Abstract
Free full text

Cell size and invasion in TGF-β–induced epithelial to mesenchymal transition is regulated by activation of the mTOR pathway
Abstract
Epithelial to mesenchymal transition (EMT) occurs during development and cancer progression to metastasis and results in enhanced cell motility and invasion. Transforming growth factor-β (TGF-β) induces EMT through Smads, leading to transcriptional regulation, and through non-Smad pathways. We observe that TGF-β induces increased cell size and protein content during EMT. This translational regulation results from activation by TGF-β of mammalian target of rapamycin (mTOR) through phosphatidylinositol 3-kinase and Akt, leading to the phosphorylation of S6 kinase 1 and eukaryotic initiation factor 4E–binding protein 1, which are direct regulators of translation initiation. Rapamycin, a specific inhibitor of mTOR complex 1, inhibits the TGF-β–induced translation pathway and increase in cell size without affecting the EMT phenotype. Additionally, rapamycin decreases the migratory and invasive behavior of cells that accompany TGF-β–induced EMT. The TGF-β–induced translation pathway through mTOR complements the transcription pathway through Smads. Activation of mTOR by TGF-β, which leads to increased cell size and invasion, adds to the role of TGF-β–induced EMT in cancer progression and may represent a therapeutic opportunity for rapamycin analogues in cancer.
Introduction
Translational control and regulation of cell size are essential cellular processes that govern the development and homeostasis of cells and tissues (Ruvinsky and Meyuhas, 2006). The protein synthesis machinery has been largely considered an autonomous entity whose overall output is subject to a limited number of control mechanisms. However, several components of the translational machinery and, consequently, the process of protein biosynthesis are controlled by signaling pathways and transcriptional regulation (Hay and Sonenberg, 2004). In addition, changes in the control of translation are associated with carcinogenesis. Specifically, ribosome function can be modulated by tumor suppressors and oncogenes, whereas certain signaling pathways enhance the translational capacity of the cell. Deregulation of one or more steps that control protein biosynthesis has been associated with alterations in cell cycle progression and cell growth (Ruggero and Pandolfi, 2003).
Activation of mammalian target of rapamycin (mTOR) has emerged as a regulatory mechanism that is conserved from yeast to mammals in the control of protein biosynthesis and cell size (Wullschleger et al., 2006). mTOR is a large serine/threonine protein kinase that is found in two distinct multiprotein complexes: mTOR complex 1 (mTORC1; containing mLST8 and raptor), which has been implicated in translational regulation (Wullschleger et al., 2006), and mTORC2 (containing mLST8, mSin1, and rictor; Sarbassov et al., 2004; Frias et al., 2006). Rapamycin in complex with FKBP12 interacts with mTOR and inhibits its activity when mTOR is part of mTORC1 (Wullschleger et al., 2006). mTOR activity is increased in many tumors, which is consistent with its pivotal role in protein biosynthesis, and specific inhibition of mTOR function through the use of rapamycin analogues is considered a promising avenue for cancer treatment (Faivre et al., 2006; Hynes and Boulay, 2006; Smolewski, 2006).
The best-characterized effectors of mTOR in the rapamycin-sensitive complex are S6 kinase 1 (S6K1) and the eukaryotic initiation factor 4E–binding protein 1 (4E-BP1). Phosphorylation of S6K1 by mTOR enhances the translational capacity by acting on translation initiation complex assembly (Hay and Sonenberg, 2004; Holz et al., 2005). Phosphorylation of 4E-BP1 by mTOR induces the dissociation of eukaryotic initiation factor 4E from 4E-BP1, which enhances the cap-dependent initiation of mRNA translation (Hay and Sonenberg, 2004).
mTOR serves as a sensor and integrator of multiple stimuli induced by growth factors, nutrients, energy, or stress. The best-characterized signaling pathway that regulates mTOR activity in mTORC1 is initiated by the activation of phosphatidylinositol 3-kinase (PI3K), which enhances the phosphorylation of Akt (also known as PKB; Fingar and Blenis, 2004; Hay and Sonenberg, 2004). Akt phosphorylation inactivates the tuberous sclerosis complex (TSC) formed by hamartin (TSC1) and tuberin (TSC2; Manning and Cantley, 2003), leading to accumulation of the GTP-bound form of the small G protein Rheb that activates mTOR (Fingar and Blenis, 2004). The pathway from PI3K to mTOR is up-regulated in many cancers, as reflected by the increased phosphorylation of PI3K and Akt, which correlates with increased mTOR activity (Guertin and Sabatini, 2005; Faivre et al., 2006). Growth factors that act through tyrosine kinase receptors have the ability to activate PI3K. Most prominent among these are insulin and insulin-like growth factor-1 (IGF-1; Grimberg, 2003). The up-regulation of IGF-1 expression and autocrine responses in many tumors may well be a major factor in the increased PI3K signaling and mTOR activity in cancers. Inhibitors of PI3K are accordingly pursued for targeted cancer therapy (Kim et al., 2005; Faivre et al., 2006).
TGF-β, a secreted cytokine, regulates a variety of processes in development and cancer and exerts many autocrine activities. The expression of TGF-β, specifically TGF-β1, is up-regulated in many, if not most, cancers and is thought to play a key role in cancer progression (Bierie and Moses, 2006). In early tumor progression, deregulation of TGF-β signaling leads to a loss of its autocrine antiproliferative effect, resulting in increased cell proliferation. However, the increased TGF-β expression by tumor cells provides distinct advantages for cancer progression (e.g., by inducing localized immunosuppression or enhanced angiogenesis; Bierie and Moses, 2006). In addition, this increased TGF-β expression benefits cancer progression through autocrine effects on the tumor cells. Specifically, TGF-β can induce an epithelial to mesenchymal transition (EMT) of carcinoma cells, which leads to invasion and metastasis (Derynck et al., 2001; Zavadil and Bottinger, 2005; Thiery and Sleeman, 2006). EMT is a complex process that involves cytoskeletal remodeling, cell–cell and cell–matrix adhesion, and transcriptional regulation, leading to the transition from a polarized epithelial phenotype to an elongated fibroblastoid phenotype (Zavadil and Bottinger, 2005; Thiery and Sleeman, 2006). A disassembly of cell–cell junctions, including the relocalization of E-cadherin and zonula occludens-1 (ZO-1), occurs during EMT and allows for an increase in cell motility. As a result of EMT, the fibroblastoid cells degrade the extracellular matrix and show invasive behavior. The key role of this TGF-β–mediated process in cancer is illustrated by the ability of TGF-β inhibitors to diminish cancer progression and metastasis (Arteaga, 2006).
TGF-β signals through a complex of two types of serine/threonine kinase receptors. Upon ligand binding, the type II receptors activate the type I receptors, which recruit and activate the intracellular mediators Smad2 and Smad3. Smad2 and Smad3 combine with Smad4 to form complexes that translocate into the nucleus to activate or repress gene expression (Shi and Massagué, 2003; Feng and Derynck, 2005). Increasing evidence shows that Smad signaling leading to transcription responses is complemented by non-Smad signaling mechanisms that are also activated by TGF-β (Derynck and Zhang, 2003; Moustakas and Heldin, 2005). TGF-β–induced EMT, which is largely studied using NMuMG epithelial cells as a model, integrates Smad as well as non-Smad signaling, including signaling through small GTPases and the Erk and p38 MAPK pathways (Moustakas and Heldin, 2005; Zavadil and Bottinger, 2005).
In our studies of TGF-β–induced EMT using NMuMG cells as a model, we observed that the typical loss of the epithelial phenotype with concomitant acquisition of the spindle-shaped fibroblastoid phenotype was accompanied by an increase in cell size and protein content. The TGF-β–induced increases in cell size and protein synthesis correlated with a rapid activation of mTOR and phosphorylation of its effectors S6K1 and 4E-BP1 through the PI3K–Akt pathway. Inhibition of mTOR by rapamycin blocked the TGF-β–induced increases in cell size and protein synthesis without affecting the EMT phenotype and inhibited cell migration, adhesion, and invasion. Although these results establish mTOR activation as a novel non-Smad TGF-β signaling pathway, they also reveal the direct regulation of protein synthesis by TGF-β as an effector pathway that complements the transcriptional regulation through Smads. The autocrine response of cells to TGF-β–induced mTOR activation and protein synthesis may represent a novel mechanism through which the increased TGF-β expression in tumor cells contributes to cancer progression.
Results
The TGF-β–induced EMT is associated with an increase in cell size and protein content
Murine mammary epithelial NMuMG cells are known to undergo an EMT that is readily apparent at 36 h after TGF-β treatment (Miettinen et al., 1994). As a result of EMT, the cells adopt a spindlelike shape with actin reorganization and E-cadherin delocalization from the adherens junctions (Fig. 1, A and B). To better characterize the EMT in NMuMG cells, we used time-lapse microscopy during the first 36 h after adding TGF-β. No major change in cell morphology was apparent during the first 24 h of TGF-β treatment, and the treated cells elongated between 24 and 36 h after adding TGF-β (Videos 1 and 2, available at http://www.jcb.org/cgi/content/full/jcb.200611146/DC1).
