Abstract
Free full text

The anticancer immune response: indispensable for therapeutic success?
Abstract
Although the impact of tumor immunology on the clinical management of most cancers is still negligible, there is increasing evidence that anticancer immune responses may contribute to the control of cancer after conventional chemotherapy. Thus, radiotherapy and some chemotherapeutic agents, in particular anthracyclines, can induce specific immune responses that result either in immunogenic cancer cell death or in immunostimulatory side effects. This anticancer immune response then helps to eliminate residual cancer cells (those that fail to be killed by chemotherapy) or maintains micrometastases in a stage of dormancy. Based on these premises, in this Review we address the question, How may it be possible to ameliorate conventional therapies by stimulating the anticancer immune response? Moreover, we discuss the rationale of clinical trials to evaluate and eventually increase the contribution of antitumor immune responses to the therapeutic management of neoplasia.
A daunting diversity of distinct molecular etiologies gives rise to one class of life-threatening diseases — cancer (1) — which affects half of the inhabitants of developed countries during their lifetime and kills one-third of them. The changes in the cell biology of tumor cells are conditioned by epigenetic and genetic reprogramming, genomic instability being an essential feature of both oncogenesis and tumor progression. This epigenetic and genetic modification of cancer cells is often accompanied by the emission of “danger signals,” as well as the expression of ectopic or mutated proteins. Thus, the antigenic characteristics of tumor cells can be perceived by the innate and cognate immune systems.
As defined primarily by Hanahan and Weinberg, the tumorigenic process stems from six hallmark criteria: i.e., growth signal self-sufficiency, resistance to growth-inhibitory signals, resistance to apoptosis, limitless growth potential, sustained angiogenesis, and metastasizing potential (1). A seventh potential hallmark of cancer, avoidance of immunosurveillance (2), allowing tumor cells to escape anticancer immune responses or to actively suppress them (2, 3), has come under close scrutiny. The question as to whether and to what extent immunosurveillance controls and shapes the development of human cancers has been examined in several recent reviews (4–6). There is increasing evidence that tumors develop more frequently in immunodeficient patients, for instance, in transplant recipients (7). This strongly suggests that at least part of the vast evidence in favor of an important role for immunosurveillance in oncogenesis and tumor progression, as obtained in mouse models, can be extrapolated to the human system.
Our ever-expanding understanding of the molecular and cellular etiology of cancer has not yet been accompanied by a parallel improvement in therapeutic outcome. The purpose of this review is to raise a series of related questions: Is the success (or the lack thereof) of anticancer chemotherapy or radiotherapy conditioned by contribution of the immune system? And, if so, is it possible to enhance conventional therapies by stimulating the anticancer immune response? How could we best design clinical trials to interrogate (and eventually increase) the contribution of antitumor immune responses to the therapeutic management of neoplastic disease?
Relationship between cancer and the immune system: a quick guide
As previously discussed in a recent review (4), the immune system may prevent tumor outgrowth. Thus, occult cancer becomes manifest in mice after ablation of T cells and/or injection of anti–IFN-γ antibodies, indicating that the adaptive immune response can keep cancer in check during the equilibrium state (6). However, cancer cells escape the innate and adaptive immune responses (immunosurveillance) by immunoselection (selection of nonimmunogenic tumor cell variants, also known as immunoediting) or immunosubversion (active suppression of the immune response).
Although the concept of immunoediting has been developed in mice, there is evidence that this idea may also apply to humans. Unstable microsatellite tumors in humans (which can be expected to carry more neoantigens than tumors with chromosomal instability) are prominently infiltrated by CTLs and are associated with a favorable prognosis (8–10). Tumor infiltration by T, NK, and NKT cells is a sign of improved prognosis in multiple human neoplasias, including melanoma (11), colon (12), and ovarian cancers (13). Spontaneous tumor regression coupled to massive lymphocyte infiltration has been noted in individual patients with basal cell carcinoma (14), Merkel cell carcinoma (15), and lung carcinoma (16). High levels of antibodies reactive against the tumor suppressor protein p53 have a positive prognostic value in ovarian (17) and gastric cancer (18). In patients with early breast cancer, survival is favorably influenced by a natural humoral immune response to mucin. Indeed, mucin MUC1, a heterodimeric transmembrane glycoprotein aberrantly overexpressed by most human carcinomas, is a tumor antigen recognized by T cells and is shared by different tumors such as breast, colon, pancreas, ovary, and lung carcinoma and may be tumor specific due to its differential glycosylation in normal versus tumor cells (19).
One important question concerns the impact of conventional anticancer chemotherapy on the relationship between the tumor and the immune system. Therapy that is applied during the tumor escape phase not only affects the tumor but also modulates the relationship between the tumor and the immune system. Thus, chemotherapy can, by simply reducing the tumor mass (debulking), reduce its immunosuppressive properties. As a proof of principle, the mere surgical removal of the primary tumor (mechanical debulking) can reverse tumor-induced immune tolerance, restoring the antibody- and cell-mediated immune responses, even in animals bearing metastatic breast cancer (20). By enforcing the selection of chemotherapy-resistant tumor cells and by inducing additional mutations (chemotherapy often involves mutagenic agents), therapy can induce the expression of new tumor antigens. Chemotherapy can cause immunogenic cancer cell stress or death and hence mediate a sort of cancer vaccination effect (as discussed below). Furthermore, chemotherapy can stimulate the immune system, either via a direct effect on immune effectors or regulatory mechanisms or indirectly, by causing lymphopenia followed by homeostatic proliferation of immune effectors that may be particularly active in the anticancer response. The combination of these effects may “reset” the relationship between the tumor and the immune system from the latest stage (escape) to a preceding state (elimination or equilibrium).
