Abstract
Free full text

Mutagenesis and stress responses induced in Escherichia coli by hydrogen peroxide.
Abstract
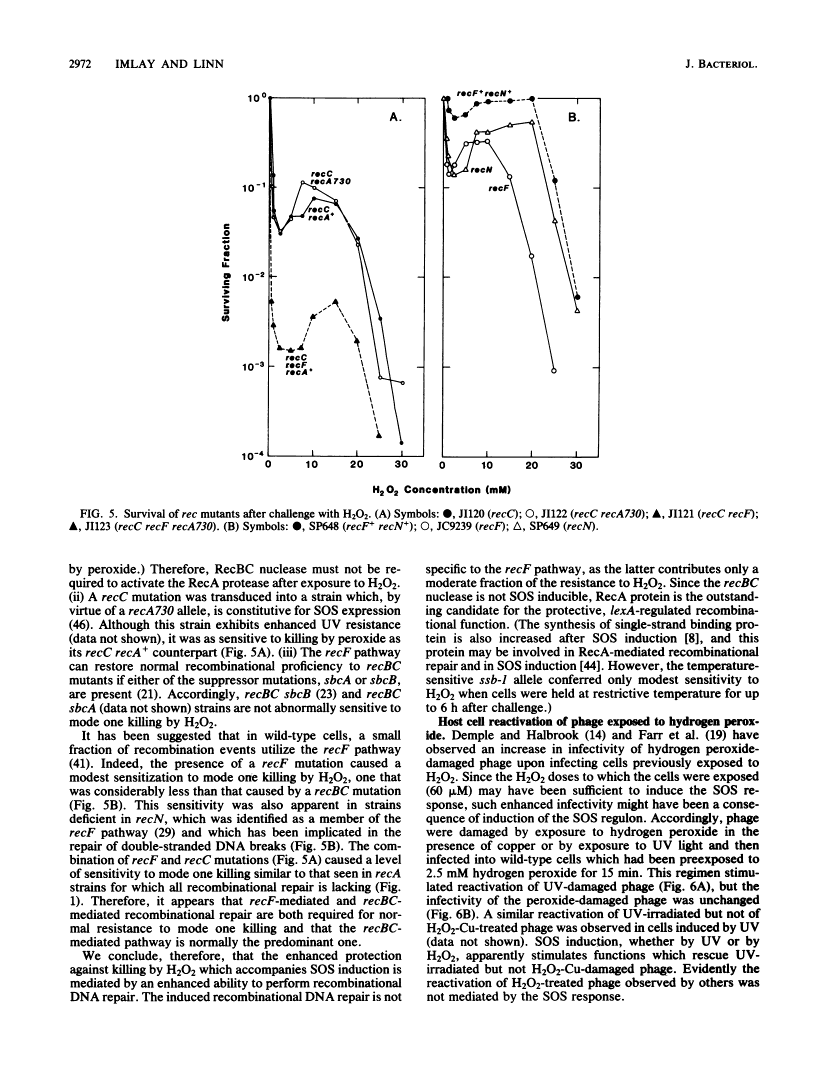
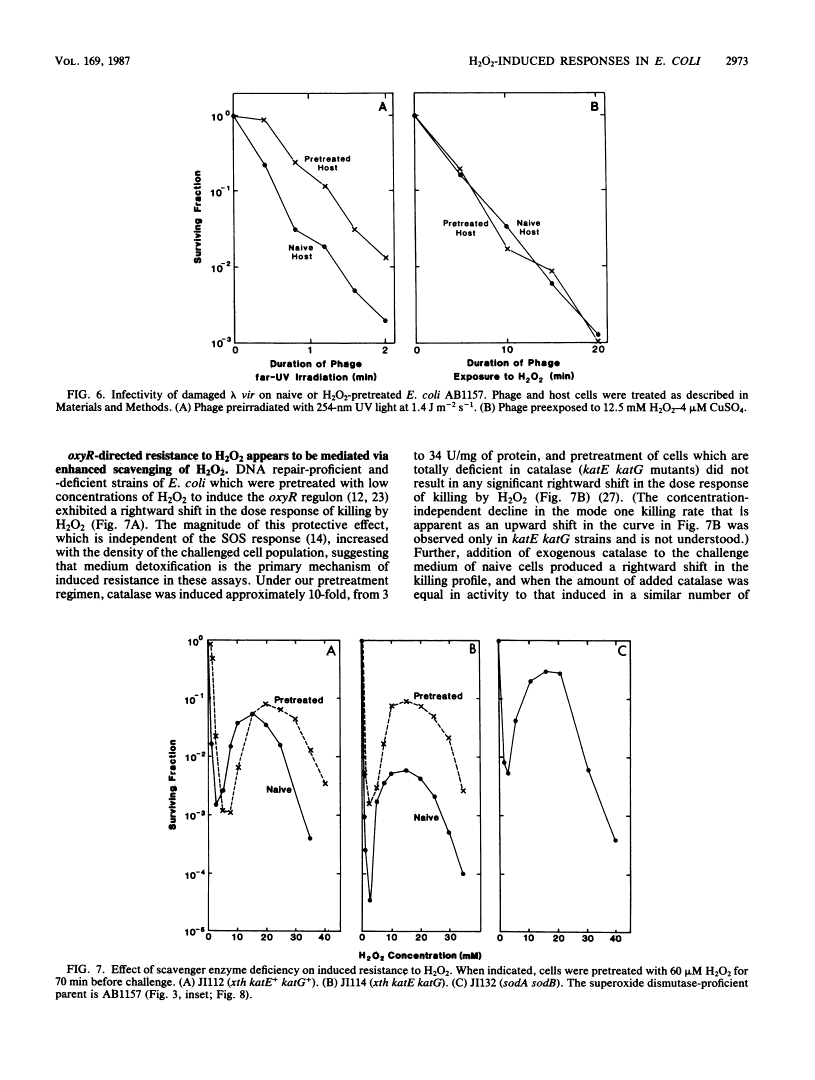
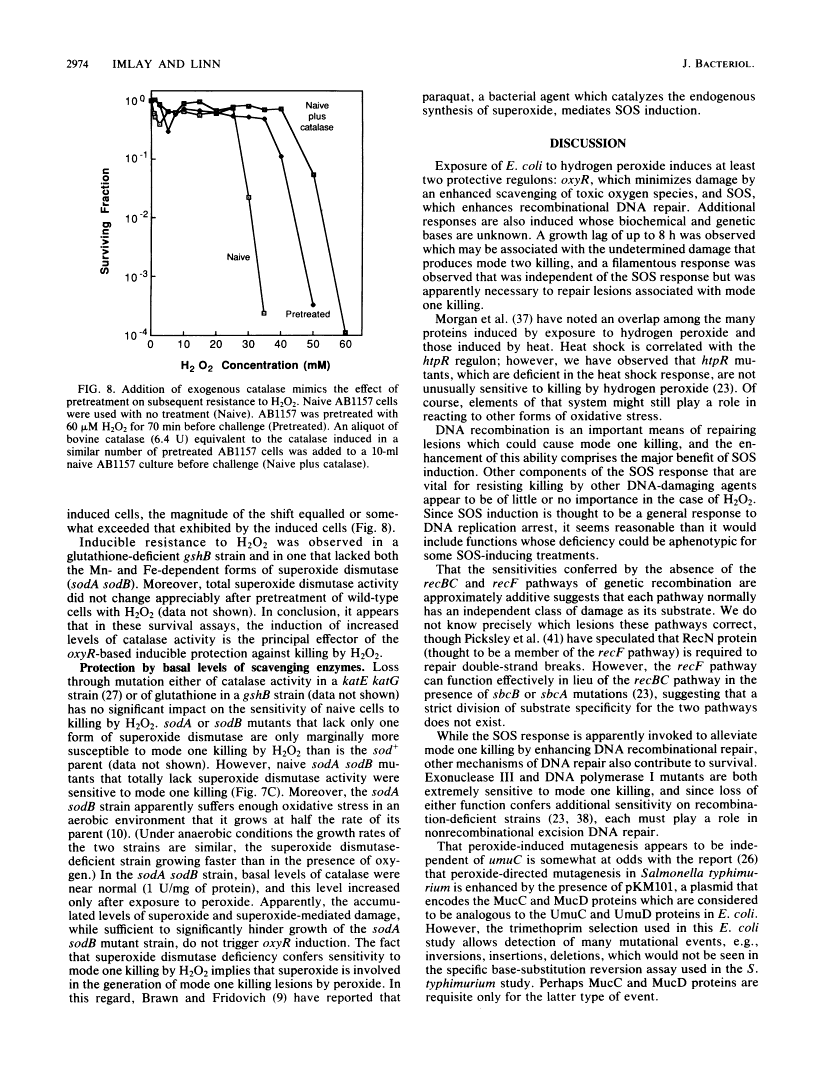
Killing of Escherichia coli by hydrogen peroxide proceeds by two modes. Mode one killing appears to be due to DNA damage, has a maximum near 1 to 3 mM H2O2, and requires active metabolism during exposure. Mode two killing is due to uncharacterized damage, occurs in the absence of metabolism, and exhibits a classical multiple-order dose-response curve up to at least 50 mM H2O2 (J. A. Imlay and S. Linn, J. Bacteriol. 166:519-527, 1986). H2O2 induces the SOS response in proportion to the degree of killing by the mode one pathway, i.e., induction is maximal after exposure to 1 to 3 mM H2O2. Mutant strains that cannot induce the SOS regulon are hypersensitive to peroxide. Analysis of the sensitivities of mutants that are deficient in individual SOS-regulated functions suggested that the SOS-mediated protection is due to the enhanced synthesis of recA protein, which is rate limiting for recombinational DNA repair. Specifically, strains wholly blocked in both SOS induction and DNA recombination were no more sensitive than mutants that are blocked in only one of these two functions, and strains carrying mutations in uvrA, -B, -C, or -D, sfiA, umuC or -D, ssb, or dinA, -B, -D, -F, -G, -H, -I, or -J were not abnormally sensitive to killing by H2O2. After exposure to H2O2, mutagenesis and filamentation also occurred with the dose response characteristic of SOS induction and mode one killing, but these responses were not dependent on the lexA-regulated umuC mutagenesis or sfiA filamentation functions, respectively. Exposure of E. coli to H2O2 also resulted in the induction of functions under control of the oxyR regulon that enhance the scavenging of active oxygen species, thereby reducing the sensitivity to H2O2. Catalase levels increased 10-fold during this induction, and katE katG mutants, which totally lack catalase, while not abnormally sensitive to killing by H2O2 in the naive state, did not exhibit the induced protective response. Protection equal to that observed during oxyR induction could be achieved by the addition of catalase to cultures of