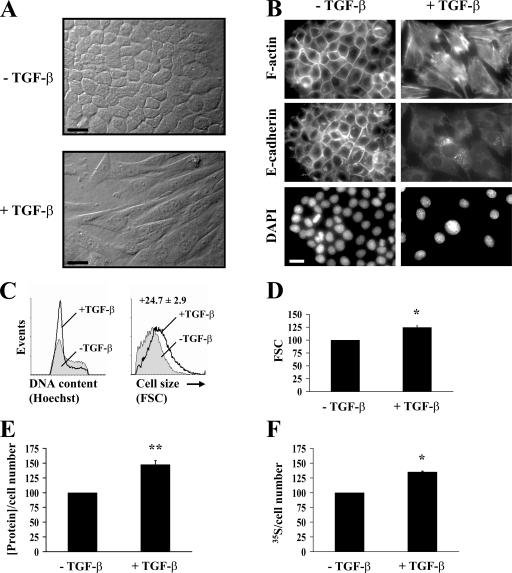
Increase in cell size and protein content during the TGF-β–induced EMT in NMuMG cells. (A) Cells were treated or not treated with TGF-β for 48 h before photography. Original magnification was 40×. See Videos 1 and 2 (available at http://www.jcb.org/cgi/content/full/jcb.200611146/DC1). (B) Cells treated or not treated with TGF-β for 48 h were stained for F-actin or E-cadherin. Nuclei were visualized using DAPI staining. Original magnification was 100×. (C) After 48 h of TGF-β treatment or no treatment, the cell cycle distribution was determined by flow cytometry analysis (left), and the size distribution of cells in G1 phase was determined by flow cytometry using the forward scatter parameter (FSC; right). (D) Quantification of the forward scatter normalized to untreated cells shows relative mean values of one representative experiment out of seven with SD (error bars) for duplicates (*, P < 0.05 vs. control). (E) 24 h after adding TGF-β, the total protein content was measured and normalized for cell number, as shown relative to untreated cells. One representative experiment out of five is shown with SD for triplicates (**, P < 0.01 vs. control). (F) 24 h after adding TGF-β, cells were [35S]-methionine/cysteine labeled, and trichloroacetic acid–precipitated proteins were quantified. The newly synthesized protein value is normalized to the cell number and shown relative to untreated cells. One out of two representative experiments is shown with SD for triplicates (*, P < 0.05 vs. control). Bars (A), 50 μm; (B) 20 μm.
Concomitantly with the change in phenotype, the cells appeared to increase in size (Fig. 1, A and B). To assess whether this was indeed the result of an increase in cell size rather than a more spread phenotype, we evaluated the size of treated and untreated cells by flow cytometry using forward scatter as the parameter indicative of cell size. Furthermore, because cell size varies with cell cycle progression (Grebien et al., 2005), we measured the size of only the cells in the G1 phase. At 24 h after adding TGF-β, no difference in cell size was apparent between TGF-β–treated and untreated NMuMG cells (unpublished data). In contrast, after 48 h, TGF-β–treated cells consistently showed an increase in cell size of 10–30% (Fig. 1, C and D). We observed that the TGF-β–treated cells were also larger in the S and G2/M phases of the cell cycle (unpublished data). After EMT, the removal of TGF-β resulted in enhanced proliferation and mesenchymal to epithelial transition (unpublished data). This reversibility of the phenotype suggests that the increase in cell size did not result from senescence, which is consistent with the lack of senescence-associated β-galactosidase staining (Dimri et al., 1995; unpublished data).
Because changes in cell size often correlate with differences in protein content, we measured the protein content in untreated and TGF-β–treated NMuMG cells. TGF-β increased the protein synthesis in NMuMG cells from 20 to 60% after 24 h (Fig. 1 E), whereas no effect of TGF-β on protein synthesis was observed after 6 and 12 h (not depicted). To confirm the effect of TGF-β on protein synthesis, we measured the incorporation of 35S-labeled methionine into newly synthesized proteins at 24 h after TGF-β treatment. As shown in Fig. 1 F, we found that TGF-β induces an increase in the synthesis of new proteins.
TGF-β activates the mTOR pathway
mTOR signaling has been implicated in the control of protein synthesis through the phosphorylation of S6K1 and 4E-BP1 (Hay and Sonenberg, 2004). Because TGF-β increases protein synthesis, we explored the effect of TGF-β on S6K1 phosphorylation in NMuMG cells. Immunoblot analyses with antibodies specific to the phosphorylated form of S6K1 showed that TGF-β induced the phosphorylation of S6K1 within 1 h after adding TGF-β, reaching maximum phosphorylation at 24 h (Fig. 2 A). TGF-β also induced the phosphorylation of 4E-BP1 with a peak at 1 h of treatment, as apparent from immunoblot analyses using an antibody to phospho-Ser65 4E-BP1 (Fig. 2 B). The TGF-β–induced phosphorylation of 4E-BP1 appeared more transient than the S6K1 phosphorylation and was no longer apparent at 24 h.
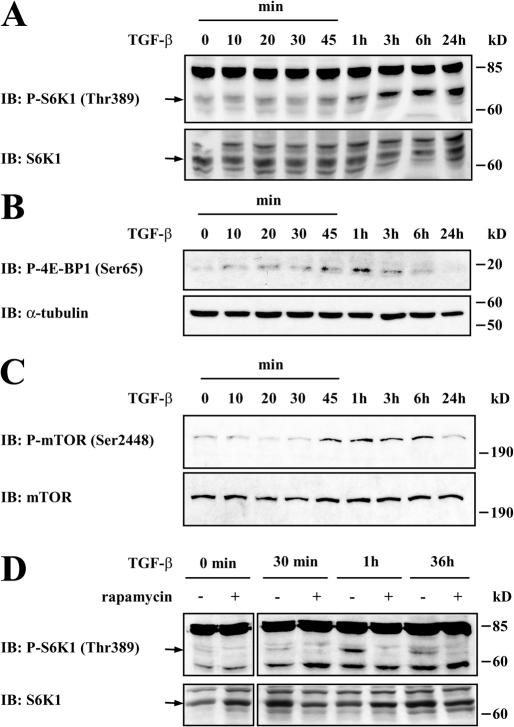
TGF-β induces mTOR, S6K1, and 4E-BP1 phosphorylation. (A–C) NMuMG cells were treated with TGF-β for the indicated times before lysis, SDS-PAGE, and immunoblotting (IB) with an antibody to phospho-S6K1 (A), phospho–4E-BP1 or α-tubulin (B), or phospho-mTOR (C). The phospho-S6K1 and phospho-mTOR immunoblots were reprobed with S6K1 and mTOR antibody, respectively. (D) NMuMG cells were pretreated without or with rapamycin for 1 h, and TGF-β was added for the indicated times. Cell lysates were separated by SDS-PAGE and immunoblotted with phospho-S6K1 antibody, stripped, and reblotted with S6K1 antibody.
In response to insulin or other inducers of protein synthesis, the phosphorylation of S6K1 and 4E-BP1 results from mTOR activation (Hay and Sonenberg, 2004). It has been shown that the phosphorylation of mTOR on Ser2448 correlates with its activation in response to insulin or growth factor stimulation (Fingar and Blenis, 2004). Therefore, we examined whether mTOR is phosphorylated on Ser2448 in response to TGF-β using a phospho-Ser2448–specific antibody. As shown in Fig. 2 C, TGF-β induced the phosphorylation of mTOR with kinetics similar to the TGF-β–induced phosphorylation of S6K1 and 4E-BP1. To establish a causal relationship, we examined the effect of rapamycin, an inhibitor of mTOR activation, on S6K1 phosphorylation in response to TGF-β. As shown in Fig. 2 D, TGF-β did not induce S6K1 phosphorylation in the presence of rapamycin after 1 h, implicating mTOR activation in TGF-β–induced S6K1 phosphorylation. The effect of rapamycin on TGF-β–induced S6K1 phosphorylation was still apparent at 36 h (Fig. 2 D).
mTOR and S6K1 activation by TGF-β is mediated by TβRI, PI3K, and Akt
mTOR activation in response to tyrosine kinase receptors has been shown to result from the activation of PI3K, which, in turn, leads to the activation of Akt (Hay and Sonenberg, 2004). We investigated whether the activation of mTOR by TGF-β occurs through the activation of PI3K, which then activates Akt. Because PI3K activates Akt through phosphorylation on Ser473, we examined the level of Akt activation in response to TGF-β in NMuMG cells using an antibody against phospho-Ser473 Akt. We found that TGF-β led to Akt phosphorylation after 30 min with a peak at 1 h (Fig. 3 A, top). This activation was inhibited by LY294002 or wortmannin, which are two inhibitors of PI3K, indicating that the TGF-β–induced phosphorylation of Akt occurred through the activation of PI3K (Fig. 3 B, top). Stimulation of NMuMG cells with TGF-β also resulted in the phosphorylation of Smad3. A peak level for TGF-β–induced Smad3 phosphorylation was reached after 30 min of stimulation (Fig. 3 A, bottom). Thus, Akt activation in response to TGF-β occurred with similar kinetics, albeit possibly somewhat slower than Smad3 activation. The PI3K inhibitors did not affect the TGF-β–induced phosphorylation of Smad3 (Fig. 3 B, bottom).
