Abstract
Free full text

Development and differentiation of the intestinal epithelium
Abstract
The gastrointestinal tract develops from a simple tube to a complex organ with patterns of differentiation along four axes of asymmetry. The organ is composed of all three germ layers signaling to each other during development to form the adult structure. The gut epithelium is a constitutively developing tissue, constantly differentiating from a stem cell in a progenitor pool throughout the life of the organism. Signals from the adjacent mesoderm and between epithelial cells are required for the normal orderly development/differentiation, homeostasis, and apoptosis. Embryonically important patterning factors are used during adult stages for these processes. Such critical pathways as the hedgehog, bone morphogenetic protein, Notch, SOX, and WNT systems are used both in embryologic and adult times of gut development. We will focus and review the roles of these factors in gut epithelial cell development and differentiation.
Introduction
This review summarizes advances in the understanding of the molecular control of intestinal epithelial cell differentiation. Recently some elegant multi-disciplinarily studies have been published that progress our understanding of endoderm development, intestinal epithelium differentiation, and its homeostasis [1–7]. Different molecular pathways and transcription factors have been described and studies in these processes. In order to clarify this review, we have decided to focus on events that we have studied and the pathways that are best understood developmentally. Key molecular pathways that we will commented include the hedgehog (Hh), bone morphogenetic protein (BMP), and Notch signaling pathways, the HOX and SOX transcription factors, the Eph receptors/ephrin ligands (Eph-ephrin) signaling system, the Wnt/β-catenin and TCP signaling pathways. Many of these systems are best known as critical control factors in general body plan developmental processes as well as role in organ pattern formation including having key roles in gastrointestinal development. These developmentally critical pathways continue to be important in cell differentiation, homeostasis, and apoptosis in the adult intestinal epithelium. Adult gut epithelium may be viewed as a “developmental” system; analogous in many ways to embryonic developmental systems. Understanding these pathways and how they may interact should provide insight to diseases found associated with gastrointestinal morphogenic defects and epithelial differentiation perturbations.
Embryonic development of the intestinal endoderm
The vertebrate gastrointestinal tract (GI tract) is a remarkably complex, three dimensional, specialized and vital organ system derived from a simple tubal structure. The GI tract includes the lumenal digestive system of the esophagus, stomach, intestines, and colon (which we will designate as “gut”) and GI tract derivatives. GI derivates essentially bud off ventrally from the early gut endoderm and will form the thyroid, lungs, and liver. The pancreas develops from the fusion of distinct dorsal and ventral diverticula that originally arise from the gut endoderm posterior to the stomach. The gut is composed of the three germ layers - endoderm (which forms the epithelial lining of the lumen), mesoderm (which forms the smooth muscle layers), and ectoderm (which includes the most anterior and posterior luminal digestive structure and the enteric nervous system).
Morphologic GI tract development has been found to be very similar in all vertebrate species studied. At the end of gastrulation, the endoderm is phenotypically homogenous until morphogenetic movements occur in cranial and caudal areas. The vertebrate gut tube develops from two ventral invaginations, one at the anterior (anterior intestinal portal, AIP) and the other at the posterior (caudal intestinal portal, CIP) end of the embryo. These invaginations elongate in the endodemal layer and fuse in the midline of the embryo to form a straight tube. During this process lateral plate derived splanchnic mesoderm surrounds the endoderm. Later in gut development, neural crest derived cells migrate into and colonize the gut to form the enteric nervous system (ENS). The ENS arises from the neural crest cells that delaminate from the dorsal region of the neural tube and colonize the whole gut to establish its innervation [8,9].
Early in embryonic development the gut becomes patterned in - the AP axis, the dorsoventral (DV) axis, the left-right (LR) axis, and later the radial (RAD) axis. Regional specific morphologic development and differentiation along the anterior-posterior (AP) axis will give rise to the formation of three regions: the foregut, the midgut, and the hindgut. These structures respectively will give rise to the adult gut: pharynx, esophagus, and stomach (for the foregut), to the small intestines (for the midgut) and the colon (for the hindgut). The LR axis is relatively evidenced early on, by the characteristic turning and looping of the gut in which the stomach is generally positioned on the left side of the organism and the gut loops in a counterclockwise direction. The endoderm remains uniform in its morphology (undifferentiated appearing stratified cuboidal cells) throughout all axes of the gut until midgestation in most vertebrates when epithelial-mesenchymal (EM) interactions direct endodermal differentiation (fig. 1). Finally the endoderm differentiates from signals provided by the mesoderm directed by its AP and DV specific location. Finally, the endodermal pattern becomes phenotypically specific in AP, DV, and RAD axes. The mature (adult) gut has a morphologic and functional pattern clearly identifiable in all four axes.
