Abstract
Free full text

Human immune disorder arising from mutation of the α chain of the interleukin-2 receptor
receptor
Abstract
Profound cellular immunodeficiency occurs as the result of mutations in proteins involved in both the differentiation and function of mature lymphoid cells. We describe here a novel human immune aberration arising from a truncation mutation of the interleukin-2 receptor α chain (CD25), a subunit of the tripartite high-affinity receptor for interleukin 2. This immunodeficiency is characterized by decreased numbers of peripheral T cells displaying abnormal proliferation but normal B cell development. Extensive lymphocytic infiltration of tissues, including lung, liver, gut, and bone, is observed, accompanied by tissue atrophy and inflammation. Although mature T cells are present, the absence of CD25 does affect the differentiation of thymocytes. While displaying normal development of CD2, CD3, CD4, and CD8 expression, CD25-deficient cortical thymocytes do not express CD1, and furthermore they fail to normally down-regulate levels of the anti-apoptotic protein bcl-2.
The high-affinity receptor for interleukin 2 (IL-2) (1) is composed of three subunits: α (CD25), β (CD122), and γ (γcommon). While the β and γ chains are constitutively expressed upon T lymphocytes, α expression is restricted to the early stages of thymocyte development and to activated mature T lymphocytes. Although the β and γ chains together can form an IL-2 receptor (IL-2R) of low affinity, the α chain cannot form a functional receptor in the absence of the others (1). The presence of the high-affinity receptor upon activated peripheral T cells is believed to be necessary for optimal proliferative responses to IL-2 after stimulation of the T cell antigen receptor; however the role of the αβγ complex upon triple-negative thymocytes is less certain.
Deletion of the IL-2R γ chain in humans results in the development of severe combined immunodeficiency (2) (X-linked) characterized by a great reduction in, or absence of, peripheral T lymphocytes (3), due to the participation of the γ chain in multiple cytokine receptor complexes (i.e., with interleukins 2, 4, 7, 9, and 15). In mice, B cell development is also ablated (4). In sharp contrast, the absence of CD122 manifests in the constitutive activation of B and T lymphocytes accompanied by the development of autoimmune responses resulting in premature death (5). The absence of CD25 in mice would appear to cause the least severe impairment of the three. Young CD25-null mice have phenotypically normal T and B cell populations, while older animals exhibit enlarged lymphoid glands due to increased B and T cell populations (as the result of inefficient activation-induced cell death) and a propensity to develop autoimmune disorders (6).
We describe here a human mutation of CD25 ablating its expression and the consequences upon immune function. Analysis of this patient suggests that expression of the IL-2R α chain is necessary not only in the functional responses of mature T cells but also in preventing the generation and/or controlling of autoreactive T lymphocytes.
MATERIALS AND METHODS
Tissue Section Preparation and Staining.
Thymic biopsies from the patient and a normal control undergoing cardiac surgery were snap frozen. Serial cryostat sections (4 μm) were mounted on glass slides and air-dried. Tissue sections were either stained with hematoxylin and eosin or immunostained with antibodies raised against CD1, CD25, bcl-2, or CD3, using a three-stage biotin–avidin–peroxidase staining procedure.
Flow Cytometry.
Epstein–Barr virus (EBV)-transformed B cells were stained with monoclonal anti-CD25 conjugated with phycoerythrin or with isotype-matched control antibodies (Coulter) and were analyzed on an Epics Coulter V flow cytometer.
Western Blots.
Peripheral blood lymphocytes (2 × 106) were lysed in SDS-sample buffer, electrophoresed on SDS/8% polyacrylamide gels, and transferred to nitrocellulose membranes (Hybond-C, Amersham). After nonspecific blocking with 5% milk solids in PBS, immunoblots with anti-IL-2Rα and anti-IL-2Rβ (Santa Cruz Biotechnology) were performed in PBS containing 0.05% Tween-20 and 1% milk solids. Bound antibody was detected with the secondary reagent horseradish peroxidase conjugated with donkey antibody to rabbit immunoglobulin (Amersham) and developed by enhanced chemiluminescence (ECL; Amersham).