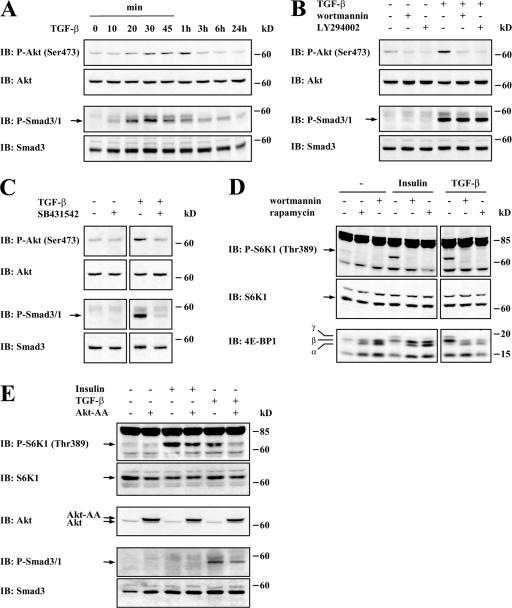
The TGF-β–induced mTOR pathway is activated by TβRI, PI3K, and Akt. (A) NMuMG cells were stimulated with TGF-β for the indicated times. Cell lysates were separated by SDS-PAGE and subjected to immunoblotting (IB) with phospho-Akt or phospho-Smad3/Smad1 antibodies and subsequent reblotting with Akt or Smad3 antibodies, respectively. (B) NMuMG cells were pretreated without or with wortmannin or LY294002 for 1 h, and TGF-β was added or not added to the medium for 1 h. Cell lysates were immunoblotted with antibodies to phospho-Akt or phospho-Smad3/Smad1 and reblotted with antibodies to Akt or Smad3, respectively. (C) NMuMG cells were treated without or with SB431542 for 1 h, and TGF-β was added or not added to the medium for 1 h. Cell lysates were immunoblotted with antibodies to phospho-Akt or phospho-Smad3/Smad1 and reprobed with antibodies to Akt or Smad3, respectively. (D) NMuMG cells were treated with or without rapamycin or wortmannin for 1 h, and TGF-β or 5 μg/ml insulin was added or not added to the medium for 1 h. Cell lysates were immunoblotted with antibodies to phospho-S6K1 or 4E-BP1 and reprobed with S6K1 antibody. (E) NMuMG cells were transfected to express a dominant-negative Akt mutant with Ser473 and Thr308 substituted by alanines. Cells were stimulated or not stimulated with TGF-β or 5 μg/ml insulin for 1 h. Cell lysates were immunoblotted with antibodies to phospho-S6K1, Akt, or phospho-Smad3/Smad1; phospho-S6K1 and phospho-Smad3/Smad1 immunoblots were reprobed with S6K1 or Smad3 antibodies, respectively.
TGF-β–induced Smad signaling results from activation of the type I TGF-β receptor (TβRI), which acts epistatically downstream from the type II receptor (a result of TβRII-mediated activation of the TβRI kinase). Whereas Smads are activated by TβRI, several responses have recently been directly linked to type II receptors without the functional requirement of the TβRI kinase activity (Ozdamar et al., 2005). Therefore, we examined whether the TGF-β–induced activation of Akt depended directly on the kinase activity of TβRI using the kinase inhibitor SB431542, which specifically binds the ATP-binding site of TβRI and does not inhibit TβRII. As shown in Fig. 3 C, SB431542 prevented the TGF-β–induced activation of Akt as well as Smad3 phosphorylation. We conclude that TGF-β induces Akt activation through activation of the TβRI kinase.
To further define the activating pathway leading to S6K1 and 4E-BP1 phosphorylation upon TGF-β treatment, we examined whether the phosphorylation of S6K1 and 4E-BP1 in response to TGF-β requires the activation of PI3K. To this end, we examined the effect of TGF-β on the phosphorylation of S6K1 and 4E-BP1 in the absence or presence of wortmannin, an inhibitor of PI3K, using insulin as a positive control to induce S6K1 and 4E-BP1 phosphorylation. Wortmannin inhibited the TGF-β–induced S6K1 and 4E-BP1 phosphorylation similar to its effect on insulin-induced phosphorylation and to the same extent as rapamycin, the inhibitor of mTOR (Fig. 3 D). Moreover, activation of Akt was required for TGF-β–induced S6K1 phosphorylation as for insulin-induced S6K1 phosphorylation because expression of an Akt mutant, in which both Ser473 and Thr308 have been replaced by alanines, blocked TGF-β–induced S6K1 phosphorylation (Fig. 3 E). This Akt-AA mutant has previously been shown to prevent Akt activation through dominant-negative interference (Stokoe et al., 1997). Together, these data illustrate that TGF-β induces the activation of mTOR and phosphorylation of its downstream targets S6K1 and 4E-BP1 through the activation of PI3K and Akt. Rapamycin decreases the TGF-β–dependent increase of cell size and protein synthesis.
The increase in cell size and protein synthesis concomitantly with activation of the mTOR–S6K1 pathway in response to TGF-β raises the question of how much the activation of mTOR accounts for the TGF-β–induced increase in cell size and protein synthesis. Therefore, we examined the effect of rapamycin on the TGF-β–induced cell size increase. Rapamycin inhibited the TGF-β–induced increase in cell size, as assessed by forward scatter in FACS analysis (Fig. 4 A). Because autocrine TGF-β signaling occurs in most, if not all, cells in culture, including NMuMG cells, we set out to define the contribution of autocrine TGF-β signaling by assessing in parallel the cellular response to SB431542, which prevents the TGF-β–induced activation of Akt and is expected to block autocrine TGF-β signaling. As shown in Fig. 4 B, SB431542 treatment in the absence of added TGF-β resulted in a decreased cell size. These results suggest that autocrine TGF-β signaling contributes to the control of cell size.
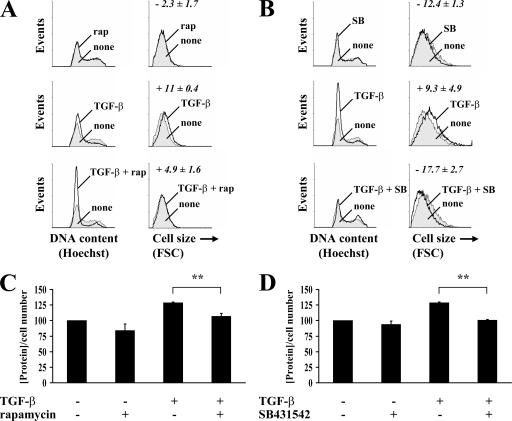
Effect of rapamycin on TGF-β–induced cell size and protein synthesis of NMuMG cells. (A and B) Cells were pretreated with or without rapamycin (A) or SB431542 (B) for 1 h, and TGF-β was added or not added to the medium for 48 h. The cell cycle distribution was determined by flow cytometry (left), and the size distribution of cells in G1 phase was determined by flow cytometry using the forward scatter parameter (FSC; right). The numbers represent the mean forward scatter percentage of cell size increase (+) or decrease (−) normalized to untreated cells. One representative experiment out of two independent experiments is shown with SD (error bars) for triplicates. (C and D) Cells were pretreated with or without rapamycin (C) or SB431542 (D) for 1 h, and TGF-β was added or not added to the medium. After 24 h, the cells were trypsinized, and the cell number was determined. The total protein content normalized for cell number is shown relative to untreated cells. One representative experiment out of two independent experiments is shown with SD for triplicates (**, P < 0.01).
Similar experiments were performed to evaluate the effects of rapamycin and SB431542 on protein synthesis. Consistent with the results on cell size, rapamycin and SB431542 both inhibited the TGF-β–induced increase in protein content (Fig. 4, C and D). Furthermore, SB431542 moderately decreased the protein content of NMuMG cells in the absence of added TGF-β. We conclude that similar to the control of cell size, autocrine TGF-β signaling through mTOR contributes to the control of protein synthesis.
Rapamycin does not affect the TGF-β–induced EMT phenotype
We compared the effects of rapamycin and SB431542 on TGF-β–induced EMT. As expected, inhibition of the kinase activity of TβRI using SB431542 prevented TGF-β from inducing EMT, and the cells maintained their epithelial phenotype. In contrast, rapamycin did not affect TGF-β–induced EMT. Thus, the cells acquired their elongated shape with a loss of E-cadherin localization at cell–cell junction and actin reorganization (Fig. 5 A). EMT is accompanied by the disruption of ZO-1 from tight junctions and increased N-cadherin and fibronectin expression (Bhowmick et al., 2001; Thiery and Sleeman, 2006). Rapamycin did not affect the changes in ZO-1, fibronectin, and N-cadherin subcellular localization that accompanied TGF-β–induced EMT as assessed by immunofluorescence nor did it affect the epithelial phenotype in the absence of added TGF-β (Fig. 5 B). Because rapamycin blocked the TGF-β–induced increase in protein content and size, the cells that underwent EMT in response to TGF-β and in the presence of rapamycin were smaller than in the absence of rapamycin (Fig. 4, A and C). Our results indicate that inhibition of the mTOR pathway does not affect the morphological changes associated with EMT but inhibits the TGF-β–induced increase in protein content and cell size that accompanies EMT of NMuMG cells.
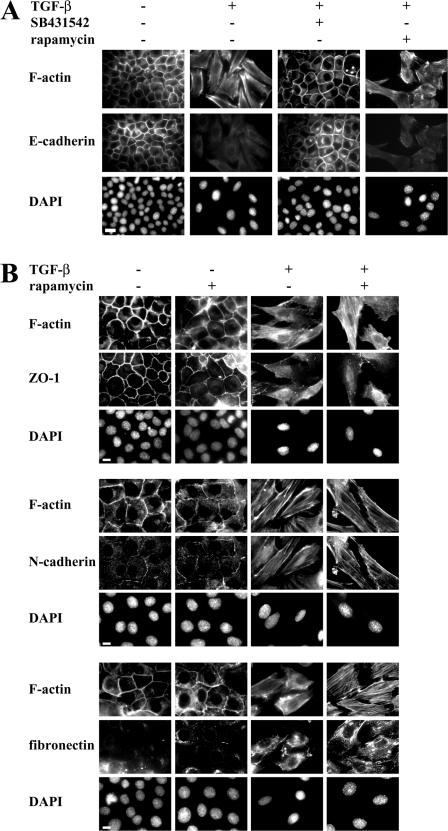
Effect of rapamycin on TGF-β– induced EMT. (A) NMuMG cells were treated with or without rapamycin or SB431542 for 1 h, and TGF-β was added or not added to the medium. After 48 h, the cells were stained for F-actin and E-cadherin. DAPI was used to stain the nuclei. Original magnification was 100×. (B) Cells were treated with or without rapamycin for 1 h, and TGF-β was added or not added to the medium. After 48 h, the cells were stained for F-actin, ZO-1, N-cadherin, or fibronectin. DAPI was used to stain the nuclei. Original magnification was 100×. Bars (A), 20 μm; (B) 10 μm.
