Abstract
Free full text

New thermosensitive plasmid for gram-positive bacteria.
Abstract
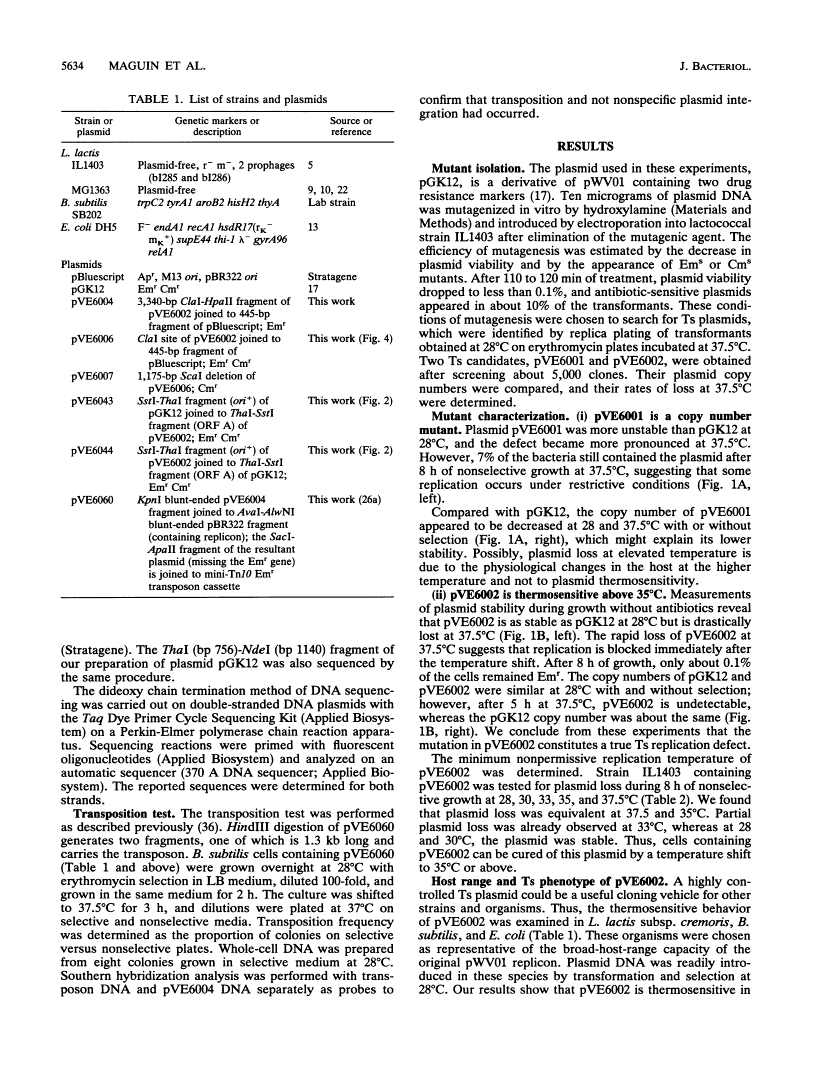
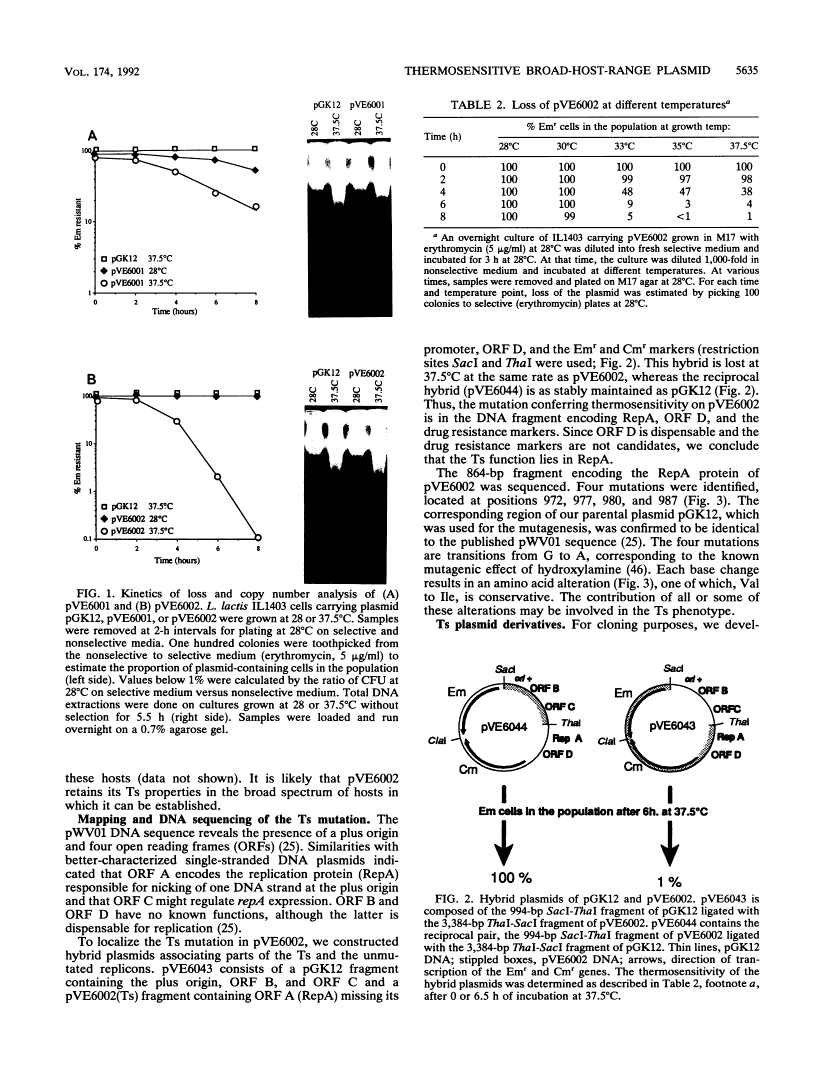
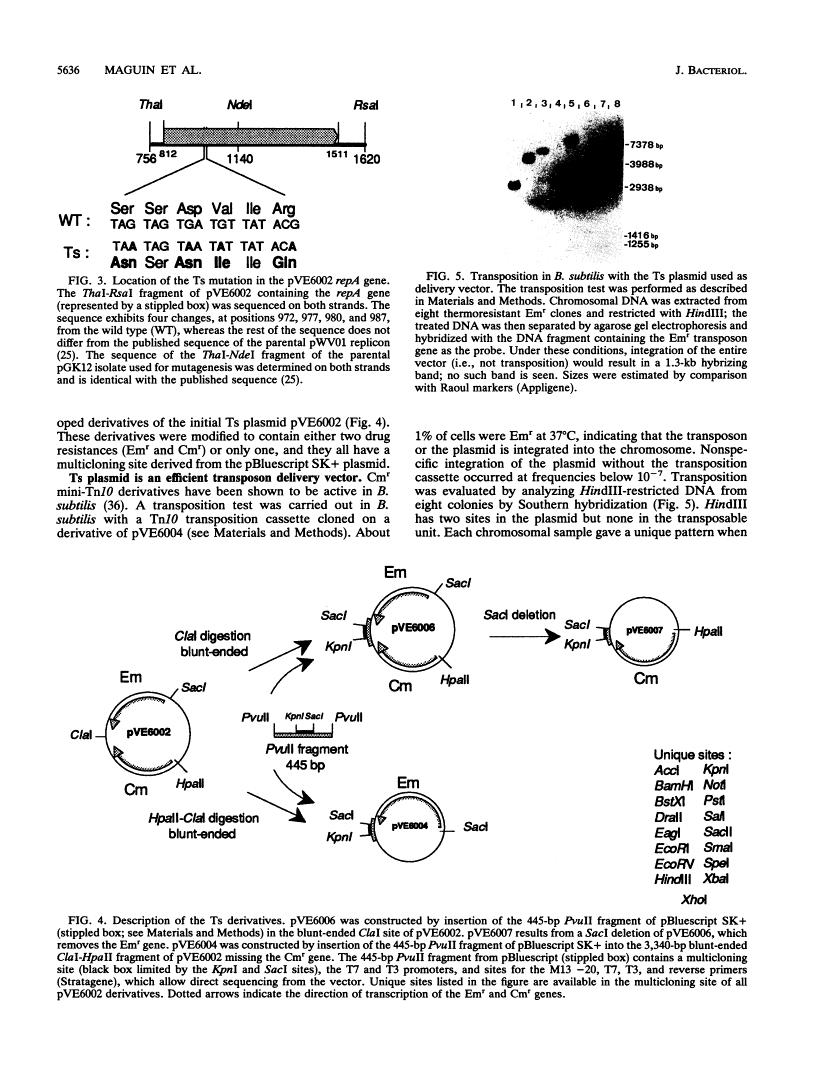
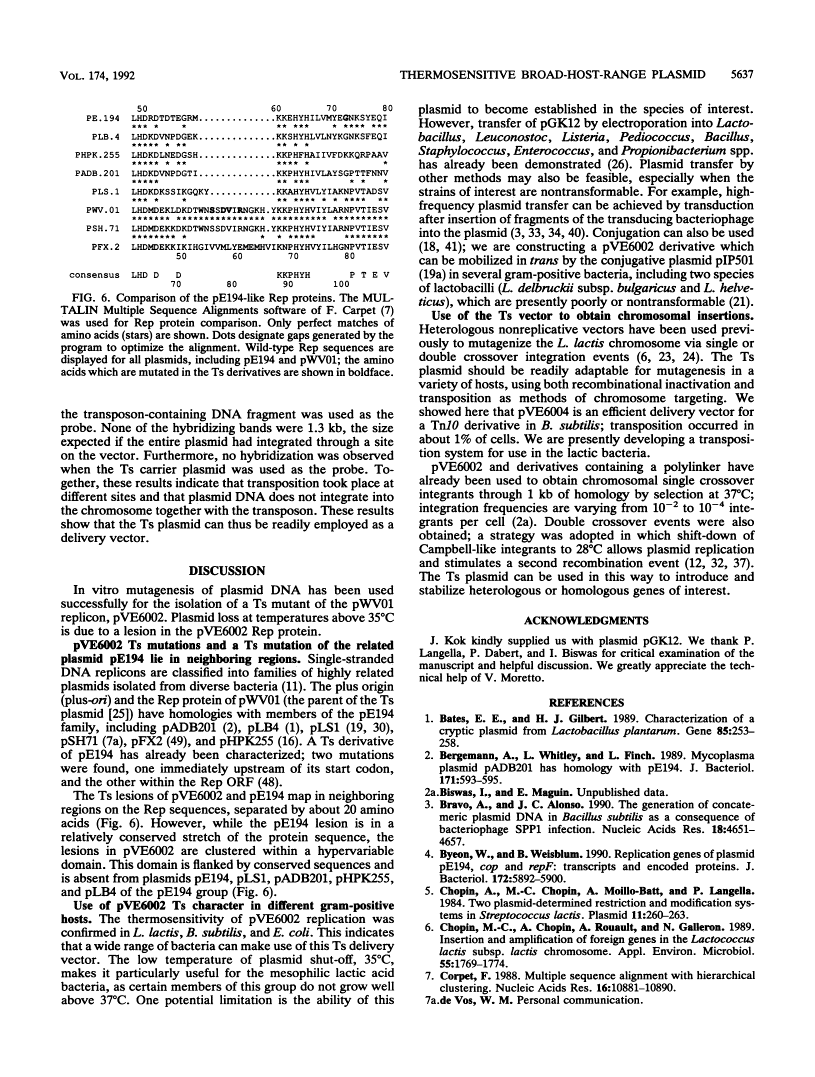
We isolated a replication-thermosensitive mutant of the broad-host-range replicon pWV01. The mutant pVE6002 is fully thermosensitive above 35 degrees C in both gram-negative and gram-positive bacteria. Four clustered mutations were identified in the gene encoding the replication protein of pVE6002. The thermosensitive derivative of the related plasmid pE194 carries a mutation in the analogous region but not in the same position. Derivatives of the thermosensitive plasmid convenient for cloning purposes have been constructed. The low shut-off temperature of pVE6002 makes it a useful suicide vector for bacteria which are limited in their own temperature growth range. Using pVE6002 as the delivery vector for a transposon Tn10 derivative in Bacillus subtilis, we observed transposition frequencies of about 1%.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.3M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bates EE, Gilbert HJ. Characterization of a cryptic plasmid from Lactobacillus plantarum. Gene. 1989 Dec 21;85(1):253–258. [Abstract] [Google Scholar]
- Bergemann AD, Whitley JC, Finch LR. Homology of mycoplasma plasmid pADB201 and staphylococcal plasmid pE194. J Bacteriol. 1989 Jan;171(1):593–595. [Europe PMC free article] [Abstract] [Google Scholar]
- Bravo A, Alonso JC. The generation of concatemeric plasmid DNA in Bacillus subtilis as a consequence of bacteriophage SPP1 infection. Nucleic Acids Res. 1990 Aug 25;18(16):4651–4657. [Europe PMC free article] [Abstract] [Google Scholar]
- Byeon WH, Weisblum B. Replication genes of plasmid pE194-cop and repF: transcripts and encoded proteins. J Bacteriol. 1990 Oct;172(10):5892–5900. [Europe PMC free article] [Abstract] [Google Scholar]