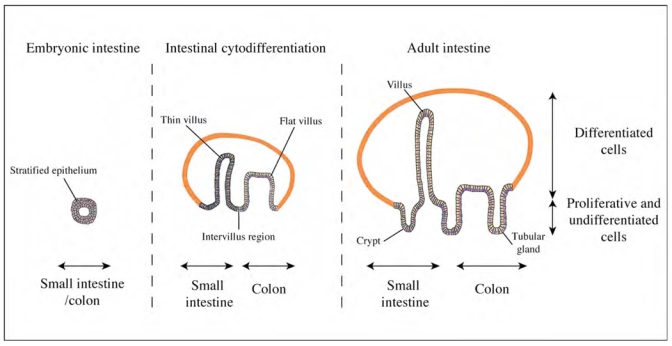
From development to differentiation of the intestinal epithelium. During early embryonic development, the visceral endoderm appears uniform and presents stratified cell layer. Intestinal epithelial cytodifferentiation occurs during foetal development and is marked by mesodermal growing into the lumen and villi formations. These villi are separated by proliferative intervillus epithelium. AP axis differences appear and are characterized by long and thin villi in the small intestine and by transitory wide and flat villi in the colon. The intervillus epithelium of the small intestine is reshaped downward forming crypts. In human, final architecture of the small intestine is reached before birth and is characterized by the crypt-villus unit. The colonic villi disappeared at the time of birth and the mature colonic epithelium present tubular glands (crypts). [Adapted from Expert reviews in molecular medecine (2001) (01)00362-3a.pdf: fig. 2].
Transcription factors involved during gut endoderm development
Genetic controls of endoderm development have been less well studied as the mesoderm or ectoderm [10]. Numerous factors involved in the specification of the endoderm layer have been described and reviewed (for review see [11]). Other factors, such as Sox17, first have been identified as early endodermal specification factors, but also its action during gut endoderm development was recently demonstrated [12, 13]. Sox17 is a high-mobility group (HMG) transcription factor gene related to the sex-determining factor gene SRY [14]. SOX genes have been identified as key players in numerous developmental processes: sex determination [15, 16], neurogenesis [17], muscle differentiation [18], and chondrogenesis [19]. SOX genes are also involved in pathologic development as well [20] shown to be involved in pathways controlling cellular proliferation [21] and oncogenesis [22]. Early endoderm formation is under control of Sox17 expression as demonstrated in Xenopus [12, 23]. Recently in zebrafish, casanova, a novel member of SOX family gene acts upstream of zebrafish Sox17 related gene and as Sox17 is sufficient to induce early endodermal formation [24–28]. Murine knockouts for Sox17 show that Sox17 is essential for embryonic cells to acquire endodermal cell fate but also suggests a potential redundancy with other SOX gene expressed on overlapping endodermal territory [13]. Disruption of Sox 17 gene in the murine system has an impact on endoderm development but does not affect the formation of the anterior definitive endoderm [13]. Published and unpublished observations have demonstrated the presence of at least 5 different SOX genes expressed in the gut endoderm: Sox2 [29], Sox7 [30, 31], Sox9 (P. de Santa Barbara unpublished data), Sox17 [13, 32], and Sox18 [33] suggesting that SOX family genes may be important in the gut endoderm development. Additive functions of SOX genes in intestinal epithelium are suggested by different expressional studies [31–33]. Moreover, in our experiments we shown that SOX9 expression changes during the differentiation state of the intestinal epithelium, with restriction of then along villi axis, indicating a potential function of SOX gene during the epithelial differentiation process (P. de Santa Barbara unpublished data).
Hox genes are homeobox containing transcription factors conserved across divergent species [34, 35]. Hox genes function in pattern formation of many aspects of development including the overall body plan [36, 37], limb [38], CNS [39, 40], and viscera [41–44]. Mesodermal expression of specific Hox genes play an important role in patterning the gut along the AP axis in both the gross morphology of the gut and later the epithelial-mesenchymal interactions responsible for normal gut epithelial differentiation [42, 45]. The genes of the AbdB class include the most 5′ of the vertebrate Hox genes. These vertebrate Hox genes are expressed spatially in the most posterior body regions and subregions [41]. In the gut, these Hox genes are expressed in a spatially and temporally specific manner in the posterior mesoderm of the gut, from the post-umbilical portion of the midgut through the hindgut [41–43, 45]. Hoxa13 and Hoxd13 are co-expressed in the distal most hindgut mesoderm (anorectal mesoderm in the mouse and cloacal mesoderm in the chick) and uniquely throughout the hindgut endoderm [42, 46]. In mouse Hoxa13(+/−)/Hoxd13(−/−) mutants have gastrointestinal malformations of the muscular and epithelial layers of the rectum [44]. The tissue specific roles of these genes were not dissected. Were the anomalies seen in the null mice due to absence of Hox function in the mesoderm, the endoderm, or both? Recently, the role of Hoxa13 in the posterior endoderm was investigated using the avian system. A Hoxa13 mutant protein, which behaves as a dominant-negative, was specifically expressed in the early developing chick posterior endoderm [45]. This resulted in decreased wild type protein and the chicks developed with a dramatic malformation in the gut and genitourinary system with atresia of the hindgut anterior to the cloaca, cystic mesonephric maldevelopment, atresia of the distal Müllerian ducts. This was the first time that a specific endodermal function of a Hox gene was described. Different Hox genes also were found expressed in the small and large intestine endoderm, such as Hoxa8, however no functional studies were made [47, 48]. Their expressions let us hypothesize specific functions of intestinal endodermally expressed Hox genes, but still to be described.