Activation of mTOR during TGF-β–induced EMT in human HaCaT cells
To assess whether the data using murine NMuMG cells can be extended to other cell systems, we evaluated the human HaCaT keratinocytes, which also undergo EMT in response to TGF-β (Zavadil et al., 2001). As shown in Fig. 6 A, TGF-β induced the elongated morphology characteristic of EMT after 72 h of TGF-β treatment. The idea that HaCaT cells underwent EMT in response to TGF-β was also apparent from the filamentous actin (F-actin) stress fiber formation and E-cadherin relocalization from the junctions (Fig. 6 C).
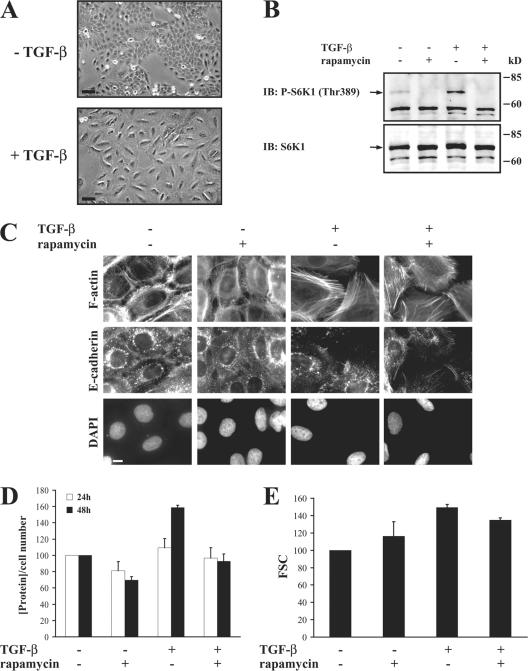
TGF-β activates the mTOR pathway during EMT in HaCaT cells. (A) Cells were treated or not treated with TGF-β for 72 h before photography. Original magnification was 20×. (B) HaCaT cells were pretreated with or without rapamycin for 1 h, and TGF-β was added or not added to the medium for 1 h. Cell lysates were separated by SDS-PAGE and immunoblotted (IB) with phospho-S6K1 antibody. The filter was then stripped and reblotted with S6K1 antibody. (C) Cells were treated with or without rapamycin for 1 h, and TGF-β was added or not added to the medium. After 72 h, the cells were stained for F-actin and E-cadherin. DAPI was used to stain the nuclei. Original magnification was 100×. (D) Cells were pretreated without or with rapamycin for 1 h, and TGF-β was added or not added to the medium. After 24 and 48 h, the cells were trypsinized, and the cell number was determined. The total protein content normalized for cell number is shown relative to untreated cells. One experiment out of two independent experiments is shown with SD (error bars) for triplicates. (E) Cells were pretreated without or with rapamycin for 1 h, and TGF-β was added or not added to the medium for 72 h. The size distribution of cells in G1 phase was determined by flow cytometry using the forward scatter (FSC) parameter. Quantification of the FSC normalized to untreated cells shows relative mean values of one experiment out of two with SD for triplicates. Bars (A), 50 μm; (C) 10 μm.
As in NMuMG cells, TGF-β induced the phosphorylation of S6K1 in HaCaT cells as assessed using a phospho-Thr389–specific antibody (Fig. 6 B). Rapamycin inhibited the TGF-β–induced phosphorylation of S6K1 (Fig. 6 B), confirming activation of the mTOR pathway by TGF-β during TGF-β–induced EMT. The rapamycin-induced decrease in S6K1 phosphorylation in the absence of TGF-β may reflect the autocrine TGF-β stimulation. Consistent with our results with NMuMG cells (Fig. 5), rapamycin did not inhibit the TGF-β–induced EMT phenotype in HaCaT cells (Fig. 6 C). It should be noted that the TGF-β–induced phosphorylation of S6K1 as assessed using a phospho-Thr389–specific antibody was not restricted to NMuMG and HaCaT cells, which undergo EMT in response to TGF-β, but also occurred in C2C12 mouse myoblasts, NRK-49F rat kidney fibroblasts, and 3T3-F442A mouse preadipocytes (unpublished data).
Consistent with the results of NMuMG cells, in HaCaT cells, TGF-β induced an increase in protein synthesis of ~60% after 48 h, whereas no difference was observed after 24 h (Fig. 6 D), and induced an increase in cell size of ~50% after 72 h (Fig. 6 E), whereas no difference was observed after 48 h (not depicted). The TGF-β–induced increases in protein synthesis and cell size were inhibited by rapamycin (Fig. 6, D and E). Thus, as in NMuMG cells, mTOR activation is required for the TGF-β– induced increases in protein synthesis and cell size but does not affect the TGF-β–induced EMT phenotype in HaCaT cells.
Rapamycin inhibits the enhanced migration and adhesion of NMuMG cells associated with TGF-β–induced EMT
Besides inducing the phenotypic characteristics of EMT, TGF-β treatment of NMuMG cells also results in behavioral changes that are associated with EMT and are important aspects of TGF-β's stimulatory role in tumorigenesis, such as an increase in cell motility and invasion (Bakin et al., 2000; Zavadil and Bottinger, 2005; Thiery and Sleeman, 2006). To evaluate whether the mTOR pathway plays additional roles in the EMT process of NMuMG cells in addition to its effect on protein content and cell size, we examined the effect of rapamycin on NMuMG cells treated with TGF-β to induce EMT.
We first examined the effect of rapamycin on the increased motility of cells in response to TGF-β using the monolayer wound assay (Pilkington et al., 1997). Thus, NMuMG cells were treated with or without TGF-β in the presence or absence of rapamycin for 48 h, at which time all TGF-β–treated cells had undergone EMT. At that time, the cell monolayers were replenished with fresh medium and wounded with a pipette tip, and closing of the wound as a result of cell migration was analyzed by time-lapse microscopy (Fig. 7 A and Videos 3 and 4, available at http://www.jcb.org/cgi/content/full/jcb.200611146/DC1). After 12 h, cells that had been treated with TGF-β in the absence of rapamycin and had undergone EMT showed a 77% wound closure. In contrast, cells that were not treated with TGF-β and thus had maintained their epithelial phenotype showed a 56% wound closure (Fig. 7 B). Exposure to rapamycin concomitantly with TGF-β treatment reduced the wound closure of cells that had undergone EMT from 77 to 42%. In cells that had not been exposed to TGF-β and thus had maintained their epithelial phenotype, rapamycin reduced the wound healing from 56 to 47% (Fig. 7 B). We also measured the migration speed of the cells in this experiment. We found that treatment with rapamycin concomitantly with TGF-β treatment and consequent EMT reduced the migration rate from 11.9 ± 1.5 to 6.5 ± 1.6 μm/h for cells that had been exposed to TGF-β.

Effect of rapamycin on migration and adhesion of NMuMG cells after TGF-β–induced EMT. (A) Cells were treated with or without rapamycin for 1 h, and TGF-β was added or not added to the medium for 48 h. The cell monolayer was then scratched to create a wound, and the migration of the cells was observed by time-lapse microscopy and photography at 0 and 12 h after wounding. Original magnification was 10×. See Videos 3 and 4 (available at http://www.jcb.org/cgi/content/full/jcb.200611146/DC1. (B) The percentage of wound healing was calculated from the mean of eight wound widths per condition with SD (error bars) measured at 12 h. One representative experiment out of three independent experiments is shown (*, P < 0.05; **, P < 0.01). (C) Cells were treated with or without rapamycin for 1 h, and TGF-β was added or not added to the medium. After 36 h, the fluorophore DiI was added for an additional 12 h. The cells were then trypsinized and placed in transwell filter chambers. Fluorescent measurements of migration of DiI-labeled cells through the transwell membrane were taken at 6 and 24 h. The result shows one representative experiment out of two independent experiments with SD for triplicates. (D) Cells were treated with or without rapamycin for 1 h, and TGF-β was added or not added to the medium. After 48 h, the cells were trypsinized and reseeded on plastic culture plates, and nonadherent cells were removed by washing the plates with PBS at 30 min after reseeding. The number of adherent cells was determined. One representative experiment out of two independent experiments is shown with SD for triplicates (**, P < 0.01). Bar, 200 μm.
We also measured the effect of rapamycin treatment with or without TGF-β on the migration of cells toward 10% FBS in transwell chambers (Pilkington et al., 1997). Confirming the monolayer wound-healing results, rapamycin reduced the enhanced migration of cells that were treated with TGF-β to a level that is comparable with that of NMuMG cells that were not treated with TGF-β and therefore had maintained their epithelial phenotype. This effect was apparent at 24 h after initiation of the transwell migration assay but not at 6 h (Fig. 7 C). We conclude that activation of the mTOR pathway in response to TGF-β plays an essential role in the increased motility and migration of cells that have undergone EMT in response to TGF-β.
Migration involves discrete phases of adhesion and de- adhesion (Angers-Loustau et al., 1999). Therefore, we examined whether rapamycin treatment affected the adhesion of cells to the culture plates. In this assay, cells were treated with or without TGF-β in the presence or absence of rapamycin for 48 h (as for the wound-healing and migration assays), dissociated using trypsin, and seeded back in plastic culture plates. After 30 min, nonadherent cells were removed by washing, and the adhered cells were released from the plastic and counted. Cells that were treated with TGF-β and thus had EMT conversion showed increased adhesion. In contrast, cells that had been treated with rapamycin together with TGF-β showed reduced adhesion (Fig. 7 D). Thus, inhibition by rapamycin of the increased cell motility and migration in response to TGF-β is associated with (and likely, in part, is the result of) effects of rapamycin on the TGF-β–induced increase in cell adhesion.