- Chopin A, Chopin MC, Moillo-Batt A, Langella P. Two plasmid-determined restriction and modification systems in Streptococcus lactis. Plasmid. 1984 May;11(3):260–263. [Abstract] [Google Scholar]
- Chopin MC, Chopin A, Rouault A, Galleron N. Insertion and amplification of foreign genes in the Lactococcus lactis subsp. lactis chromosome. Appl Environ Microbiol. 1989 Jul;55(7):1769–1774. [Europe PMC free article] [Abstract] [Google Scholar]
- Corpet F. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 1988 Nov 25;16(22):10881–10890. [Europe PMC free article] [Abstract] [Google Scholar]
- Gasson MJ. In vivo genetic systems in lactic acid bacteria. FEMS Microbiol Rev. 1990 Sep;7(1-2):43–60. [Abstract] [Google Scholar]
- Gasson MJ. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J Bacteriol. 1983 Apr;154(1):1–9. [Europe PMC free article] [Abstract] [Google Scholar]
- Gruss A, Ehrlich SD. The family of highly interrelated single-stranded deoxyribonucleic acid plasmids. Microbiol Rev. 1989 Jun;53(2):231–241. [Europe PMC free article] [Abstract] [Google Scholar]
- Hamilton CM, Aldea M, Washburn BK, Babitzke P, Kushner SR. New method for generating deletions and gene replacements in Escherichia coli. J Bacteriol. 1989 Sep;171(9):4617–4622. [Europe PMC free article] [Abstract] [Google Scholar]
- Holo H, Nes IF. High-Frequency Transformation, by Electroporation, of Lactococcus lactis subsp. cremoris Grown with Glycine in Osmotically Stabilized Media. Appl Environ Microbiol. 1989 Dec;55(12):3119–3123. [Europe PMC free article] [Abstract] [Google Scholar]
- Horinouchi S, Weisblum B. Nucleotide sequence and functional map of pE194, a plasmid that specifies inducible resistance to macrolide, lincosamide, and streptogramin type B antibodies. J Bacteriol. 1982 May;150(2):804–814. [Europe PMC free article] [Abstract] [Google Scholar]
- Kleanthous H, Clayton CL, Tabaqchali S. Characterization of a plasmid from Helicobacter pylori encoding a replication protein common to plasmids in gram-positive bacteria. Mol Microbiol. 1991 Oct;5(10):2377–2389. [Abstract] [Google Scholar]