naive cells in an amount equivalent to that induced by the oxyR response. Thus, the induction of catalase is necessary and sufficient for the observed oxyR-directed resistance to killing by H2O2. Although superoxide dismutase appeared to be uninvolved in this enhanced protective response, sodA sodB mutants, which totally lack superoxide dismutase, were especially sensitive to mode one killing by H2O2 in the naive state. gshB mutants, which lack glutathione, were not abnormally sensitive to killing by H2O2.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.6M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann BJ. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. [Europe PMC free article] [Abstract] [Google Scholar]
- Bagg A, Kenyon CJ, Walker GC. Inducibility of a gene product required for UV and chemical mutagenesis in Escherichia coli. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5749–5753. [Europe PMC free article] [Abstract] [Google Scholar]
- Barbour SD, Clark AJ. Biochemical and genetic studies of recombination proficiency in Escherichia coli. I. Enzymatic activity associated with recB+ and recC+ genes. Proc Natl Acad Sci U S A. 1970 Apr;65(4):955–961. [Europe PMC free article] [Abstract] [Google Scholar]
- Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. [Abstract] [Google Scholar]
- BEERS RF, Jr, SIZER IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952 Mar;195(1):133–140. [Abstract] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. [Abstract] [Google Scholar]
- Brandsma JA, Bosch D, Backendorf C, van de Putte P. A common regulatory region shared by divergently transcribed genes of the Escherichia coli SOS system. Nature. 1983 Sep 15;305(5931):243–245. [Abstract] [Google Scholar]
- Brawn MK, Fridovich I. Increased superoxide radical production evokes inducible DNA repair in Escherichia coli. J Biol Chem. 1985 Jan 25;260(2):922–925. [Abstract] [Google Scholar]
- Carlioz A, Touati D. Isolation of superoxide dismutase mutants in Escherichia coli: is superoxide dismutase necessary for aerobic life? EMBO J. 1986 Mar;5(3):623–630. [Europe PMC free article] [Abstract] [Google Scholar]
- Casadaban MJ, Cohen SN. Lactose genes fused to exogenous promoters in one step using a Mu-lac bacteriophage: in vivo probe for transcriptional control sequences. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4530–4533. [Europe PMC free article] [Abstract] [Google Scholar]