Rapamycin decreases the invasive behavior of NMuMG cells after TGF-β–induced EMT
Because rapamycin inhibited the migration of NMuMG cells that have undergone TGF-β–induced EMT, we also tested its effect on the invasive behavior of these cells. We used a standard in vitro invasion assay in which cells migrate through Matrigel and through the pores of a nitrocellulose filter toward 10% serum and are then counted (Albini et al., 2004). Cells that had undergone EMT showed a much higher level of invasive behavior than those that had not been exposed to TGF-β and thus had not undergone EMT (Fig. 8, A and B). In contrast, the addition of rapamycin concomitantly with TGF-β treatment to induce EMT resulted in a much lower level of invasion than cells that underwent TGF-β–induced EMT in the absence of rapamycin. In fact, the invasiveness of the TGF-β/rapamycin-treated cells was similar to that of the NMuMG cells that were not exposed to TGF-β and did not undergo EMT. Therefore, we conclude that rapamycin inhibits the TGF-β–induced invasion associated with cells that have undergone EMT in response to TGF-β and, thus, that activation of the mTOR pathway by TGF-β plays an essential role in the increased invasive behavior of NMuMG cells with TGF-β–induced EMT.
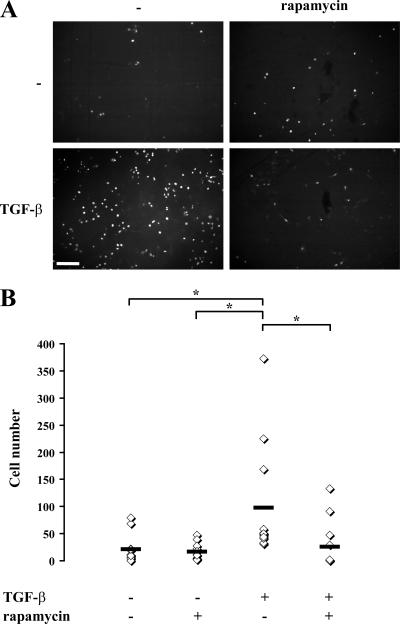
Effect of rapamycin on the invasion of NMuMG cells after TGF-β–induced EMT. NMuMG cells were treated with or without rapamycin for 1 h, and TGF-β was added or not added to the medium. After 48 h, cells were trypsinized and placed in Matrigel-coated transwell filter chambers and incubated for 24 h. The noninvading cells were removed from the upper surface of the membrane, cells that adhered to the lower surface were fixed, and the nuclei were stained with DAPI. (A) Photography of the DAPI staining using fluorescence microscopy. The result shows a field of one filter per condition in one representative experiment out of three independent experiments. Original magnification was 10×. (B) The DAPI-stained nuclei were counted in four fields per filter in triplicate in one representative experiment out of three independent experiments (*, P < 0.05). The horizontal bars represent the cell number mean of 12 fields for each condition. Bar, 200 μm.
Discussion
Although TGF-β signaling plays an important role as a tumor suppressor pathway, increased TGF-β expression by tumor cells is an important hallmark in cancer progression, as it contributes through autocrine and paracrine mechanisms to tumor development, invasion, and metastasis (Derynck et al., 2001; Bierie and Moses, 2006). A key role of TGF-β in cancer progression results from its ability to induce EMT, which confers not only changes in cell phenotype but also enhances cell migration and invasion (Zavadil and Bottinger, 2005; Thiery and Sleeman, 2006). Using NMuMG epithelial cells, which are the commonly used model for TGF-β–induced EMT (Miettinen et al., 1994; Thuault et al., 2006), and human HaCaT cells that also undergo EMT in the presence of TGF-β (Zavadil et al., 2001), we found that the phenotypic changes in response to TGF-β are accompanied by an increase in cell size and protein content. We also showed that TGF-β activates the mTOR pathway via PI3K and Akt and that its activation is responsible for the effect of TGF-β on cell size and protein synthesis. Thus, our results demonstrate that in addition to regulating transcription through Smads, TGF-β can induce enhanced translation by inducing phosphorylation of the major targets of mTOR, S6K1, and 4E-BP1. Finally, we show that blocking the mTOR pathway during TGF-β–induced EMT decreases the migration and invasion of the cells that have undergone EMT.
Increased cell size and protein synthesis during TGF-β–induced EMT
During cancer progression, changes in the regulation of protein synthesis have been shown to occur. In several types of cancers, translation initiation and protein synthesis are up-regulated through cell-autonomous changes in the translation machinery, whereas in other scenarios, enhanced levels of c-myc or increased IGF-1 secretion and associated autocrine responses are thought to lead to enhanced cell size and protein synthesis (Ruggero and Pandolfi, 2003). In turn, increases in cell size and protein synthesis enhance the proliferation of cancer cells because the control of protein synthesis and cell proliferation are intimately linked (Fingar and Blenis, 2004). Increased TGF-β expression by tumor cells was hitherto seen to contribute to cancer progression through its ability to induce EMT and through paracrine effects on the tumor microenvironment (Derynck et al., 2001; Bierie and Moses, 2006). The increased cell size and protein synthesis induced by TGF-β concomitantly with EMT is likely to represent another important contributing factor through which TGF-β stimulates cancer progression.
The TGF-β–induced increase in cell size during EMT is consistent with several other observations of cell size regulation by TGF-β family proteins. In Caenorhabditis elegans, TGF-β family signaling regulates body size (Krishna et al., 1999; Suzuki et al., 1999). In vertebrates, bone morphogenetic protein signaling participates in chondrocyte hypertrophy (Leboy et al., 1997; Volk et al., 1998), and myostatin/growth and differentiation factor-8 acts as a negative regulator of the skeletal muscle mass through effects on the size and number of myofibers (McPherron et al., 1997). Under pathological conditions, TGF-β can increase smooth muscle mass of the bronchi in a mouse model to study asthma (Goldsmith et al., 2006) and is also thought to be involved in the hypertrophy of cardiomyocytes (Brand and Schneider, 1995). We now present evidence that TGF-β induces increased cell size and protein content during EMT.
TGF-β activates the mTOR pathway during EMT
In studying the mechanisms of the TGF-β–induced cell size increase, we found that TGF-β rapidly activates mTOR and that this activation leads to the phosphorylation of two major mTOR targets, S6K1 and 4E-BP1. Growth factors such as insulin and IGF-1 that signal through receptor tyrosine kinases activate mTOR through PI3K and Akt activation (Hay and Sonenberg, 2004). Although TGF-β acts through serine/threonine kinase receptors, we found that TGF-β rapidly induces Akt phosphorylation. PI3K, Akt, mTOR, S6K1, and 4E-EBP1 are linked in one pathway activated by TGF-β, and this is supported by several observations. First, wortmannin and LY294002, which are two inhibitors of PI3K, prevent TGF-β–induced Akt phosphorylation. In addition, wortmannin, the mTOR inhibitor rapamycin, and a dominant-negative version of Akt all inhibit TGF-β–induced S6K1 phosphorylation. Finally, inhibition of the TβRI kinase using SB431542 prevents TGF-β–induced Akt phosphorylation. Therefore, we conclude that in response to TGF-β, TβRI activates the pathway that connects PI3K and Akt with mTOR, S6K1, and 4E-BP1. These results are consistent with the recent observation that the catalytic subunit of PI3K can interact with TβRI, leading to PI3K activation upon TGF-β stimulation (Yi et al., 2005).
In accordance with the induction of increased cell size through the mTOR pathway, rapamycin prevented the TGF-β– induced increase in cell size during EMT without affecting the associated morphological changes. Thus, in the presence of rapamycin, NMuMG and HaCaT cells underwent apparent EMT in response to TGF-β but were smaller than those cells that were not exposed to rapamycin. These results suggest that the rapamycin-sensitive mTORC1 pathway is not involved in the EMT phenotype itself, which is in agreement with other observations that rapamycin does not affect EMT (Bakin et al., 2000; Aguilera et al., 2005). These data also suggest that activation of PI3K and Akt leading to mTOR activation may not be involved in the morphological changes of EMT. This would be consistent with the inability of wortmannin to block EMT (Bakin et al., 2000).
The inhibition of TGF-β–induced S6K1 and 4E-BP1 phosphorylation by rapamycin suggests that TGF-β induces increased cell size through its effects on the rapamycin-sensitive mTORC1. Thus, activation of S6K1 by TGF-β is expected to enhance the translational machinery, and induction of 4E-BP1 phosphorylation leads to the enhanced translation of capped mRNAs (Hay and Sonenberg, 2004). Although S6K1 and 4E-BP1 appear to act as effectors in the increased protein translation upon TGF-β treatment, the effect of TGF-β on mTORC2 is currently unknown. mTORC2 regulates the actin cytoskeleton, and its activity may be regulated through PKCα and the small GTPases Rho and Rac (Wullschleger et al., 2006). Because EMT involves dramatic changes in the cytoskeleton that result from the activities of small GTPases, it will be important to evaluate whether TGF-β signaling regulates the activity of this complex and whether such regulation may play a role in EMT.