- Kok J, van der Vossen JM, Venema G. Construction of plasmid cloning vectors for lactic streptococci which also replicate in Bacillus subtilis and Escherichia coli. Appl Environ Microbiol. 1984 Oct;48(4):726–731. [Europe PMC free article] [Abstract] [Google Scholar]
- Krah ER, 3rd, Macrina FL. Identification of a region that influences host range of the streptococcal conjugative plasmid pIP501. Plasmid. 1991 Jan;25(1):64–69. [Abstract] [Google Scholar]
- Lacks SA, Lopez P, Greenberg B, Espinosa M. Identification and analysis of genes for tetracycline resistance and replication functions in the broad-host-range plasmid pLS1. J Mol Biol. 1986 Dec 20;192(4):753–765. [Abstract] [Google Scholar]
- Langella P, Chopin A. Conjugal transfer of plasmid pIP501 from Lactococcus lactis to Lactobacillus delbrückii subsp. bulgaricus and Lactobacillus helveticus. FEMS Microbiol Lett. 1989 Jul 15;51(1):149–152. [Abstract] [Google Scholar]
- Le Bourgeois P, Mata M, Ritzenthaler P. Genome comparison of Lactococcus strains by pulsed-field gel electrophoresis. FEMS Microbiol Lett. 1989 May;50(1-2):65–69. [Abstract] [Google Scholar]
- Leenhouts KJ, Kok J, Venema G. Campbell-like integration of heterologous plasmid DNA into the chromosome of Lactococcus lactis subsp. lactis. Appl Environ Microbiol. 1989 Feb;55(2):394–400. [Europe PMC free article] [Abstract] [Google Scholar]
- Leenhouts KJ, Tolner B, Bron S, Kok J, Venema G, Seegers JF. Nucleotide sequence and characterization of the broad-host-range lactococcal plasmid pWVO1. Plasmid. 1991 Jul;26(1):55–66. [Abstract] [Google Scholar]
- Luchansky JB, Muriana PM, Klaenhammer TR. Application of electroporation for transfer of plasmid DNA to Lactobacillus, Lactococcus, Leuconostoc, Listeria, Pediococcus, Bacillus, Staphylococcus, Enterococcus and Propionibacterium. Mol Microbiol. 1988 Sep;2(5):637–646. [Abstract] [Google Scholar]
- McKay LL, Baldwin KA. Applications for biotechnology: present and future improvements in lactic acid bacteria. FEMS Microbiol Rev. 1990 Sep;7(1-2):3–14. [Abstract] [Google Scholar]