Immunostimulatory side effects of anticancer drugs
Although cytotoxic anticancer drugs as well as so-called targeted agents act mostly through direct effects on cancer cells, many among these agents have additional effects on the immune system that may contribute to their therapeutic efficacy (Figure (Figure1). 1).
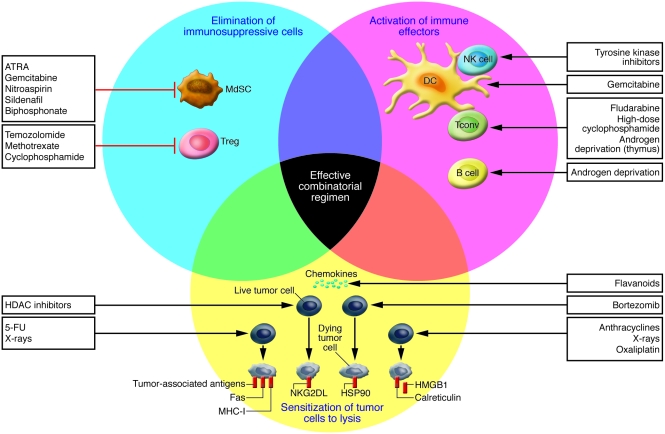
Anticancer therapeutics can inhibit suppressive mechanisms of tumor-induced immune tolerance (blue circle), boost T and/or B cell responses (pink circle), or stress tumor cells in such a way that tumor cells become immunogenic and sensitive to lysis (yellow circle). The main drugs driving these effects are also shown. Cyclophosphamide at low doses, gemcitabine, and all-trans-retinoic acid (ATRA) act on immunosuppressive cells such as Tregs or myeloid suppressor cells (MdSC) to facilitate tumor attack by conventional effectors (Tconv). Pharmacological inhibition of MdSCs can also be achieved by nitroaspirin (96), sildenafil (97), and biphosphonate (98). Androgen deprivation boosts T and B cell responses. Strategies leading to lymphodepletion allow the establishment of memory effector T cells efficient in long-term protection against tumor cells. Tyrosine kinase inhibitors boost DC/NK cell crosstalk. The proteasome inhibitor bortezomib induces myeloma cell–surface expression of the molecular chaperone protein HSP90, which leads to DC uptake, antigen processing, and DC maturation. Anthracyclines, oxaliplatin, and irradiation promote tumor membrane expression of CRT and release of HMGB1 by tumor cells, which are required events for DC-mediated phagocytosis of dying tumors and cross-presentation of tumor antigens to T cells, respectively. Inhibitors of histone deacetylases (HDACs) promote the expression of NKG2D ligands (NKG2DL), sensitizing the tumor cell to NK cell–mediated lysis. Tumor cells exposed to x-rays express increased numbers of MHC class I molecules, tumor antigens, and Fas, favoring CTL attack. Flavanoid-mediated production of chemokines favors attraction of immune effectors into tumor beds. Ideally, an appropriate combination of chemotherapeutic agents could achieve all of these three types of beneficial effects.
Transient lymphopenia (abnormally low levels of blood lymphocytes) may activate homeostatic mechanisms that finally stimulate tumor-specific effector T cells. Thus, the therapeutic induction of lymphopenia has raised considerable interest in the context of adoptive cell transfer (ACT) therapies (which involve ex vivo generation of tumor-reactive lymphocytes from endogenous tumor-infiltrating lymphocytes and their activation and expansion before reinfusion into the tumor-bearing host) and vaccination against melanoma (21). Animal studies demonstrated that lymphoablation enhances the effectiveness of adoptively transferred tumor-specific CD8+ T cells (22). These experimental data have been tested in a clinical trial on 35 patients with metastatic melanoma refractory to conventional treatments. A highly lymphodepleting conditioning regimen was followed by ACT with tumor-infiltrating lymphocytes (TILs), resulting in 51% objective response rates associated with a persistent clonal lymphocytosis and/or antimelanocyte autoimmunity (23). In mice, lymphodepletion can be combined with vaccination strategies to promote the differentiation of central memory T cells specific for tumor antigens (24–27). The clinical relevance of these findings has been validated in a randomized clinical trial in myeloma (28). The in vivo–primed T cells of patients who were immunized with the 7-valent pneumococcal conjugate vaccine (PCV; Prevnar) were first collected following vaccination. Patients who then received high-dose chemotherapy and autologous stem cell transplantation as well as an infusion of these in vivo–primed and in vitro–expanded T cells, followed by subsequent booster immunizations, had a robust reconstitution of clinically relevant antimicrobial immunity within a month after transplantation (28). Defeating T cell fatigue after ACT against cancer or HIV has also been achieved with IL-15 (29), anti-CD40 (30), or programmed cell death 1 (PD-1) blockade (31). These findings suggest that, after some additional refinement, chemotherapy or irradiation-induced lymphodepletion, combined with additional manipulations such as ACT and immunopharmacological interventions, might achieve antitumor immune responses.
Hormonotherapy, the use of hormonal manipulation, is part of the clinical armamentarium for the management of breast and prostate cancer. The influence of androgens on lymphocyte development and activation has been reviewed (32). Experimental data indicate that androgen deprivation increases the number of naive T cells exported from the thymus and may therefore contribute to broadening the repertoire of T cell immunity, leading to effective antitumor immune responses and breaking of tumor-induced tolerance (33, 34). Moreover, androgen deprivation enhances the production of newly generated IgM+ naive B cells from bone marrow (35). Androgen ablation in prostate cancer patients may activate immune responses to some prostate tumor antigens by increasing the pool of naive T cells (as demonstrated by TCR rearrangement excision circle [TREC] analyses; TRECs are stable DNA circles excised from T cell germline DNA to allow for TCR formation during early T cell development and exist in high concentrations in thymic emigrants), decreasing and/or diluting the population of Tregs, and favoring the concomitant infiltration of T cells into tumor beds (36, 37). One study performed on 73 men bearing nonmetastatic prostate cancer and 50 controls showed that neoadjuvant hormonal and radiation therapy (but not radical prostatectomy) can elicit a tumor-specific autoantibody response (38). Humoral responses arose early and were durable in most cases, prompting the manipulation of B cell responses by immunotherapy.
