| PMC full text: |
|
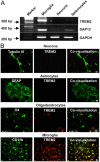
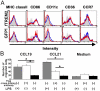
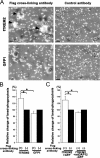
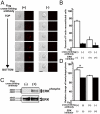
Figure 8.
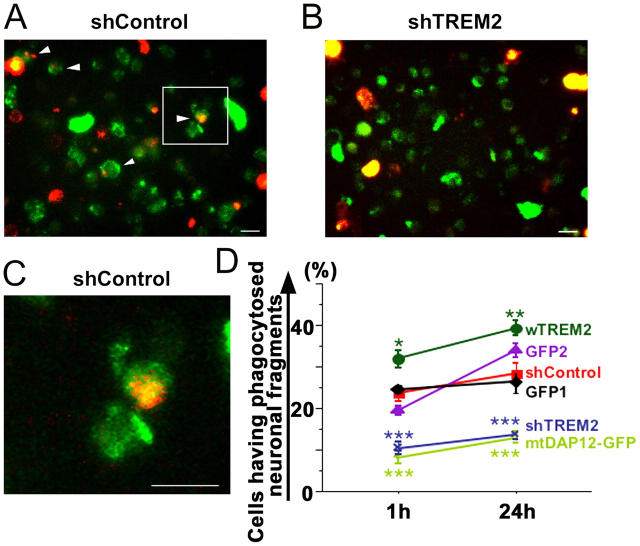
TREM2 expression of microglia determines phagocytosis of apoptotic neuronal membranes. Cultured neurons were labeled with a red fluorescent membrane dye, treated with okadaic acid to induce apoptosis, and cocultured with microglia transduced either with a lentiviral vector producing short hairpin RNAs specific for TREM2 (shTREM2), irrelevant short hairpin RNAs (shControl), wTREM2, control GFP2 vector, mtDAP12 (mtDAP12-GFP), or control GFP1 vector. Cells were analyzed by fluorescence microscopy and flow cytometry. (A) Microglia (green fluorescent protein) lentivirally transduced with the control vector (shControl) phagocytosed apoptotic neuronal membrane fragments (red fluorescent dye) as shown by confocal images. Inset as indicated. Bar, 10 μm. (B) No obvious phagocytosis was observed in TREM2 knockdown microglia. Bar, 10 μm. (C) High magnification of the shControl image. Bar, 10 μm. (D) The percentage of cells having phagocytosed apoptotic neuronal membrane fragments was quantified by flow cytometry at 1 and 24 h of coculture. Microglia were transduced with lentiviral vectors producing short hairpin RNAs specific for TREM2 (shTREM2), irrelevant short hairpin RNAs (shControl), wTREM2, control GFP2, mtDAP12 (mtDAP12-GFP), or control GFP1. wTREM2 vector (wTREM2)-transduced microglia phagocytosed significantly more apoptotic neuronal material after 1 and 24 h compared with GFP- (GFP1 or GFP2) or control vector (shControl)-transduced microglia. Control vector (shControl)- and GFP (GFP1 or GFP2)-transduced microglia phagocytosed more apoptotic neuronal material after 1 and 24 h compared with microglia transduced with the TREM2 knockdown (shTREM2) or mtDAP12 (mtDAP12-GFP) vector. Data are presented as mean ± SEM. *, P < 0.02; **, P < 0.05; ***, P < 0.01; Mann-Whitney U test.