Reverse Transcriptase–Polymerase Chain Reaction.
RNA was extracted from patient peripheral blood lymphocytes by using Trizol (GIBCO/BRL). Random priming was used in first strand cDNA synthesis and PCR was performed with primers corresponding to GGTCCCAAGGCTCAGGAAGATG and TGGTAAGAAGCCGGGAACAGACACAG, comprising CD25 5′ and 3′ untranslated sequences, respectively. The amplification conditions of 94°C denaturation for 30 sec, 60°C annealing for 30 sec, and 72°C extension for 60 sec were used for 35 cycles in a Perkin–Elmer 9600 thermal cycler. PCR products were cloned in pUC19 and sequenced with the Sequenase kit (United States Biochemical) accoding to the instructions of the supplier.
RESULTS AND DISCUSSION
Immunological Phenotype.
A male child of first cousin parentage presented with increased susceptibility to viral, bacterial, and fungal infections, suffering from cytomegalovirus (CMV) pneumonitis, persistent oral thrush, and Candida esophagitis at the age of 6 months. He also had adenovirus gastroenteritis, developed chronic diarrhea, and failed to thrive. From the age of 8 months lymphadenopathy and hepatosplenomegaly became increasingly apparent, with no significant abnormality of liver function. He subsequently required hospitalization for recurrent exacerbation of lung disease. By the age of 3 years he had developed gingivitis, iron deficiency anemia with no evidence of hemolytic anemia, chronic inflammation of his mandible, and chronic lung disease involving the right upper lobe. While serum IgM levels were normal (0.2–1.5 g/liter) and IgG somewhat increased (7–15 g/liter; normal age-matched values are 4.5–14.3 g/liter), IgA levels were low (<0.07 g/liter, normal 0.2–1 g/liter). The absolute number of peripheral CD3+ T lymphocytes was relatively low (922 CD3+ cells per μl, normal range 885–2270), with a significant reduction in CD3+ CD4+ cells (30% of peripheral mononuclear cells, normal range 60–85%) resulting in an abnormal CD4/CD8 ratio of 1:1 (normal 2:1). In vitro assays of peripheral lymphocyte function demonstrated a reduced responsiveness to stimulation by anti-CD3 (11% of control), phytohemagglutinin (20% of control), and other mitogens. Addition of exogenous IL-2 (500 units/ml) could not significantly rescue these proliferative responses. Severe immunological dysfunction was proven by the patient’s inability to reject an allogeneic skin graft. Consequently the patient underwent an allogeneic bone marrow transplant following cytoreduction. Engraftment was rapid and a complete resolution of symptoms ensued.
Absence of CD1 Expression on Cortical Thymocytes.
Atypically for profound cellular immunodeficiency, the thymus was of normal size, although displaying minimal Hassall’s corpuscles and a lack of distinct cortical–medullary demarcation (Fig. (Fig.1,1, top row). Whereas immunohistochemical staining of thymus sections for CD3 (Fig. (Fig.11 bottom row), CD2, CD4, CD8, and class I and class II major histocompatibility complex proteins (not shown) revealed normal patterns of expression, staining for CD1 was completely negative (Fig. (Fig.11 second row).
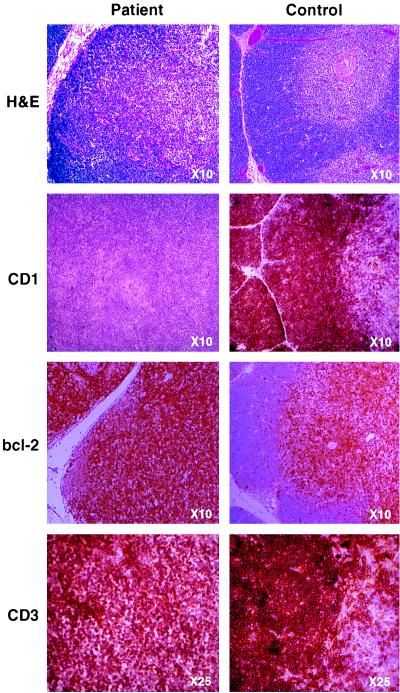
In immunohistological comparison of patient (Left) and normal age-matched (Right) thymus sections, hematoxylin and eosin (H&E) staining revealed fibrous septa separating lobules of lymphoid tissue as seen in normal thymus. However, although cortex and medulla are present in the patient thymus, there is a loss of the normally distinct demarcation between the two areas. Immunohistochemical analysis clearly showed lack of expression of CD1 (second row) but normal staining for CD3 (bottom row), while control samples stained strongly for all three markers. bcl-2 expression in the normal thymus is restricted to the medulla, with few positive cells present within the cortex, whereas the patient’s sample displays a uniformly high level of bcl-2 expression (third row). (Figure is printed at 74% of original magnification.)