Some agents (in particular cyclophosphamide but also fludarabine, gemcitabine, oxaliplatin, and 5-fluorouracil [5-FU]) can at least partially deplete or transiently inactivate tumor-protective Tregs (39–41). Several clinical trials have combined low doses of cyclophosphamide and antitumor vaccination for the treatment of metastatic melanoma, without any clear results, perhaps due to the lack of statistical power (42–46). In a small, randomized study performed on 42 metastatic breast cancer patients, cyclophosphamide was combined with a vaccine composed of a synthetic sialyl-Tn epitope linked to keyhole limpet hemocyanin (KLH) and an adjuvant. KLH is a natural protein isolated from the marine mollusk keyhole limpet and used as a carrier protein together with weak antigens to boost immune responses to haptens, self antigens, and idiotype proteins. A statistically significant increase in median survival from 12 months to 20 months was reported (47). A similar trend was observed in renal cell carcinoma patients immunized with allogeneic, mature DCs pulsed with tumor lysates and KLH and also receiving 300 mg/m2 i.v. cyclophosphamide 4 and 3 days before each monthly vaccination (48). In yet another study, 10 end-stage cancer patients received 1 daily dose of 100 mg cyclophosphamide, every other week, for 4 weeks (49). This “metronomic” (i.e., administered orally daily) cyclophosphamide treatment suppressed Treg inhibitory functions and restored the proliferative capacity of effector T cells as well as the cytotoxicity of NK cells (49, 50).
Many other anticancer agents also have immunostimulatory effects that have been demonstrated at the clinical level. For example, gemcitabine has been reported to enhance the frequency of IFN-γ–producing T cells and/or CD69+ activated cells in pancreatic cancer patients (51). In a phase I trial, gemcitabine elicited cellular immune responses in non–small cell lung cancers (52). One clinical trial examined the combined effects of gemcitabine and cytokines (GM-CSF and low doses of IL-2) in 42 patients presenting with advanced colorectal cancers. The objective responses rates and time to progression were encouraging and associated with increased tumor-specific CTL precursor frequencies in responders (53), prompting the initiation of a phase III trial. Another example is provided by a study of imatinib mesylate (IM; also known as STI571 and Gleevec), an inhibitor of the tyrosine kinases BCR-ABL, PDGFR, and KIT. Some patients with gastrointestinal stromal tumors (GISTs) that have no c-KIT mutation (and hence lack the IM target) responded to IM (54), suggesting that IM can exert indirect antitumor effects. Indeed, in a fraction of patients with GIST, IM causes an increase in NK activity. The activation of NK cells induced by IM constitutes a positive prognostic parameter (54), indicating that immunostimulation may contribute to the therapeutic effect of IM in patients, as has been suggested by animal experiments (55).
Immunogenic cancer cell stress and death
Transforming cells have to overcome both intrinsic (cell-autonomous) and extrinsic (immune-mediated) barriers to tumorigenesis. One important intrinsic barrier against transformation includes the activation of a DNA damage response after oncogenic stress, resulting in the activation of ataxia telangiectasia mutated (ATM), checkpoint kinase–1 (CHK1), and finally, p53-dependent apoptosis (56) or senescence (57). As a result, preneoplastic lesions and in situ carcinomas often manifest the activation of ATM, CHK1, and p53, while advanced cancers suppress or lose this DNA damage response. An extrinsic barrier against tumor growth consists of the elimination of transforming cells by cells from the innate and cognate immune systems. Intriguingly, these barriers may be linked in molecular terms (3, 58). Thus, the DNA damage response induces expression of NK cell group 2D (NKG2D) ligands on tumor cells in an ATM- and CHK1-dependent (but p53-independent) fashion (59). NKG2D is an activating receptor involved in tumor immunosurveillance that is expressed on NK, NKT, and γδT cells and resting (in mice) and/or activated (in humans) CD8+ T cells. The expression of NKG2D ligands on the surface of transforming cells thus can be expected to play a major role in tumor surveillance (Figure (Figure1). 1).
Although p53 is not required for the expression of NKG2D ligands in cells undergoing DNA damage (59), a recent study highlights an important cooperation between p53-induced tumor cell senescence and the innate immune system (57). Restoration of p53 function in established liver cancers led to tumor regression, but only when the mice carried an intact immune system. Thus, the reactivation of p53 led to the remission of liver cancer, an effect that was lost after ablation of NK cells and macrophages (57). These examples illustrate how molecules that are activated during early tumorigenesis (e.g., ATM, CHK1, p53) can alert the immune system to mediate an antitumor response. As chemotherapy with DNA-damaging agents often activates ATM, CHK1, and p53, it appears plausible, yet remains to be proven, that such agents also elicit ATM-, CHK1-, and p53-dependent immune effects.
Chemotherapeutic agents and radiation can increase the immunogenic properties of tumor cells by enhancing MHC class I expression (60), thereby increasing their vulnerability to CTLs. Similarly, some chemotherapeutic agents (such as genotoxic agents and histone deacetylase inhibitors) increase the expression of NKG2D ligands (59, 61), thus facilitating tumor cell lysis by NKG2D-expressing lymphocytes (including NK cells, NKT cells, and CTLs). Yet another frequent effect of DNA damage inflicted by radiotherapy or chemotherapy is the increase in the expression of death receptors (in particular Fas/CD95 and TNF-related apoptosis-inducing ligand [TRAIL] receptors) (62), enabling lysis of the tumor cells by Fas/CD95 ligand and TRAIL-positive immune effectors (Figure (Figure1). 1).