Another level of regulation by TGF-β: translational control
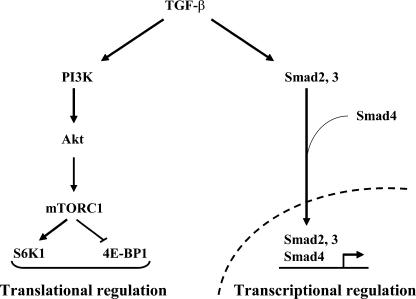
TGF-β family proteins signal through Smads, which combine with DNA sequence-specific transcription factors to activate or repress transcription (Feng and Derynck, 2005; Massagué et al., 2005). The Smad pathway, which, in the case of TGF-β, is mediated by Smad2 and Smad3 in combination with Smad4, is considered to be the major TGF-β family signaling pathway and accounts for the many changes in gene expression observed in response to TGF-β family proteins. TGF-β–induced non-Smad pathways have been identified and lead to the activation of Erk and JNK MAPK or RhoA, but how these pathways are activated in response to TGF-β is not well understood (Derynck and Zhang, 2003; Moustakas and Heldin, 2005). In TGF-β– induced EMT, Smad signaling represents an essential pathway that confers changes in gene expression through cooperation with transcription factors such as Snail, Slug, and/or Id (Zavadil and Bottinger, 2005). Non-Smad signaling in response to TGF-β (e.g., activation of RhoA) also contributes to EMT and is important for the associated cytoskeletal and phenotypic changes (Bhowmick et al., 2001). Our results now show that TGF-β can increase protein synthesis in EMT through the mTOR pathway, leading to the regulation of S6K1 and 4E-BP1 activities. The activation by TGF-β of a pathway that leads directly to increased protein synthesis stands in contrast with the changes in gene transcription through the Smad pathway (Fig. 9). Thus, in addition to changes in gene expression, TGF-β signaling through mTOR leads to the enhanced translation of proteins that contribute to the behavior of cells that undergo EMT. Accordingly, studies using rapamycin have implicated mTOR in the regulation of collagen synthesis (Shegogue and Trojanowska, 2004). A characterization of the relative changes in protein levels that are independent of changes in gene expression and can be blocked by rapamycin will provide insight into the contribution of mTOR signaling to the cell's response to TGF-β.
Rapamycin decreases the invasive behavior of the cells after TGF-β–induced EMT
Although rapamycin did not block the TGF-β–induced EMT morphology, it inhibited the increased migration and invasion of NMuMG cells that accompanies EMT. These data suggest that mTORC1 plays a role in defining the migration and invasion of cells that have undergone EMT in response to TGF-β. Thus, rapamycin not only blocks the increased cell size but also the invasive behavior of cells with EMT without affecting the EMT-associated morphological changes. The mechanisms through which rapamycin inhibits invasion remain to be clarified, although the induction of expression of the metalloproteinase ADAM-12 and the metalloproteinase inhibitor TIMP-3 by TGF-β was recently reported to be inhibited by rapamycin (Le Pabic et al., 2005; Qureshi et al., 2007). Perhaps the mTOR pathway selectively regulates the translation of matrix metalloproteases and other proteins that allow for increased invasion in response to TGF-β. Our observation that rapamycin inhibits the migration and invasion of cells after TGF-β–induced EMT agrees with recent observations that rapamycin inhibits the induced motility of some cancer cells (Liu et al., 2006).
As PI3K and mTOR activities are commonly up-regulated in various cancers, PI3K inhibitors and rapamycin analogues are investigated as inhibitors of cancer progression in preclinical and clinical trials (Faivre et al., 2006; Hynes and Boulay, 2006; Smolewski, 2006). Our observations on the activation of mTOR signaling and the increase of cell size in response to TGF-β during EMT may have considerable clinical relevance. Indeed, increased autocrine TGF-β expression and responsiveness leading to TGF-β–induced EMT are considered to be important initiators of the invasive behavior of cancers during cancer progression. Our findings that TGF-β–induced EMT is accompanied by increased cell size and that the increased cell size and the EMT-associated increase in invasive behavior are mediated by increased mTOR signaling in response to TGF-β suggest that rapamycin analogues can be used to antagonize some key features of EMT that contribute to cancer progression.
Materials and methods
Cell culture, treatments, and transfection
NMuMG and HaCaT cells were cultured in DME with glucose (4.5 g/liter) and 10% FBS (Equitech-Bio, Inc.), 100 U/ml penicillin, and 100 mg/ml streptomycin. The medium to culture NMuMG cells was supplemented with 10 μg/ml insulin (Sigma-Aldrich). Images of the cells were captured at room temperature using a Spot RT slider camera (2.3.0; Diagnostic Instruments) on a microscope (Axiovert S-100; Carl Zeiss MicroImaging, Inc.) with a 40× Hoffmann modulation objective and Openlab software (Improvision) or a camera (D2x; Nikon) on a microscope (Diaphot; Nikon) with a 20× NA 0.4 air objective and Photoshop software (Adobe).
Cells were stimulated with 2–10 ng/ml TGF-β (PeproTech) for 10 min to 48 or 72 h. After incubation in a medium without insulin overnight, control cells were treated with 5 μg/ml insulin for 1 h to activate mTOR. To inhibit signaling effectors, the cells were treated with 100 nM rapamycin and 100 nM wortmannin (Calbiochem) or 5 μM SB431542 and 10 μM LY294002 (Sigma-Aldrich) for 1 h before TGF-β or insulin treatment, and DMSO was used as a control. To inhibit Akt signaling, cells were transfected using LipofectAMINE PLUS (Invitrogen) with a plasmid expressing an Akt mutant in which Ser473 and Thr308 were replaced by alanines (Stokoe et al., 1997).
Immunofluorescence microscopy
Cells were fixed with 4% PFA for 30 min, permeabilized in 2% PFA and 0.2% Triton X-100 for 10 min, and incubated in a PBS and 3% BSA blocking solution for 1 h. The slides were incubated for 2 h with anti–E-cadherin, anti–N-cadherin (BD Biosciences), anti–ZO-1 (Zymed Laboratories), or antifibronectin (Sigma-Aldrich) diluted (1:400, 1:500, 1:200, or 1:400, respectively) in PBS and 3% BSA and were stained for 1 h with FITC-conjugated secondary antibody (1:500; Invitrogen) or rhodamine-conjugated phalloidin (1:500; Invitrogen) to visualize actin filaments. The slides were incubated with DAPI (1:10,000; Sigma-Aldrich) for 10 min to stain nuclei. After mounting the slides (Cytoseal 280 mounting medium; Richard-Allan Scientific), the cells were viewed at room temperature by epifluorescence microscopy with a microscope (Axiophot; Carl Zeiss MicroImaging, Inc.) using a 100× NA 1.3 oil objective (Carl Zeiss MicroImaging, Inc.) or a microscope (Eclipse TE2000-E; Nikon) using a 100× NA 1.49 oil objective (Nikon), and images were analyzed using Spot (Diagnostic Instruments), NIS-Elements (Nikon), and Photoshop software (Adobe).
Flow cytometry
The cells, which were treated or not treated for 48 h with rapamycin or SB431542 with or without TGF-β, were trypsinized and resuspended in PBS containing 3% FBS, and 7 μg/ml Hoechst no. 33342 (Sigma-Aldrich) was added for 45 min. 1 μg/ml propidium iodide (Sigma-Aldrich) was added just before flow cytometry, which was performed with a sorter/analyzer SE three-laser system (FACSVantage; Becton Dickinson). The cell cycle and cell size were analyzed with CellQuest (BD Biosciences) and WinMDI 2.8 software.
Protein content and metabolic labeling
To measure protein content, cells were trypsinized, and the cell number was determined. The cells were lysed in radioimmunoprecipitation assay buffer with protease inhibitors, and the protein content was quantified using protein assay (Bio-Rad Laboratories) and normalized to cell number.
For metabolic labeling of proteins, cells were preincubated in methionine–cysteine-free DME, and 150 μCi/ml [35S]methionine–[35S]cysteine (PerkinElmer) was added for 3 h. The cells were lysed in radioimmunoprecipitation assay buffer with protease inhibitors. After centrifugation for 10 min at 4°C, 1:10 of the supernatant was precipitated with 300 μl of 20% trichloroacetic acid on ice for 20 min. After filtration on 0.22-μm GS Millipore membranes, the precipitated 35S-labeled protein was quantified and normalized against cell number.
Western blotting
Cells were lysed in radioimmunoprecipitation assay buffer with protease and phosphatase inhibitors. Proteins were quantified using protein assay (Bio-Rad Laboratories), and 20 μg of protein was separated by SDS-PAGE and transferred to 0.2 μm nitrocellulose membrane. Membranes were blocked in TBS, 0.1% Tween 20, and 5% BSA for 2 h before overnight incubation with primary antibody diluted 1:1,000 in TBS, 0.1% Tween 20, and 5% BSA. Antibodies to phospho-S6K1 (Thr389), phospho–4E-BP1 (Ser65), phospho-mTOR (Ser2448), mTOR, phospho-Akt (Ser473), Akt, and phospho-Smad3 (Ser433/435)/Smad1 (Ser463/465) were obtained from Cell Signaling Technology. Antibodies to S6K1, 4E-BP1, and Smad3 were purchased from Santa Cruz Biotechnology, Inc., Bethyl Laboratories, and Zymed Laboratories, respectively. Antibody to α-tubulin was purchased from Sigma-Aldrich and diluted 1:4,000 in TBS, 0.1% Tween 20, and 5% BSA. The membranes were incubated for 1 h in HRP-conjugated secondary antibody diluted at 1:2,000–1:10,000 in TBS and 0.1% Tween 20. Immunoreactive protein was detected using ECL (GE Healthcare) and BioMax film (Kodak).
Migration assay
Cell monolayers were wounded with a plastic tip at 48 h after the initiation of treatment (Pilkington et al., 1997). The migration was followed for 12 h at 37°C and photographed using a Spot RT slider camera (2.3.0; Diagnostic Instruments) mounted on a microscope (Axiovert S-100; Carl Zeiss MicroImaging, Inc.) with a 10× Hoffmann modulation objective and Openlab software (Improvision).