- Christman MF, Morgan RW, Jacobson FS, Ames BN. Positive control of a regulon for defenses against oxidative stress and some heat-shock proteins in Salmonella typhimurium. Cell. 1985 Jul;41(3):753–762. [Abstract] [Google Scholar]
- CLARK AJ, MARGULIES AD. ISOLATION AND CHARACTERIZATION OF RECOMBINATION-DEFICIENT MUTANTS OF ESCHERICHIA COLI K12. Proc Natl Acad Sci U S A. 1965 Feb;53:451–459. [Europe PMC free article] [Abstract] [Google Scholar]
- Demple B, Halbrook J. Inducible repair of oxidative DNA damage in Escherichia coli. Nature. 1983 Aug 4;304(5925):466–468. [Abstract] [Google Scholar]
- Devoret R, Pierre M, Moreau PL. Prophage phi 80 is induced in Escherichia coli K12 recA430. Mol Gen Genet. 1983;189(2):199–206. [Abstract] [Google Scholar]
- Elledge SJ, Walker GC. Proteins required for ultraviolet light and chemical mutagenesis. Identification of the products of the umuC locus of Escherichia coli. J Mol Biol. 1983 Feb 25;164(2):175–192. [Abstract] [Google Scholar]
- Farr SB, D'Ari R, Touati D. Oxygen-dependent mutagenesis in Escherichia coli lacking superoxide dismutase. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8268–8272. [Europe PMC free article] [Abstract] [Google Scholar]
- Farr SB, Natvig DO, Kogoma T. Toxicity and mutagenicity of plumbagin and the induction of a possible new DNA repair pathway in Escherichia coli. J Bacteriol. 1985 Dec;164(3):1309–1316. [Europe PMC free article] [Abstract] [Google Scholar]
- Horii Z, Clark AJ. Genetic analysis of the recF pathway to genetic recombination in Escherichia coli K12: isolation and characterization of mutants. J Mol Biol. 1973 Oct 25;80(2):327–344. [Abstract] [Google Scholar]
- Huisman O, D'Ari R. An inducible DNA replication-cell division coupling mechanism in E. coli. Nature. 1981 Apr 30;290(5809):797–799. [Abstract] [Google Scholar]
- Imlay JA, Linn S. Bimodal pattern of killing of DNA-repair-defective or anoxically grown Escherichia coli by hydrogen peroxide. J Bacteriol. 1986 May;166(2):519–527. [Europe PMC free article] [Abstract] [Google Scholar]
- Kenyon CJ, Walker GC. DNA-damaging agents stimulate gene expression at specific loci in Escherichia coli. Proc Natl Acad Sci U S A. 1980 May;77(5):2819–2823. [Europe PMC free article] [Abstract] [Google Scholar]