Tumor cell demise often occurs through apoptosis or necrosis. Although there are teleological arguments to suggest that apoptosis must be nonimmunogenic or even tolerogenic (because physiological cell death occurs through apoptosis but does not lead to autoimmunity), and although necrosis (which is often pathological) has been considered as being proinflammatory, the theoretical equations in which apoptosis equals nonimmunogenicity and necrosis equals immunogenicity do not withstand experimental verification. Instead, it seems that apoptosis is nonuniform in biochemical terms, meaning that various pathways can lead to cell death and induce stimulus-specific changes. Anthracyclines, oxaliplatin, and ionizing irradiation have the exceptional capacity to induce immunogenic cell death, while the vast majority of chemotherapeutic agents induce nonimmunogenic cell death (63–66). Anthracyclines, oxaliplatin, and ionizing irradiation also have the particular ability to induce the early, preapoptotic translocation of the chaperone calreticulin (CRT) from the lumen of the endoplasmic reticulum (endo-CRT) to the plasma membrane (ecto-CRT) (64, 65, 67). Ecto-CRT then facilitates the engulfment of the tumor cell by DCs yet does not induce DC maturation. Neutralization or knockdown of CRT abolishes the immunogenicity of tumor cell death, while exogenous supply of recombinant CRT can restore the immunogenicity per se of nonimmunogenic cell death (64, 65, 67) (Figure (Figure1). 1).
Another chaperone, HSP90, has recently been reported to be involved in the immunogenicity of human myeloma cell death elicited by the proteasome inhibitor bortezomib (68). In this in vitro model, HSP90 is exposed on the plasma membrane surface of myeloma cells following exposure to bortezomib and induces the phenotypic maturation of DCs (Figure (Figure1).1). Yet another factor that acts on DCs is high mobility group box 1 protein (HMGB1), a chromatin-binding protein that is released from cells during the late stages of cell death induced by anthracyclines, oxaliplatin, and ionizing irradiation. HMGB1 then interacts with TLR4 on the surface of DCs and affects the intracellular fate of engulfed antigen from dying tumor cells. Ligation of TLR4 by HMGB1 inhibits the fusion of phagosomes (which contain antigenic cargo) with lysosomes, thereby allowing the antigen to traffic toward the antigen-presenting compartment (66). Neutralization or knockdown of HMGB1 abolishes the capacity of dying tumor cells to elicit anticancer immune responses, underscoring its contribution to tumor immunogenicity (66). The aforementioned examples illustrate that, depending on the cell death inducer, tumor cells can expose or release factors that affect their uptake by DCs (e.g., CRT), the maturation of DCs (e.g., HSP90), or antigen presentation by DCs (e.g., HMGB1).
Molecular and/or epidemiological evidence for an immune response during therapy
Pioneering work has highlighted a contribution of T cells to the antitumor effects mediated by cytotoxic agents (Tables (Tables11 and and2)2) (69). An EL4 thymoma cell clone that was relatively resistant to the anthracycline doxorubicin in vitro gave rise to doxorubicin-sensitive tumors when implanted into immunocompetent C57BL/6 mice. This unexpected therapeutic effect, however, appeared to depend on splenocytes, suggesting that immunity could be indispensable to the antitumor effects mediated by anthracyclines (69). While this contention was not always supported by experimental data (Table (Table1),1), similar results have been obtained by other investigators in several other cancers (Table (Table2),2), provided that immunogenic chemotherapies (for example, anthracyclines or oxaliplatin) were administered. Thus, EL4 thymoma, Glasgow osteosarcomas, and CT26 colon cancer treated with oxaliplatin, as well as CT26 colon cancers and MCA205 fibrosarcomas treated with anthracyclines, exhibit much better therapeutic responses when the host is immunocompetent than when it is immunodeficient (e.g., athymic nu/nu mice) (64, 66, 67, 70). Similarly, the depletion of CD4+ or CD8+ T cells by injection of specific monoclonal antibodies compromises the therapeutic efficacy of doxorubicin on CT26 tumors (64). TS/A, a cell line derived from a spontaneous mammary adenocarcinoma in BALB/c mice, also responded much better to local radiotherapy in immunocompetent as compared with athymic hosts (66). Together, these results demonstrate that the efficacy of chemotherapy and radiotherapy is influenced by the immune system.
Table 1
Mouse models in which chemotherapy efficacy does not involve immunity
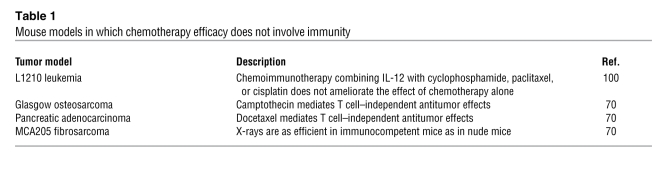
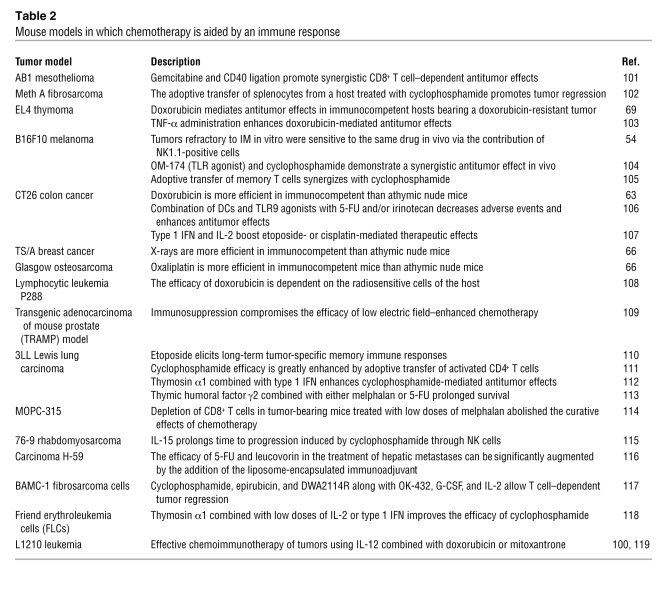
Table 2
Mouse models in which chemotherapy is aided by an immune response
The aforementioned results suggest that induction of immunogenic tumor cell death by anthracyclines, oxaliplatin, or ionizing radiation stimulates an immune response that helps keep the tumor in check. If this speculation were true, one would expect that tumor-derived proteins such as CRT and HMGB1 (which dictate the immunogenicity of tumor cell death) and the presence of their receptors on antigen-presenting cells would be required for full therapeutic success. Accordingly, the knockout of TLR4 and that of its downstream effector MyD88 (but not that of TIR domain–containing adapter-inducing IFN-β [TRIF], an adapter responding to activation of TLRs that mediates a signaling cascade distinct from that mediated by MyD88) reduced the therapeutic efficacy of immunogenic chemotherapies in mice bearing CT26 colon cancers, TS/A mammary cancers, EL4 thymomas, or Glasgow osteosarcomas (66).