For cell migration in the transwell assay (Pilkington et al., 1997), cells were labeled with 5 μg/ml of the fluorophore DiI (Invitrogen) for 12 h starting at 36 h after the initiation of treatment. The cells were then trypsinized, and 50,000 cells suspended in DME and 0.2% FBS were added to transwell inserts (8-μm pore size; Falcon HTSF Fluoroblok inserts; Becton Dickinson), held in 24-well companion plates with DME and 10% FBS, and incubated for 6–24 h. Migration of cells to the lower culture plate was assessed by the fluorescence of DiI using a fluorimeter (SpectraMax M5; Molecular Devices).
Adhesion assay
Cells were trypsinized and seeded onto multiwells for 30 min. Unattached cells were removed by washing twice with PBS, and the attached cells were counted after trypsinization.
Invasion assay
Cells were trypsinized, and 50,000 cells resuspended in DME with 0.2% FBS were added to rehydrated Matrigel-coated inserts (BioCoat Matrigel Invasion Chamber; Becton Dickinson) and placed in 24-well companion plates with DME and 10% FBS (Albini et al., 2004). After 24 h, the cells and Matrigel in the upper chambers were removed using a cotton tip. The filters were fixed in methanol for 5 min at −20°C, incubated in DAPI (1:5,000; Sigma-Aldrich) for 10 min, and mounted (Cytoseal 280 mounting medium; Richard-Allan Scientific). The invading cells were counted at room temperature using an epifluorescence microscope (Axiophot; Carl Zeiss MicroImaging, Inc.) with a 10× NA 0.3 air objective, and images were analyzed using Spot (Diagnostic Instruments) and Photoshop software (Adobe).
Online supplemental material
Time-lapse video microscopy (Fig. 1) of untreated or TGF-β–treated NMuMG cells is shown in Videos 1 and 2, respectively, to show changes in morphology and cell size during EMT. Images were captured at 20-min intervals for 36 h at 37°C in growth medium. Time-lapse video microscopy (Fig. 7) of a wound-healing experiment of TGF-β–treated NMuMG cells in the absence or presence of rapamycin is shown in Videos 3 and 4, respectively, to show cell migration after TGF-β–induced EMT. Images were captured at 15-min intervals for 12 h at 37°C in growth medium. Images were captured using a Spot RT slider camera (2.3.0; Diagnostic Instruments) mounted on a microscope (Axiovert S-100; Carl Zeiss MicroImaging, Inc.) with a 10× Hoffmann modulation objective and operated using Openlab software (Improvision). Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200611146/DC1.
Acknowledgments
We thank D. Stokoe for providing us with plasmids, helpful discussions, and critical reading of the manuscript, J. Blenis for providing reagents, P. Dazin for his skillful technical help with flow cytometry analysis, D. Sabatini, J. Campisi, and S. Souchelnytskyi for their advice, B. Bilanges for his help, R. Shaw for access to fluorescence microscopy, and members of the D. Barber group for help with time-lapse microscopy. We are grateful to J. LaMarre and J. Smyth for their critical reviews of the manuscript.
This research was supported by National Institutes of Health grant PO1 HL60231 (project III) to R. Derynck and a postdoctoral fellowship (grant 5566-07) from the Leukemia and Lymphoma Society to S. Lamouille.
Notes
Abbreviations used in this paper: 4E-BP1, eukaryotic initiation factor 4E–binding protein 1; EMT, epithelial to mesenchymal transition; F-actin, filamentous actin; IGF-1, insulin-like growth factor-1; mTOR, mammalian target of rapamycin; mTORC, mTOR complex; PI3K, phosphatidylinositol 3-kinase; S6K1, S6 kinase 1; TβR, TGF-β receptor; TSC, tuberous sclerosis complex; ZO-1, zonula occludens-1.
References
- Aguilera, A., L.S. Aroeira, M. Ramirez-Huesca, M.L. Perez-Lozano, A. Cirugeda, M.A. Bajo, G. Del Peso, A. Valenzuela-Fernandez, J.A. Sanchez-Tomero, M. Lopez-Cabrera, and R. Selgas. 2005. Effects of rapamycin on the epithelial-to-mesenchymal transition of human peritoneal mesothelial cells. Int. J. Artif. Organs. 28:164–169. [Abstract] [Google Scholar]
- Albini, A., R. Benelli, D.M. Noonan, and C. Brigati. 2004. The “chemoinvasion assay”: a tool to study tumor and endothelial cell invasion of basement membranes. Int. J. Dev. Biol. 48:563–571. [Abstract] [Google Scholar]
- Angers-Loustau, A., J.F. Cote, and M.L. Tremblay. 1999. Roles of protein tyrosine phosphatases in cell migration and adhesion. Biochem. Cell Biol. 77:493–505. [Abstract] [Google Scholar]
- Arteaga, C.L. 2006. Inhibition of TGFβ signaling in cancer therapy. Curr. Opin. Genet. Dev. 16:30–37. [Abstract] [Google Scholar]
- Bakin, A.V., A.K. Tomlinson, N.A. Bhowmick, H.L. Moses, and C.L. Arteaga. 2000. Phosphatidylinositol 3-kinase function is required for transforming growth factor β-mediated epithelial to mesenchymal transition and cell migration. J. Biol. Chem. 275:36803–36810. [Abstract] [Google Scholar]
- Bhowmick, N.A., M. Ghiassi, A. Bakin, M. Aakre, C.A. Lundquist, M.E. Engel, C.L. Arteaga, and H.L. Moses. 2001. Transforming growth factor-β1 mediates epithelial to mesenchymal transdifferentiation through a RhoA- dependent mechanism. Mol. Biol. Cell. 12:27–36. [Europe PMC free article] [Abstract] [Google Scholar]
- Bierie, B., and H.L. Moses. 2006. TGF-β and cancer. Cytokine Growth Factor Rev. 17:29–40. [Abstract] [Google Scholar]
- Brand, T., and M.D. Schneider. 1995. The TGFβ superfamily in myocardium: ligands, receptors, transduction, and function. J. Mol. Cell. Cardiol. 27:5–18. [Abstract] [Google Scholar]
- Derynck, R., and Y.E. Zhang. 2003. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature. 425:577–584. [Abstract] [Google Scholar]
- Derynck, R., R.J. Akhurst, and A. Balmain. 2001. TGF-β signaling in tumor suppression and cancer progression. Nat. Genet. 29:117–129. [Abstract] [Google Scholar]
- Dimri, G.P., X. Lee, G. Basile, M. Acosta, G. Scott, C. Roskelley, E.E. Medrano, M. Linskens, I. Rubelj, O. Pereira-Smith, et al. 1995. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA. 92:9363–9367. [Europe PMC free article] [Abstract] [Google Scholar]
- Faivre, S., G. Kroemer, and E. Raymond. 2006. Current development of mTOR inhibitors as anticancer agents. Nat. Rev. Drug Discov. 5:671–688. [Abstract] [Google Scholar]
- Feng, X.-H., and R. Derynck. 2005. Specificity and versatility in TGF-β signaling through Smads. Annu. Rev. Cell Dev. Biol. 21:659–693. [Abstract] [Google Scholar]
- Fingar, D.C., and J. Blenis. 2004. Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene. 23:3151–3171. [Abstract] [Google Scholar]
- Frias, M.A., C.C. Thoreen, J.D. Jaffe, W. Schroder, T. Sculley, S.A. Carr, and D.M. Sabatini. 2006. mSin1 is necessary for Akt/PKB phosphorylation, and its isoforms define three distinct mTORC2s. Curr. Biol. 16:1865–1870. [Abstract] [Google Scholar]
- Goldsmith, A.M., J.K. Bentley, L. Zhou, Y. Jia, K.N. Bitar, D.C. Fingar, and M.B. Hershenson. 2006. Transforming growth factor-β induces airway smooth muscle hypertrophy. Am. J. Respir. Cell Mol. Biol. 34:247–254. [Europe PMC free article] [Abstract] [Google Scholar]
- Grebien, F., H. Dolznig, H. Beug, and E.W. Mullner. 2005. Cell size control: new evidence for a general mechanism. Cell Cycle. 4:418–421. [Abstract] [Google Scholar]
- Grimberg, A. 2003. Mechanisms by which IGF-I may promote cancer. Cancer Biol. Ther. 2:630–635. [Europe PMC free article] [Abstract] [Google Scholar]
- Guertin, D.A., and D.M. Sabatini. 2005. An expanding role for mTOR in cancer. Trends Mol. Med. 11:353–361. [Abstract] [Google Scholar]
- Hay, N., and N. Sonenberg. 2004. Upstream and downstream of mTOR. Genes Dev. 18:1926–1945. [Abstract] [Google Scholar]
- Holz, M.K., B.A. Ballif, S.P. Gygi, and J. Blenis. 2005. mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell. 123:569–580. [Abstract] [Google Scholar]
- Hynes, N.E., and A. Boulay. 2006. The mTOR pathway in breast cancer. J. Mammary Gland Biol. Neoplasia. 11:53–61. [Abstract] [Google Scholar]
- Kim, D., G.Z. Cheng, C.W. Lindsley, H. Yang, and J.Q. Cheng. 2005. Targeting the phosphatidylinositol-3 kinase/Akt pathway for the treatment of cancer. Curr. Opin. Investig. Drugs. 6:1250–1258. [Abstract] [Google Scholar]