- Kumura K, Sekiguchi M, Steinum AL, Seeberg E. Stimulation of the UvrABC enzyme-catalyzed repair reactions by the UvrD protein (DNA helicase II). Nucleic Acids Res. 1985 Mar 11;13(5):1483–1492. [Europe PMC free article] [Abstract] [Google Scholar]
- Levin DE, Hollstein M, Christman MF, Schwiers EA, Ames BN. A new Salmonella tester strain (TA102) with A X T base pairs at the site of mutation detects oxidative mutagens. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7445–7449. [Europe PMC free article] [Abstract] [Google Scholar]
- Linn S, Imlay JA. Toxicity, mutagenesis and stress responses induced in Escherichia coli by hydrogen peroxide. J Cell Sci Suppl. 1987;6:289–301. [Abstract] [Google Scholar]
- Little JW, Edmiston SH, Pacelli LZ, Mount DW. Cleavage of the Escherichia coli lexA protein by the recA protease. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3225–3229. [Europe PMC free article] [Abstract] [Google Scholar]
- Lloyd RG, Picksley SM, Prescott C. Inducible expression of a gene specific to the RecF pathway for recombination in Escherichia coli K12. Mol Gen Genet. 1983;190(1):162–167. [Abstract] [Google Scholar]
- Loewen PC. Isolation of catalase-deficient Escherichia coli mutants and genetic mapping of katE, a locus that affects catalase activity. J Bacteriol. 1984 Feb;157(2):622–626. [Europe PMC free article] [Abstract] [Google Scholar]
- Markham BE, Little JW, Mount DW. Nucleotide sequence of the lexA gene of Escherichia coli K-12. Nucleic Acids Res. 1981 Aug 25;9(16):4149–4161. [Europe PMC free article] [Abstract] [Google Scholar]
- McCord JM. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985 Jan 17;312(3):159–163. [Abstract] [Google Scholar]
- McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [Abstract] [Google Scholar]
- McPartland A, Green L, Echols H. Control of recA gene RNA in E. coli: regulatory and signal genes. Cell. 1980 Jul;20(3):731–737. [Abstract] [Google Scholar]
- Milcarek C, Weiss B. Mutants of Escherichia coli with altered deoxyribonucleases. I. Isolation and characterization of mutants for exonuclease 3. J Mol Biol. 1972 Jul 21;68(2):303–318. [Abstract] [Google Scholar]