In individuals of mixed European descent, there is a sequence polymorphism in TLR4 (896A→G, Asp299Gly, RefSNP identification number rs4986790) with an allelic frequency of approximately 6% (71, 72) that prevents the binding of HMGB1 to TLR4 in a dominant negative fashion (66, 70). A clinical study indicated that breast cancer patients that carry the Asp299Gly TLR4 allele do not differ from patients displaying the normal TLR4 allele for any of the classical prognostic factors. However, the Kaplan-Meier estimate of metastasis-free survival after local radiotherapy and systemic anthracycline chemotherapy indicated that the Asp299Gly TLR4 allele (but not the TLR4 mutation, RefSNP identification number rs1927911) is an independent predictive factor of early relapse (66, 70). In metastatic renal cancer, a polymorphism in the IL-4 promoter strongly influences prognosis (73).
Many investigators use the term immunochemotherapy to describe the therapeutic instillation of monoclonal antibodies that recognize tumor antigens. Of note, the efficacy of immunochemotherapy in rituximab (anti-CD20)–treated follicular lymphoma is, in part, dictated by the efficacy of antibody-dependent cellular cytotoxicity, as determined by the Fc receptor IIIA genotype (74, 75) and the content of tumor-associated macrophages (76).
For technical reasons, there are very few studies that address the frequency of tumor-specific effector or memory T cells or antibody titers before and after chemotherapy. In infants with acute myeloid leukemia (AML), the emergence of antileukemia precursors after chemotherapy correlates with the maintenance of hematologic remission (77). Recently, a dynamic immunomonitoring study reported the presence of leukemia-specific, TNF-α–producing CD4+ T cells in patients bearing chronic myelogenous leukemia and successfully treated with IM (78). Spontaneous and induced T cell reactivities were associated with hematological and cytogenetic responses in a majority of patients. Interestingly, antibody titers against antiphospholipids were associated with prolonged survival after retinoid acid treatment in promyelocytic leukemia (79, 80). Galanis et al. studied the prognostic value of neuronal autoantibodies in small cell lung carcinoma patients. Although the titers of the paraneoplastic autoantibodies were associated with less-extensive disease at presentation, the cisplatin-based chemotherapy caused patients to become immunocompromised, and time to progression could not be predicted according to a rise in the antibody titers (81).
Future investigation should address the question, In which cancers does the induction of specific antitumor immune responses by immunogenic chemotherapies or radiotherapy have a prognostic impact?
Immunochemotherapy
Based on the aforementioned premises, oncologists and immunotherapy experts might discuss the opportunity to optimize their current anticancer approaches. Such an optimization might stem from the sequential strategy depicted in Figure Figure2,2, associating: (a) suppressors of tolerogenic pathways; (b) priming of naive T and B cells with vaccines; (c) induction of cell death with cytotoxic drugs; (d) agents rendering nonimmunogenic cell death immunogenic; (e) chloroquine (to combat host defects in TLR4 or block autophagy and multidrug resistance); (f) and sustaining immune effectors with NK, NKT, or DC adjuvants (e.g., TLR agonists, glycolipids, cytokines, chemokines).
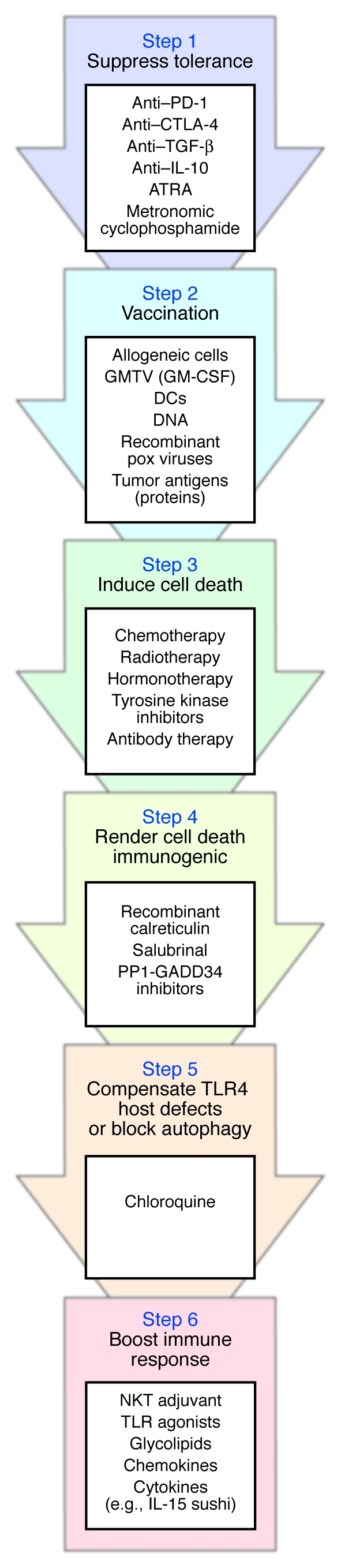
The immune system of cancer-bearing individuals suffers from tumor-induced tolerance, which should be alleviated (Step 1) before induction of an active immune response with tumor vaccines (Step 2). Some evidence suggests that prior vaccination (Step 2) favors the antitumor effects of chemotherapeutic agents (Step 3). Cell death triggered by chemotherapy or radiotherapy (Step 3) should then be rendered immunogenic via addition of compounds that enhance calreticulin expression at the tumor cell membrane (Step 4). To overcome putative TLR4 host defects, which can compromise the developing immune response, administration of chloroquine is indicated (Step 5). Finally, immune adjuvants should be given to sustain and enhance the ensuing antitumor immune response (Step 6). Potential mediators at each step are listed. GMTV, genetically modified tumor vaccines; PP1-GADD34, protein phosphatase 1 complexed to GADD34; IL-15 sushi, sushi domain of soluble IL-15 receptor α (99).