- Mercenier A, Chassy BM. Strategies for the development of bacterial transformation systems. Biochimie. 1988 Apr;70(4):503–517. [Abstract] [Google Scholar]
- Minton NP, Oultram JD, Brehm JK, Atkinson T. The replication proteins of plasmids pE194 and pLS1 have N-terminal homology. Nucleic Acids Res. 1988 Apr 11;16(7):3101–3101. [Europe PMC free article] [Abstract] [Google Scholar]
- Niaudet B, Ehrlich SD. In vitro genetic labeling of Bacillus subtilis cryptic plasmid pHV400. Plasmid. 1979 Jan;2(1):48–58. [Abstract] [Google Scholar]
- Noirot P, Petit MA, Ehrlich SD. Plasmid replication stimulates DNA recombination in Bacillus subtilis. J Mol Biol. 1987 Jul 5;196(1):39–48. [Abstract] [Google Scholar]
- Novick RP. Penicillinase plasmids of Staphylococcus aureus. Fed Proc. 1967 Jan-Feb;26(1):29–38. [Abstract] [Google Scholar]
- Novick RP, Edelman I, Lofdahl S. Small Staphylococcus aureus plasmids are transduced as linear multimers that are formed and resolved by replicative processes. J Mol Biol. 1986 Nov 20;192(2):209–220. [Abstract] [Google Scholar]
- Otto R, de Vos WM, Gavrieli J. Plasmid DNA in Streptococcus cremoris Wg2: Influence of pH on Selection in Chemostats of a Variant Lacking a Protease Plasmid. Appl Environ Microbiol. 1982 Jun;43(6):1272–1277. [Europe PMC free article] [Abstract] [Google Scholar]
- Petit MA, Bruand C, Jannière L, Ehrlich SD. Tn10-derived transposons active in Bacillus subtilis. J Bacteriol. 1990 Dec;172(12):6736–6740. [Europe PMC free article] [Abstract] [Google Scholar]
- Petit MA, Mesas JM, Noirot P, Morel-Deville F, Ehrlich SD. Induction of DNA amplification in the Bacillus subtilis chromosome. EMBO J. 1992 Apr;11(4):1317–1326. [Europe PMC free article] [Abstract] [Google Scholar]
- Projan SJ, Carleton S, Novick RP. Determination of plasmid copy number by fluorescence densitometry. Plasmid. 1983 Mar;9(2):182–190. [Abstract] [Google Scholar]