The CD1 family consists of five members (a–e), all structurally related to class I major histocompatibility complex proteins (7). In normal thymus CD1a is highly expressed on cortical thymocytes and dramatically down-regulated upon progression into the medulla. These proteins may function as nonclassical antigen-presenting molecules, as CD1b has been shown to present mycobacterial lipoglycan antigens (8, 9). A mutation in CD1 was unlikely to be the primary cause of immunodeficiency, as patient monocytes up-regulated CD1 expression normally upon in vitro stimulation with granulocyte/macrophage colony-stimulating factor and IL-4 (10) (not shown). However, to conclusively exclude this possibility, sequencing of patient CD1a cDNA was performed; it was found to be normal. This suggested a failure to provide appropriate signals for CD1 up-regulation in thymocytes. Currently, the significance of CD1 deficiency during T cell differentiation is difficult to identify. It is possible that rather than serving simply as a nonclassical antigen-presenting molecule, CD1a may participate in mediating thymocyte–epithelium interactions and in its absence, be partially responsible for the loss of the distinct corticomedullary boundary.
Absence of IL-2R α Chain (CD25) Expression.
Since expression of CD25 occurs at an early stage in thymocyte differentiation, preceding CD1 up-regulation (11), we hypothesized that an IL-2 signal may be necessary for CD1 induction. Indeed, immunohistochemical analysis of patient thymus sections revealed an absence of CD25 expression (not shown). Similarly, an EBV-transformed cell line derived from patient peripheral B lymphocytes did not express detectable CD25 by flow cytometry, whereas EBV-transformed lines from normal individuals typically contained 20–40% CD25-positive cells (Fig. (Fig.22A). Western blot analysis of lysates prepared from patient peripheral blood lymphocytes confirmed the absence of CD25 protein (Fig. (Fig.22B). In contrast, expression of the IL-2R β chain was elevated in patient lymphocytes, presumably reflecting compensation for the lack of the α chain CD25.
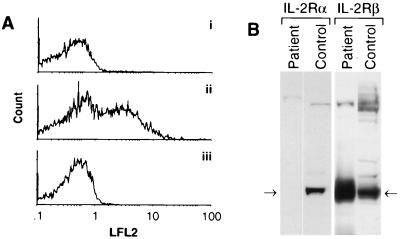
(A) EBV-transformed patient B lymphocytes (trace i) and normal control B lymphocytes (trace ii) were stained with anti-CD25 and analyzed by flow cytometry, demonstrating the absence of CD25 expression on patient cells (1.1% positive) compared with a normal control (44% positive). Isotype-matched antibody control staining of patient cells (1.8% positive) is shown in trace iii. LFL2, count. (B) Western blot analysis demonstrates the absence of IL-2Rα protein and elevated IL-2Rβ expression in patient peripheral blood lymphocytes compared with normal.
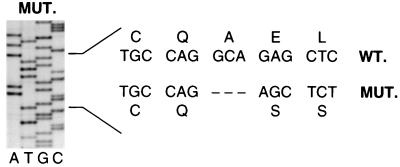
Two forms of CD25 (12–14) message were isolated from patient lymphocyte RNA by reverse transcriptase–polymerase chain reaction, representing the full-length sequence (coding the active receptor) and a secondary splicing product lacking exon 4 (coding an inactive receptor). All forms of the patient’s CD25 message were found to contain a 4-bp deletion at base pairs 60–64, resulting in a translational frameshift (Fig. (Fig.3).3). Translation proceeds for 20 amino acids before the deletion and resultant frameshift occurs, effectively ablating CD25 expression. A further 25 irrelevant amino acids are added before termination. The homozygous nature of the mutation was confirmed by analysis of sequences from the parents, who both demonstrated a normal and 4-bp-deleted allele.