Future studies should focus on combination therapies and determine the optimal schedule of chemoimmunotherapy, since the kinetics of the host and tumor response are likely critical determinants of the therapeutic response. Moreover, such combinations, while potentially effective, might also be fraught with significant toxicity (i.e., infectious diseases or autoimmunity, as have already been observed with the use of lymphodepletion or anti-CTLA4 antibodies). Phase I trials of immunochemotherapy may help define the toxicity and the dosage at which an immune response is achieved, but sizeable randomized studies will be needed to evaluate the optimal schedule of such therapeutic combinations.
To date, very few clinical studies have combined conventional chemotherapies with anticancer vaccination and/or immunostimulatory regimens (Table (Table3).3). In particular, there is no systematic or randomized assessment of the order in which cytotoxic therapies and tumor vaccines should be administered. In one trial, 29 end-stage non–small cell lung cancer patients who were resistant to first-line platin-based therapy were enrolled in a study employing a vaccination protocol involving autologous DCs infected with adenoviral vectors encoding p53 (82). Since the tumor progressed in 23 of 29 patients, salvage chemotherapy by paclitaxel or carboplatin was proposed as a second line of chemotherapy. The response rate to this second-line chemotherapy was 61.5%, and up to 38% survived at one year following vaccination. The historical controls were known to exhibit a 6%–16% objective response rate to a second-line chemotherapeutic, and less than 20% were alive beyond one year. A direct positive correlation between immune response to p53 and clinical outcome could be observed. Only 30% of patients who did not develop a p53-specific response to vaccination responded to second-line chemotherapy, whereas 75% of p53 cellular immune responders had objective clinical responses to chemotherapy after vaccination (P = 0.08) (82).
Table 3
Patient outcome after a combination of conventional chemotherapy and immunomodulation
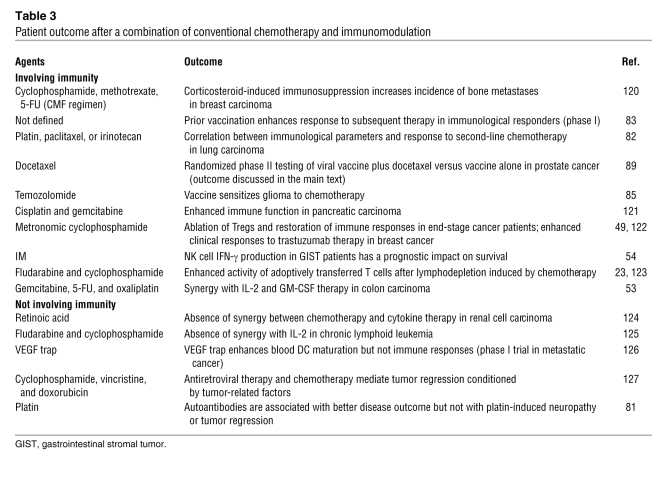
In another study, 17 patients bearing a diverse range of advanced tumors received an encapsulated DNA vaccine encoding the carcinogen activator cytochrome P450 1B1 expressed by almost all human tumors. While 10 of 11 who did not develop anti-CYP1B1–specific T cells failed to respond to salvage therapy, 5 of 6 who elicited immunity against CYP1B1 exhibited marked response to the salvage regimen for more than one year (83).
A similar conclusion was reached in a retrospective examination of the impact of vaccination based on peptide- or lysate-loaded DCs on the efficacy of conventional therapy (craniotomy, stereotaxic radiosurgery, radiotherapy, treatment with temozolomide alone or in combination with nitrosourea) against glioblastoma (84, 85). Progression rates and overall survival were compared among 12 tyrosinase-related protein 2–based (TRP-2–based) vaccine–treated patients, 13 chemotherapy-treated patients, and 13 glioblastoma patients treated with a combination of the TRP-2–based vaccine and chemotherapy. The results suggested that chemotherapy synergized with previous therapeutic vaccination to slow glioblastoma progression and significantly extend patient survival relative to individual therapies. In some cases, where the authors could establish glioblastoma cell lines before and after vaccination, the immunoselected tumor cells exhibited increased sensitivity to temozolomide and carboplatin compared with primary tumor cells. These data suggest that immunological targeting of TRP-2 increases sensitivity to cell death inducers (86).