- Puyet A, del Solar GH, Espinosa M. Identification of the origin and direction of replication of the broad-host-range plasmid pLS1. Nucleic Acids Res. 1988 Jan 11;16(1):115–133. [Europe PMC free article] [Abstract] [Google Scholar]
- Raya RR, Klaenhammer TR. High-Frequency Plasmid Transduction by Lactobacillus gasseri Bacteriophage phiadh. Appl Environ Microbiol. 1992 Jan;58(1):187–193. [Europe PMC free article] [Abstract] [Google Scholar]
- Romero DA, Slos P, Robert C, Castellino I, Mercenier A. Conjugative mobilization as an alternative vector delivery system for lactic streptococci. Appl Environ Microbiol. 1987 Oct;53(10):2405–2413. [Europe PMC free article] [Abstract] [Google Scholar]
- Scheer-Abramowitz J, Gryczan TJ, Dubnau D. Origin and mode of replication of plasmids pE194 and pUB110. Plasmid. 1981 Jul;6(1):67–77. [Abstract] [Google Scholar]
- te Riele H, Michel B, Ehrlich SD. Single-stranded plasmid DNA in Bacillus subtilis and Staphylococcus aureus. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2541–2545. [Europe PMC free article] [Abstract] [Google Scholar]
- Terzaghi BE, Sandine WE. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol. 1975 Jun;29(6):807–813. [Europe PMC free article] [Abstract] [Google Scholar]
- van der Vossen JM, van der Lelie D, Venema G. Isolation and characterization of Streptococcus cremoris Wg2-specific promoters. Appl Environ Microbiol. 1987 Oct;53(10):2452–2457. [Europe PMC free article] [Abstract] [Google Scholar]
- Villafane R, Bechhofer DH, Narayanan CS, Dubnau D. Replication control genes of plasmid pE194. J Bacteriol. 1987 Oct;169(10):4822–4829. [Europe PMC free article] [Abstract] [Google Scholar]
- Xu FF, Pearce LE, Yu PL. Genetic analysis of a lactococcal plasmid replicon. Mol Gen Genet. 1991 May;227(1):33–39. [Abstract] [Google Scholar]
Associated Data
Articles from Journal of Bacteriology are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/jb.174.17.5633-5638.1992
Read article for free, from open access legal sources, via Unpaywall:
https://jb.asm.org/content/jb/174/17/5633.full.pdf
Free to read at jb.asm.org