Dense Lymphocytic Infiltrates of Multiple Tissues Are Observed.
The phenotype of CD25 deficiency differs significantly from most other cellular immunodeficiencies, with a normal size thymus and most noticeably lymphocytic infiltration of multiple tissues (Fig. (Fig.4).4). Unlike CD25−/− mice, which have infiltrates limited to the gastrointestinal tract (6), human CD25 deficiency manifests dense lymphocytic infiltration of lung (Fig. (Fig.44 Upper), liver (Fig. (Fig.44 Lower), gut, soft tissue, and bone, accompanied by tissue atrophy and chronic inflammation. The lymphocytic infiltration observed in CD25−/− mice was suggested to occur as the result of inefficient antigen-mediated deletion of activated mature T cells (6). However, analysis of the human disease suggests that one cause of infiltration may lie in abnormal thymic differentiation.
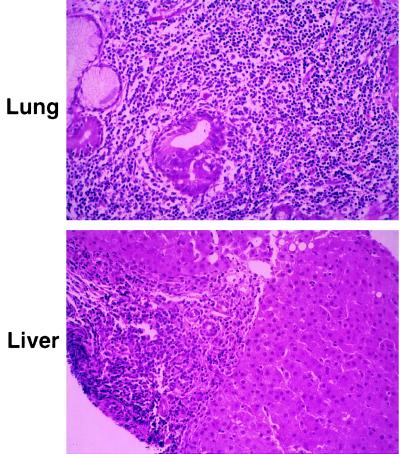
Light microscopy of hematoxylin and eosin-stained sections of patient lung biopsy (Upper; ×18,250) shows dilatation of airways, partially collapsed lung tissue, and dense infiltration of lymphocytes in the bronchial wall as well as in the lung parenchyma. No fungal elements were identified by specific staining and no viral particles were identified by electron microscopy (not shown). In addition, hepatitis B and C virus, EBV, and cytomegalovirus sequences were not detected by PCR. Light microscopy of liver sections (Lower; ×18,250) shows preserved lobular architecture, with marked infiltration of lymphocytes in the portal tracts. Consistent with the analysis of the lung biopsy, no fungal or viral elements were identified. Similar dense lymphocytic infiltrates were found in stomach and duodenal biopsies (not shown).
Failure to Decrease Cortical Thymocyte Levels of the Anti-apoptotic bcl-2 Protein.
Normally the induction of CD1 expression in cortical thymocytes precedes a dramatic decrease in the cellular level of the bcl-2 protein (15–18). The presence of the bcl-2 gene product acts to protect cells against programmed cell death, without promoting proliferation (19). Early immature triple-negative thymocytes, and more mature double-positive (CD1− CD3hi) medullary thymocytes, express high levels of bcl-2 and are relatively resistant to the induction of apoptosis (15, 16, 19). To permit immature thymocytes to undergo positive and negative selection, both of which frequently (95%) result in the induction of programmed cell death, a reduction in bcl-2 levels is believed to be required. Whereas normal thymocytes demonstrated distinct areas of low (cortical) and high (medullary) bcl-2 expression, staining of patient thymus sections revealed that all CD25-deficient thymocytes express high levels of bcl-2 protein (Fig. (Fig.11 bottom row). The lack of regulation of bcl-2 expression suggests a possible dependency upon signals from either CD1 or CD25.