Given the evidence of a role for testosterone in modulating B and T cell immune responses, there is a strong rationale for combining androgen deprivation with vaccine therapy in prostate cancer, and this is further supported by some experimental data (87). As reviewed by Aragon-Ching et al. (32), phase I and phase II trials indicated that androgen ablation may boost immune responses elicited by DCs, allogeneic prostate cancer cell lysate, or GM-CSF–secreting tumor cell–based vaccines (88). Arlen et al. (89) were the first to conduct a randomized study evaluating the use of vaccine alone versus concomitant vaccine plus weekly administration of docetaxel and steroids, with the end points of monitoring immune responses in 28 patients bearing a metastatic, androgen-resistant prostate cancer. First, they showed that a vaccine (admixed recombinant vaccinia virus expressing prostate-specific antigen [PSA]) or B7.1 followed by sequential boosts with fowlpox virus-PSA can be administered with weekly docetaxel and dexamethasone comedication without compromising the ability of the patient to mount tumor-associated antigen–specific T cell responses as compared with vaccine alone. They also evaluated clinical responses to the combination therapy, vaccine alone, and chemotherapy after progression following vaccination. Whereas progression-free survival in the combination arm was similar to that of the historical controls (median, 3.2 versus 3.7 months), there was an increase in progression-free survival in patients who received docetaxel following disease progression on vaccine alone (6.1 versus 3.7 months). Patients who crossed over to docetaxel following vaccination continued to maintain PSA-specific T cell responses that corresponded to declining serum levels of PSA. A similar trend was observed in a report by Noguchi et al., in which low-dose estramustine (a nitrogen mustard) was combined with HLA-A24–restricted peptide vaccination for prostate cancer. Combination therapy was associated with augmented CTL responses, peptide-specific IgG responses, and decreased serum levels of PSA in 13 patients (90).
The feasibility and safety of combining 5-FU–based chemotherapy with carcinoembryonic antigen–derived (CEA-derived) peptides in adjuvants was evaluated in a study of 17 patients bearing colorectal cancer (91). Eight of 17 patients developed CEA-specific CTL responses, while 6 of 17 exhibited an objective response, a rate similar to that of historical controls treated with chemotherapy alone (91). A phase II trial enrolled 32 patients treated with anti-idiotype antibody mimicking the CEA antigen, with 14 of them additionally placed on a 5-FU–based regimen. All patients developed CEA-specific humoral and cellular immune responses (92). The 5-FU–based regimen, which is the standard of care for patients with localized colon cancer with nodal involvement, did not affect the immune response. These data warrant a phase III trial for patients with resected colon cancer.
Open questions in cancer immunology
Many clinical oncologists doubt the general impact of immune parameters on the practical management of neoplasia. How can clinical studies be designed in order to provide more ample evidence of the importance of cancer immunology?
It would be important to perform large-scale epidemiological studies that link the incidence and course of cancer — including therapeutic failure — to genotypic and phenotypic markers of immunodeficiency. As mentioned above, polymorphisms in the genes encoding TLR4 and cytokines may correlate with therapeutic outcome, but larger and more systematic studies (such as genome-wide SNP correlations) will be important in this area. It would be interesting as well to correlate the susceptibility to intracellular infectious agents (including viruses, listeria, tuberculosis, mycoplasma, etc.) with poor cancer chemotherapeutic responses. Moreover, an exhaustive meta-analysis should be performed on melanoma vaccination studies. Such a meta-analysis should address the putative positive impact of vaccination on later chemotherapeutic responses.
The prognostic impact of tumor-specific gene expression or proteomic profiles should be reinterpreted by studying markers of preexisting immune responses. The molecular profiling of follicular lymphoma at diagnosis indicated that the two gene-expression signatures that predicted survival comprised genes expressed by nonmalignant tumor-infiltrating cells, most likely myeloid and T cells. The nature of the infiltrating immune cells was the predominant feature of the cancer that predicted survival (93). It would be important to perform prospective trials in which serial tumor biopsies, performed before and after chemotherapy, are evaluated for signs of local immune responses as putative prognostic factors. If anticancer immune responses dictate long-term therapeutic success, then local signs of antigen-priming (i.e., the presence of DCs in a pseudolymphoid architecture and contexture) or T and NK cell responses (with the presence of effector memory T cells, preferentially of the Th1 type, that should outnumber Tregs) would correlate with favorable responses. This working hypothesis is supported by our recent data showing a correlation between pathological complete response to neoadjuvant chemotherapy and therapy-induced high CD8+ effector T lymphocyte and low Treg infiltrate levels in a series of 56 patients bearing locally advanced breast cancer (94).
The immunosuppressive side effects of massive chemotherapy should also be reevaluated. Although one cycle of lymphodepletion may constitute a therapeutic advantage due to homeostatic T cell repletion, repeated cycles of lymphodepletion may destroy the expanding population of tumor-specific immune effectors. For instance, in breast cancer, treatment with weak, repeated doses of anthracyclines (every week, ideally without glucocorticoids) should be compared with the usual therapeutic scheme in which intense doses are administered every three weeks (and usually accompanied by high doses of glucocorticoids to reduce the chemotherapy-associated discomfort) with simultaneous monitoring of tumor size (by PET/CT) and antitumor immune responses.
Along the same lines, it may be important to readdress the therapeutic management of different cancers, given the idea that chemotherapy should elicit an immune response. Hence, neoadjuvant chemotherapy (as opposed to adjuvant chemotherapy) of small cancers (such as early breast cancers discovered by routine radiographic screening) would offer the advantage that more tumor antigen would become available for the priming of T cells, while high-dose tolerance should not constitute a problem. Similarly, it could be important to preserve the sentinel lymph node rather than remove it systematically for disease staging purposes. Indeed, the sentinel lymph node constitutes the privileged site of antigen priming, in which the presence of activated DCs (expressing DC-LAMP — a protein only expressed in mature DCs) constitutes a positive prognostic marker, at least in melanoma (95).