http://jb.asm.org/cgi/content/abstract/174/17/5633
Free after 4 months at jb.asm.org
http://jb.asm.org/cgi/reprint/174/17/5633
Citations & impact
Impact metrics
Article citations
Function and contribution of two putative Enterococcus faecalis glycosaminoglycan degrading enzymes to bacteremia and catheter-associated urinary tract infection.
Infect Immun, 92(7):e0019924, 06 Jun 2024
Cited by: 0 articles | PMID: 38842305 | PMCID: PMC11238560
Effect of sugar transporter on galactose utilization in Streptococcus thermophilus.
Front Microbiol, 14:1267237, 21 Nov 2023
Cited by: 1 article | PMID: 38075912 | PMCID: PMC10703360
The type-2 Streptococcus canis M protein SCM-2 binds fibrinogen and facilitates antiphagocytic properties.
Front Microbiol, 14:1228472, 26 Oct 2023
Cited by: 0 articles | PMID: 37965557 | PMCID: PMC10641296
Wild-type Lactococcus lactis producing bacteriocin-like prophage lysins.
Front Microbiol, 14:1219723, 14 Jul 2023
Cited by: 2 articles | PMID: 37520360 | PMCID: PMC10377672
New Effective Method of Lactococcus Genome Editing Using Guide RNA-Directed Transposition.
Int J Mol Sci, 23(22):13978, 12 Nov 2022
Cited by: 0 articles | PMID: 36430465 | PMCID: PMC9696066
Go to all (286) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Characterization of a plasmid from Helicobacter pylori encoding a replication protein common to plasmids in gram-positive bacteria.
Mol Microbiol, 5(10):2377-2389, 01 Oct 1991
Cited by: 46 articles | PMID: 1791753
The replication proteins of plasmids pE194 and pLS1 have N-terminal homology.
Nucleic Acids Res, 16(7):3101, 01 Apr 1988
Cited by: 10 articles | PMID: 3368318 | PMCID: PMC336455
Genetic analysis of a lactococcal plasmid replicon.
Mol Gen Genet, 227(1):33-39, 01 May 1991
Cited by: 26 articles | PMID: 1904536
The RepA_N replicons of Gram-positive bacteria: a family of broadly distributed but narrow host range plasmids.
Plasmid, 61(2):94-109, 06 Jan 2009
Cited by: 42 articles | PMID: 19100285 | PMCID: PMC2652615
Review Free full text in Europe PMC