It has been reported that CD25+ CD4+ T cells maintain self-tolerance in mice by suppressing autoreactive cells (20). Indeed, even in the absence of thymic irregularities, autoreactive responses emerged in the absence of CD25+ T cells (20). This suggests that the lymphocytic infiltrates observed in human CD25 deficiency could be autoimmune in nature. The persistence of high bcl-2 expression in cortical thymocytes would appear to support this view, possibly permitting some thymocytes bearing autoreactive T cell antigen receptors to escape deletion, as seen in some transgenic mice overexpressing bcl-2 (21). Furthermore, treatment of the CD25-deficient patient with corticosteroids and cyclosporin A decreased inflammation and induced a dramatic reduction of lymphadenopathy and hepatosplenomegaly, supporting the hypothesis that the cellular infiltrates were contributing to tissue damage.
It is conceivable that a combination of at least two events, one intrathymic and another peripheral, may combine to create the phenotype observed in CD25 deficiency: first, a potentially inefficient negative selection process rooted in abnormal thymic differentiation, as evinced in the failure to express CD1 and to regulate bcl-2, and second, an inability to control potential autoreactive cells within the periphery due to the absence of the CD25+ CD4+ T cell subset (20). Inefficient activation-induced cell death, as observed in the CD25 knockout mouse (6), may also contribute to the phenotype observed; but because the patient has undergone bone marrow transplantation we are now unable to examine this possibility. The lack of CD25 expression may also compromise the ability of peripheral T cells to proliferate after stimulation and thus result in the profound immunodeficiency observed.
Acknowledgments
We thank Wilson Chan and Ilan Dalal for their technical assistance and A. Cohen and M. Julius for their critical review of the manuscript. This work was supported by the Medical Research Council of Canada and the Reichmann Immunodeficiency Fund.
ABBREVIATIONS
| IL-2 | interleukin 2 |
| IL-2R | IL-2 receptor |
| EBV | Epstein–Barr virus |
References
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.94.7.3168
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc20340?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Antiproliferative and Anti-Inflammatory Effects of the Polyphenols Phloretin and Balsacone C in a Coculture of T Cells and Psoriatic Keratinocytes.
Int J Mol Sci, 25(11):5639, 22 May 2024
Cited by: 0 articles | PMID: 38891824 | PMCID: PMC11171971
Single cell characterization of blood and expanded regulatory T cells in autoimmune polyendocrine syndrome type 1.
iScience, 27(4):109610, 27 Mar 2024
Cited by: 0 articles | PMID: 38632993 | PMCID: PMC11022049
Tipping the balance in autoimmunity: are regulatory t cells the cause, the cure, or both?
Mol Cell Pediatr, 11(1):3, 20 Mar 2024
Cited by: 1 article | PMID: 38507159 | PMCID: PMC10954601
Review Free full text in Europe PMC
Low-dose Interleukin-2 Therapy: Fine-tuning Treg in Solid Organ Transplantation?
Transplantation, 108(7):1492-1508, 31 Jan 2024
Cited by: 1 article | PMID: 38294829 | PMCID: PMC11188637
Review Free full text in Europe PMC
A History and Atlas of the Human CD4+ T Helper Cell.
Biomedicines, 11(10):2608, 23 Sep 2023
Cited by: 1 article | PMID: 37892982 | PMCID: PMC10604283
Review Free full text in Europe PMC
Go to all (230) article citations
Other citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Human IL-2 receptor alpha chain deficiency.
Pediatr Res, 48(1):6-11, 01 Jul 2000
Cited by: 80 articles | PMID: 10879793
Interleukin-7 and infection itself by human immunodeficiency virus 1 favor virus persistence in mature CD4(+)CD8(-)CD3(+) thymocytes through sustained induction of Bcl-2.
Blood, 98(7):2166-2174, 01 Oct 2001
Cited by: 22 articles | PMID: 11568004
Loss of Bim results in abnormal accumulation of mature CD4-CD8-CD44-CD25- thymocytes.
Immunobiology, 212(8):629-636, 15 Jun 2007
Cited by: 15 articles | PMID: 17869640 | PMCID: PMC2074878
A novel activation pathway for mature thymocytes. Costimulation of CD2 (T,p50) and CD28 (T,p44) induces autocrine interleukin 2/interleukin 2 receptor-mediated cell proliferation.
J Exp Med, 168(4):1457-1468, 01 Oct 1988
Cited by: 34 articles | PMID: 3049912 | PMCID: PMC2189071