Future studies might also include clinical trials to attempt to correct the defective chemotherapeutic response of breast cancer patients with the Asp299Gly TLR4 allele by administering chloroquine. Oral chloroquine is a widely used antimalaria agent that acts at an acceptable level of toxicity. In mice lacking TLR4, chloroquine restores the chemotherapeutic response to oxaliplatin, presumably because it ameliorates deficient antigen presentation by DCs. In TLR4-negative DCs, phagocytic cargo containing tumor antigen is rapidly destroyed by lysosomes, and lysosomal inhibition by chloroquine restores antigen presentation. Similarly, chloroquine reestablishes the defective presentation of dying tumor cell antigens by DCs from patients carrying the Asp299Gly TLR4 allele in vitro (66).
From a biotechnological point of view, drug-screening approaches could be used to investigate the capacity of compounds to promote the translocation of CRT to the cell surface of a responding cell line (selected on its response to anthracyclines for CRT exposure). This screening could select druggable compounds based on their capacity to induce immunogenic cell death (Figure (Figure1). 1).
In conclusion, we anticipate that clinical trials will corroborate the potential of assessing and eliciting anticancer immune responses for prognostic and therapeutic purposes, provided that issues regarding intellectual property, licensing, and liability do not represent significant obstacles for industrial partners to accept the sponsoring and/or launching of combination therapies. It is our collective hope that an expanding immunostimulatory pharmacopeia, together with ever-more-sophisticated anticancer vaccination techniques, eventually will allow us to trigger tumoricidal immune responses at will.
Acknowledgments
The authors are supported by grants from the Ligue Nationale contre le Cancer (to L. Zitvogel, L. Apetoh, and G. Kroemer), the Fondation pour la Recherche Médicale (to L. Apetoh and A. Tesniere), the European Union (grants “ALLOSTEM” and “DC-THERA” to L. Zitvogel; grants “Active p53,” “Apo-Sys,” “ChemoRes,” “Death Train,” “RIGHT,” and “Trans-Death” to G. Kroemer), Cancéropôle Ile-de-France, Institut National du Cancer, and Agence Nationale de la Recherche (to G. Kroemer).
Footnotes
Nonstandard abbreviations used: ACT, adoptive cell transfer; ATM, ataxia telangiectasia mutated; CEA, carcinoembryonic antigen; CHK1, checkpoint kinase–1; CRT, calreticulin; 5-FU, 5-fluorouracil; HMGB1, high mobility group box 1 protein; IM, imatinib mesylate; KLH, keyhole limpet hemocyanin; NKG2D, NK cell group 2D; PSA, prostate-specific antigen; TRP-2, tyrosinase-related protein 2.
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 118:1991–2001 (2008). 10.1172/JCI35180.
References
Articles from The Journal of Clinical Investigation are provided here courtesy of American Society for Clinical Investigation
Full text links
Read article at publisher's site: https://doi.org/10.1172/jci35180
Read article for free, from open access legal sources, via Unpaywall:
http://www.jci.org/articles/view/35180/files/pdf
Free to read at www.jci.org
http://www.jci.org/cgi/content/abstract/118/6/1991
Free to read at www.jci.org
http://www.jci.org/cgi/content/full/118/6/1991
Free to read at www.jci.org
http://www.jci.org/cgi/reprint/118/6/1991.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/101837663
Article citations
In vitro induction of anti‑lung cancer immune response by the A549 lung cancer stem cell lysate‑sensitized dendritic cell vaccine.
Oncol Lett, 28(5):550, 13 Sep 2024
Cited by: 0 articles | PMID: 39328277 | PMCID: PMC11425031
Advancements and Challenges in Peptide-Based Cancer Vaccination: A Multidisciplinary Perspective.
Vaccines (Basel), 12(8):950, 22 Aug 2024
Cited by: 0 articles | PMID: 39204073 | PMCID: PMC11359700
Review Free full text in Europe PMC
Iron Oxide Nanoparticles Inhibit Tumor Progression and Suppress Lung Metastases in Mouse Models of Breast Cancer.
ACS Nano, 18(15):10509-10526, 02 Apr 2024
Cited by: 1 article | PMID: 38564478 | PMCID: PMC11025112
Biomaterials in Drug Delivery: Advancements in Cancer and Diverse Therapies-Review.
Int J Mol Sci, 25(6):3126, 08 Mar 2024
Cited by: 0 articles | PMID: 38542103 | PMCID: PMC10970185
Review Free full text in Europe PMC
Predictive value of stromal tumor-infiltrating lymphocytes in patients with breast cancer treated with neoadjuvant chemotherapy: A meta-analysis.
Medicine (Baltimore), 103(6):e36810, 01 Feb 2024
Cited by: 0 articles | PMID: 38335394 | PMCID: PMC10860995
Go to all (391) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
SNPs (2)
- (1 citation) dbSNP - rs1927911
- (1 citation) dbSNP - rs4986790
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Combining immunotherapy and anticancer agents: the right path to achieve cancer cure?
Ann Oncol, 26(9):1813-1823, 28 Apr 2015
Cited by: 145 articles | PMID: 25922066
Review
Does chemotherapy augment anti-tumor immunotherapy by preferential impairment of regulatory T cells?
Med Hypotheses, 71(5):802-804, 08 Aug 2008
Cited by: 10 articles | PMID: 18691831
Immunogenic chemotherapy: Dose and schedule dependence and combination with immunotherapy.
Cancer Lett, 419:210-221, 01 Apr 2018
Cited by: 154 articles | PMID: 29414305 | PMCID: PMC5818299
Review Free full text in Europe PMC
Cytokines in immunogenic cell death: Applications for cancer immunotherapy.
Cytokine, 97:123-132, 22 Jun 2017
Cited by: 126 articles | PMID: 28648866 | PMCID: PMC5572581
Review Free full text in Europe PMC