- Krishna, S., L.L. Maduzia, and R.W. Padgett. 1999. Specificity of TGFβ signaling is conferred by distinct type I receptors and their associated SMAD proteins in Caenorhabditis elegans. Development. 126:251–260. [Abstract] [Google Scholar]
- Leboy, P.S., T.A. Sullivan, M. Nooreyazdan, and R.A. Venezian. 1997. Rapid chondrocyte maturation by serum-free culture with BMP-2 and ascorbic acid. J. Cell. Biochem. 66:394–403. [Abstract] [Google Scholar]
- Le Pabic, H., A. L'Helgoualc'h, A. Coutant, U.M. Wewer, G. Baffet, B. Clement, and N. Theret. 2005. Involvement of the serine/threonine p70S6 kinase in TGF-β1-induced ADAM12 expression in cultured human hepatic stellate cells. J. Hepatol. 43:1038–1044. [Abstract] [Google Scholar]
- Liu, L., F. Li, J.A. Cardelli, K.A. Martin, J. Blenis, and S. Huang. 2006. Rapamycin inhibits cell motility by suppression of mTOR-mediated S6K1 and 4E-BP1 pathways. Oncogene. 25:7029–7040. [Abstract] [Google Scholar]
- Manning, B.D., and L.C. Cantley. 2003. United at last: the tuberous sclerosis complex gene products connect the phosphoinositide 3-kinase/Akt pathway to mammalian target of rapamycin (mTOR) signalling. Biochem. Soc. Trans. 31:573–578. [Abstract] [Google Scholar]
- Massagué, J., J. Seoane, and D. Wotton. 2005. Smad transcription factors. Genes Dev. 19:2783–2810. [Abstract] [Google Scholar]
- McPherron, A.C., A.M. Lawler, and S.J. Lee. 1997. Regulation of skeletal muscle mass in mice by a new TGF-β superfamily member. Nature. 387:83–90. [Abstract] [Google Scholar]
- Miettinen, P.J., R. Ebner, A.R. Lopez, and R. Derynck. 1994. TGF-β induced transdifferentiation of mammary epithelial cells to mesenchymal cells: involvement of type I receptors. J. Cell Biol. 127:2021–2036. [Europe PMC free article] [Abstract] [Google Scholar]
- Moustakas, A., and C.H. Heldin. 2005. Non-Smad TGF-β signals. J. Cell Sci. 118:3573–3584. [Abstract] [Google Scholar]
- Ozdamar, B., R. Bose, M. Barrios-Rodiles, H.R. Wang, Y. Zhang, and J.L. Wrana. 2005. Regulation of the polarity protein Par6 by TGFβ receptors controls epithelial cell plasticity. Science. 307:1603–1609. [Abstract] [Google Scholar]
- Pilkington, G.J., R. Bjerkvig, L. De Ridder, and P. Kaaijk. 1997. In vitro and in vivo models for the study of brain tumour invasion. Anticancer Res. 17:4107–4109. [Abstract] [Google Scholar]
- Qureshi, H.Y., R. Ahmad, J. Sylvester, and M. Zafarullah. 2007. Requirement of phosphatidylinositol 3-kinase/Akt signaling pathway for regulation of tissue inhibitor of metalloproteinases-3 gene expression by TGF-β in human chondrocytes. Cell Signal. 19:1643–1651. [Abstract] [Google Scholar]
- Ruggero, D., and P.P. Pandolfi. 2003. Does the ribosome translate cancer? Nat. Rev. Cancer. 3:179–192. [Abstract] [Google Scholar]
- Ruvinsky, I., and O. Meyuhas. 2006. Ribosomal protein S6 phosphorylation: from protein synthesis to cell size. Trends Biochem. Sci. 31:342–348. [Abstract] [Google Scholar]
- Sarbassov, D.D., S.M. Ali, D.H. Kim, D.A. Guertin, R.R. Latek, H. Erdjument-Bromage, P. Tempst, and D.M. Sabatini. 2004. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 14:1296–1302. [Abstract] [Google Scholar]
- Shegogue, D., and M. Trojanowska. 2004. Mammalian target of rapamycin positively regulates collagen type I production via a phosphatidylinositol 3-kinase-independent pathway. J. Biol. Chem. 279:23166–23175. [Abstract] [Google Scholar]
- Shi, Y., and J. Massagué. 2003. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell. 113:685–700. [Abstract] [Google Scholar]
- Smolewski, P. 2006. Recent developments in targeting the mammalian target of rapamycin (mTOR) kinase pathway. Anticancer Drugs. 17:487–494. [Abstract] [Google Scholar]
- Stokoe, D., L.R. Stephens, T. Copeland, P.R. Gaffney, C.B. Reese, G.F. Painter, A.B. Holmes, F. McCormick, and P.T. Hawkins. 1997. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science. 277:567–570. [Abstract] [Google Scholar]
- Suzuki, Y., M.D. Yandell, P.J. Roy, S. Krishna, C. Savage-Dunn, R.M. Ross, R.W. Padgett, and W.B. Wood. 1999. A BMP homolog acts as a dose- dependent regulator of body size and male tail patterning in Caenorhabditis elegans. Development. 126:241–250. [Abstract] [Google Scholar]
- Thiery, J.P., and J.P. Sleeman. 2006. Complex networks orchestrate epithelial-mesenchymal transitions. Nat. Rev. Mol. Cell Biol. 7:131–142. [Abstract] [Google Scholar]
- Thuault, S., U. Valcourt, M. Petersen, G. Manfioletti, C.H. Heldin, and A. Moustakas. 2006. Transforming growth factor-β employs HMGA2 to elicit epithelial-mesenchymal transition. J. Cell Biol. 174:175–183. [Europe PMC free article] [Abstract] [Google Scholar]
- Volk, S.W., P. Luvalle, T. Leask, and P.S. Leboy. 1998. A BMP responsive transcriptional region in the chicken type X collagen gene. J. Bone Miner. Res. 13:1521–1529. [Abstract] [Google Scholar]
- Wullschleger, S., R. Loewith, and M.N. Hall. 2006. TOR signaling in growth and metabolism. Cell. 124:471–484. [Abstract] [Google Scholar]
- Yi, J.Y., I. Shin, and C.L. Arteaga. 2005. Type I transforming growth factor β receptor binds to and activates phosphatidylinositol 3-kinase. J. Biol. Chem. 280:10870–10876. [Abstract] [Google Scholar]
- Zavadil, J., and E.P. Böttinger. 2005. TGF-β and epithelial-to-mesenchymal transitions. Oncogene. 24:5764–5774. [Abstract] [Google Scholar]
- Zavadil, J., M. Bitzer, D. Liang, Y.C. Yang, A. Massimi, S. Kneitz, E. Piek, and E.P. Böttinger. 2001. Genetic programs of epithelial cell plasticity directed by transforming growth factor-β. Proc. Natl. Acad. Sci. USA. 98:6686–6691. [Europe PMC free article] [Abstract] [Google Scholar]
Articles from The Journal of Cell Biology are provided here courtesy of The Rockefeller University Press
Full text links
Read article at publisher's site: https://doi.org/10.1083/jcb.200611146
Read article for free, from open access legal sources, via Unpaywall:
https://rupress.org/jcb/article-pdf/178/3/437/1330774/jcb_200611146.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Deoxyelephantopin induces apoptosis and cell cycle arrest in GL261 glioblastoma cells.
Naunyn Schmiedebergs Arch Pharmacol, 10 Sep 2024
Cited by: 0 articles | PMID: 39254878
Inhibition of mTOR differently modulates planar and subepithelial fibrogenesis in human conjunctival fibroblasts.
Graefes Arch Clin Exp Ophthalmol, 23 Jul 2024
Cited by: 0 articles | PMID: 39042147
Impact of microRNA variants on PI3K/AKT signaling in triple-negative breast cancer: comprehensive review.
Med Oncol, 41(9):222, 09 Aug 2024
Cited by: 1 article | PMID: 39120634
Review
Deciphering the molecular nexus of BTG2 in periodontitis and diabetic kidney disease.
BMC Med Genomics, 17(1):152, 03 Jun 2024
Cited by: 0 articles | PMID: 38831322 | PMCID: PMC11149328
TGF-β signaling regulates differentiation of MSCs in bone metabolism: disputes among viewpoints.
Stem Cell Res Ther, 15(1):156, 31 May 2024
Cited by: 2 articles | PMID: 38816830 | PMCID: PMC11140988
Review Free full text in Europe PMC
Go to all (372) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Transforming Growth Factor β1-induced Apoptosis in Podocytes via the Extracellular Signal-regulated Kinase-Mammalian Target of Rapamycin Complex 1-NADPH Oxidase 4 Axis.
J Biol Chem, 290(52):30830-30842, 12 Nov 2015
Cited by: 26 articles | PMID: 26565025 | PMCID: PMC4692212
Mammalian Target of Rapamycin (mTOR) Regulates Transforming Growth Factor-β1 (TGF-β1)-Induced Epithelial-Mesenchymal Transition via Decreased Pyruvate Kinase M2 (PKM2) Expression in Cervical Cancer Cells.
Med Sci Monit, 23:2017-2028, 27 Apr 2017
Cited by: 24 articles | PMID: 28446743 | PMCID: PMC5417590
Metformin Inhibits TGF-β1-Induced Epithelial-to-Mesenchymal Transition via PKM2 Relative-mTOR/p70s6k Signaling Pathway in Cervical Carcinoma Cells.
Int J Mol Sci, 17(12):E2000, 30 Nov 2016
Cited by: 37 articles | PMID: 27916907 | PMCID: PMC5187800
Multiple transforming growth factor-beta isoforms and receptors function during epithelial-mesenchymal cell transformation in the embryonic heart.
Cells Tissues Organs, 185(1-3):146-156, 01 Jan 2007
Cited by: 99 articles | PMID: 17587820
Review
Funding
Funders who supported this work.
NHLBI NIH HHS (2)
Grant ID: P01 HL060231
Grant ID: P01 HL60231