- Morgan RW, Christman MF, Jacobson FS, Storz G, Ames BN. Hydrogen peroxide-inducible proteins in Salmonella typhimurium overlap with heat shock and other stress proteins. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8059–8063. [Europe PMC free article] [Abstract] [Google Scholar]
- Morimyo M. Anaerobic incubation enhances the colony formation of a polA recB strain of Escherichia coli K-12. J Bacteriol. 1982 Oct;152(1):208–214. [Europe PMC free article] [Abstract] [Google Scholar]
- Mount DW, Low KB, Edmiston SJ. Dominant mutations (lex) in Escherichia coli K-12 which affect radiation sensitivity and frequency of ultraviolet lght-induced mutations. J Bacteriol. 1972 Nov;112(2):886–893. [Europe PMC free article] [Abstract] [Google Scholar]
- Pang PP, Walker GC. Identification of the uvrD gene product of Salmonella typhimurium LT2. J Bacteriol. 1983 Mar;153(3):1172–1179. [Europe PMC free article] [Abstract] [Google Scholar]
- Picksley SM, Attfield PV, Lloyd RG. Repair of DNA double-strand breaks in Escherichia coli K12 requires a functional recN product. Mol Gen Genet. 1984;195(1-2):267–274. [Abstract] [Google Scholar]
- Roberts JW, Roberts CW. Two mutations that alter the regulatory activity of E. coli recA protein. Nature. 1981 Apr 2;290(5805):422–424. [Abstract] [Google Scholar]
- Roberts JW, Roberts CW, Craig NL. Escherichia coli recA gene product inactivates phage lambda repressor. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4714–4718. [Europe PMC free article] [Abstract] [Google Scholar]
- Vales LD, Chase JW, Murphy JB. Effect of ssbA1 and lexC113 mutations on lambda prophage induction, bacteriophage growth, and cell survival. J Bacteriol. 1980 Aug;143(2):887–896. [Europe PMC free article] [Abstract] [Google Scholar]
- Walker GC. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984 Mar;48(1):60–93. [Europe PMC free article] [Abstract] [Google Scholar]
- Witkin EM, McCall JO, Volkert MR, Wermundsen IE. Constitutive expression of SOS functions and modulation of mutagenesis resulting from resolution of genetic instability at or near the recA locus of Escherichia coli. Mol Gen Genet. 1982;185(1):43–50. [Abstract] [Google Scholar]
Associated Data
Articles from Journal of Bacteriology are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/jb.169.7.2967-2976.1987
Read article for free, from open access legal sources, via Unpaywall:
