220+ cells represent B1 cells which have been shown to be positively selected on self-antigen [33]. Self-antigen has been shown to promote accumulation of B-1 cells and serum autoantibody [34]. Although it remains unknown how self-antigen becomes available in this model, the data presented here suggest both T and B cell activation in response to self-antigen.
Interestingly, in our model females were predominantly affected which is in contrast with the human case series with M:F ratio of 1.5:1. An increased expression of prostaglandin (PGS2) under the influence of sex steroid may explain the gender differences in spontaneous diabetes in NOD mice [35, 36]. Since our mice have congenic NOD background, similar phenomenon could be envisaged. Production of prostaglandins, PGE2, mediated by PSG-2 has been shown to enhance IFN-γ production by Th1 T cells [37]. Estrogen has been shown to significantly increase TNF α- production. Reduced TNF-α production was associated with reduced myocarditis in female mice [38]. After myocardial infarction, TNF-/- mice showed less inflammatory cell infiltration and cytokines in the infarct zone compared to wild type mice [39]. In the present study we did observe higher amounts of TNF-α and IFN-γ in affected female mice compared to male mice further supporting the role of sex hormones in autoimmunity. Both sexes produce IL-10. Consistent with the cytokines profile was the subclasses of cardiac myosin antibodies. Both IgG1 and IgG2b anti-cardiac myosin antibodies were produced suggesting cytokine profile skewing might lead to pathogenesis. Male mice produced slightly increased amounts of IgG1 antibodies although the difference was not significant (data not shown). There was no relationship between the female mice developing cardiomyopathy and pregnancy.
In conclusion, spontaneous cellular and humoral response to cardiac myosin suggests NOD.DQ8 mice might harbor autoreactive cells T and B cells that leads to spontaneous autoimmune myocarditis. The data presented here confirms previous findings [40, 41]. In addition our data show gender-bias in susceptibility, presence of Ttg antibodies and role of MHC polymorphism and background genes in spontaneous autoimmunity. Our findings bring forth following important observations 1) sex hormones likely contribute towards pathogenic response in this model, 2) NOD background is required for pathogenesis as background control, B10.DQ8 mice, do not develop disease, 3) DQ8 predisposes to autoimmunity since DR3 mice do not develop myocarditis, and 4) production of antibodies to tissue transglutaminase similar to human. In conclusion, this model bears similarities to human disease and should provide a valuable tool for exploring mechanisms involved in heart disease in women.
Acknowledgements
The study was supported by Investigator grant to Veena Taneja from National Arthritis Foundation. The HLA Class II transgenic mice were produced with the support from NIH grant AI 14764. Authors thank Dr Venda A. Lennon, Professor of Neurology and Immunology, for providing help with ELISA for Skeletal muscle antibodies. Authors thank Julie Hanson for maintaining and Michele Smart for screening transgenic mice.
Abbreviations
| MHC | Major Histocompatibility complex |
| PCM | porcine cardiac myosin |
| DCM | Dialated cardiomyopathy |
| DTH | Delayed type hypersensitivity |
| MBP | Major basic protein |
| Ttg | tissue transglutaminase |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.yjmcc.2007.03.898
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc1993806?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Article citations
Immuno-related cardio-vascular adverse events associated with immuno-oncological treatments: an under-estimated threat for cancer patients.
Basic Res Cardiol, 03 Sep 2024
Cited by: 0 articles | PMID: 39225869
Review
Murine MHC-Deficient Nonobese Diabetic Mice Carrying Human HLA-DQ8 Develop Severe Myocarditis and Myositis in Response to Anti-PD-1 Immune Checkpoint Inhibitor Cancer Therapy.
J Immunol, 212(8):1287-1306, 01 Apr 2024
Cited by: 1 article | PMID: 38426910
Myocarditis in Humans and in Experimental Animal Models.
Front Cardiovasc Med, 6:64, 16 May 2019
Cited by: 75 articles | PMID: 31157241 | PMCID: PMC6532015
Review Free full text in Europe PMC
Cardiac Autoimmunity: Myocarditis.
Adv Exp Med Biol, 1003:187-221, 01 Jan 2017
Cited by: 103 articles | PMID: 28667560 | PMCID: PMC5706653
Review Free full text in Europe PMC
FTY720 (Gilenya) treatment prevents spontaneous autoimmune myocarditis and dilated cardiomyopathy in transgenic HLA-DQ8-BALB/c mice.
Cardiovasc Pathol, 25(5):353-361, 17 May 2016
Cited by: 2 articles | PMID: 27288745 | PMCID: PMC5372700
Go to all (18) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Spontaneous autoimmune myocarditis and cardiomyopathy in HLA-DQ8.NODAbo transgenic mice.
J Autoimmun, 33(3-4):260-269, 07 Oct 2009
Cited by: 13 articles | PMID: 19811893 | PMCID: PMC3574900
CD4 T cells play major effector role and CD8 T cells initiating role in spontaneous autoimmune myocarditis of HLA-DQ8 transgenic IAb knockout nonobese diabetic mice.
J Immunol, 176(12):7715-7725, 01 Jun 2006
Cited by: 30 articles | PMID: 16751419
A spontaneous model for autoimmune myocarditis using the human MHC molecule HLA-DQ8.
J Immunol, 172(4):2651-2658, 01 Feb 2004
Cited by: 32 articles | PMID: 14764740
Pathogenesis of myocarditis and dilated cardiomyopathy.
Adv Immunol, 99:95-114, 01 Jan 2008
Cited by: 136 articles | PMID: 19117533
Review
Funding
Funders who supported this work.
NIAID NIH HHS (4)
Grant ID: R01 AI014764-28
Grant ID: R37 AI014764
Grant ID: AI 14764
Grant ID: R01 AI014764