https://jb.asm.org/content/jb/169/7/2967.full.pdf
Free to read at jb.asm.org
http://jb.asm.org/cgi/content/abstract/169/7/2967
Free after 4 months at jb.asm.org
http://jb.asm.org/cgi/reprint/169/7/2967
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
OxyR is required for oxidative stress resistance of the entomopathogenic bacterium Xenorhabdus nematophila and has a minor role during the bacterial interaction with its hosts.
Microbiology (Reading), 170(7), 01 Jul 2024
Cited by: 0 articles | PMID: 39058385 | PMCID: PMC11281485
A library of reporters of the global regulators of gene expression in Escherichia coli.
mSystems, 9(6):e0006524, 30 Apr 2024
Cited by: 0 articles | PMID: 38687030 | PMCID: PMC11237500
Nonlethal deleterious mutation-induced stress accelerates bacterial aging.
Proc Natl Acad Sci U S A, 121(20):e2316271121, 06 May 2024
Cited by: 1 article | PMID: 38709929 | PMCID: PMC11098108
Multi-Omics Study on Molecular Mechanisms of Single-Atom Fe-Doped Two-Dimensional Conjugated Phthalocyanine Framework for Photocatalytic Antibacterial Performance.
Molecules, 29(7):1601, 03 Apr 2024
Cited by: 0 articles | PMID: 38611880 | PMCID: PMC11013413
Bicyclomycin generates ROS and blocks cell division in Escherichia coli.
PLoS One, 19(3):e0293858, 29 Mar 2024
Cited by: 0 articles | PMID: 38551933 | PMCID: PMC10980228
Go to all (234) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Toxicity, mutagenesis and stress responses induced in Escherichia coli by hydrogen peroxide.
J Cell Sci Suppl, 6:289-301, 01 Jan 1987
Cited by: 32 articles | PMID: 3308921
Induction of the SOS response by hydrogen peroxide in various Escherichia coli mutants with altered protection against oxidative DNA damage.
J Bacteriol, 171(11):6141-6147, 01 Nov 1989
Cited by: 53 articles | PMID: 2681154 | PMCID: PMC210482
Efficient repair of hydrogen peroxide-induced DNA damage by Escherichia coli requires SOS induction of RecA and RuvA proteins.
Mutat Res, 459(3):187-194, 01 Apr 2000
Cited by: 20 articles | PMID: 10812330
Mutagenic and lethal effects of near-ultraviolet radiation (290-400 nm) on bacteria and phage.
Environ Mol Mutagen, 10(3):317-337, 01 Jan 1987
Cited by: 34 articles | PMID: 3315655
Review
Funding
Funders who supported this work.
NIEHS NIH HHS (1)
Grant ID: P30ES01896
NIGMS NIH HHS (1)
Grant ID: GM19020