Hox gene expression is principally mesodermal in the gut, and expression occurs early in gut development, before any pattern formation in the 4 axes is evident. We have previously shown that Hoxd13 and Hoxa13 have a function in the mesoderm to direct differentiation of the overlying endoderm [42, 45]. Both Hoxd13 and Hoxa13 are expressed in the distal most hindgut mesoderm [41, 43,46]. Both are also expressed in the entire hindgut endoderm [45]. When Hoxd13 and/or Hoxa13 are ectopically expressed in the midgut mesoderm the endoderm differentiated towards a hindgut phenotype [42, 45]. These put Hoxd13 and Hoxa13 as players in the hindgut mesoderm to endoderm signaling that has been shown to direct the final epithelial phenotype. Recently, the mesodermal-endodermal HOX crosstalk pathway was also observed in mouse [49] and shows a strong conserved function of Hox genes in GI tract differentiation.
Signaling pathways acting during gut endoderm development
The Hedgehog (Hh) pathway in Drosophila and vertebrates is conserved and known to play an important role in gut development [50–52]. Sonic hedgehog (Shh) is an important factor implicated in the first phase of EM signaling in the gut [41,53]. Shh is expressed early in the AIP and CIP endoderm [41, 54–56]. As the gut tube forms and undergoes morphogenesis, Shh expression expands and is maintained in the gut endoderm with the exception of the GI tract derivates [56–58]. One other member of the Hh family, Indian hedgehog (Ihh), is expressed later in the gut endoderm in a partially overlapping pattern [1]. The function of Hh signaling in the early gut endoderm layer is not well defined, but its action in the adjacent mesoderm was demonstrated. Endodermally secreted Shh acts via its mesodemal expressed receptor Patched (Ptc) to induce mesodermal expression of Bmp4 [41, 42]. Early endodermal Shh expression was suggested to act as a signal in epithelial-mesenchymal interaction in the earliest stage of hindgut formation [41].
BMPs are members of the Transforming growth factor β (TGF β) superfamily of signaling molecules that play important roles during embryogenesis and organogenesis. BMP ligands were initially identified as regulators of bone formation [59], but subsequent analyses have suggested that these ligands regulate a spectrum of developmental processes throughout embryogenesis and organogenesis (reviewed in [60]). BMPs are tightly regulated growth and differentiation morphogens, therefore to truly understand what their function any one system may be, it is extremely useful to localize the tissue/cells in which their actions are occurring [60]. BMP ligands act via specific receptors in a complex which ultimately, by phosphorylation, activates a target molecule, SMAD1/5 and 8, that in turn moves to the nucleas to activate transcription of target genes [61]. Due to the high degree of complexity of the BMP signaling pathway (numerous ligands, receptors, and processing regulations), the detection of the phosphorylated forms of Smad 1/5/8 was used to give an endogenous cartography of the BMP activation in Xenopus [62] and chick [63], that could not be predicted from ligand and antagonist expression patterns. Smadl/5/8 phosphorylations are activated in the ventral part of the foregut endoderm [63]. These data suggest an unexpected and early role of BMP signaling in the development and patterning of the endodermal AIP structure formation.
Recent investigations have highlighted the roles of BMP in patterning the gut during development. Bmp-4 is expressed throughout the mesoderm of the chick gut sparing expression only in the avian muscular stomach (gizzard) [42, 64, 65]. Retroviral misexpression experiments suggest that level of BMP activity may have fundamental roles in the control of gut muscular development, in the pyloric sphincter development, and in the stomach gland formation [42, 64–67]. Anti-phospho-Smad 1/5/8 antibodies were used to study the endogenous BMP pathway activation in the developing GI tract in chick (P. de Santa Barbara, S. Faure and DJ. Roberts, unpublished data). Endogenous activation of this pathway is specifically found in the gut mesenchyme layer but also in the developing endoderm. The localization of activated-Smad1 in the mesoderm of the midgut is consistent with the expression of Bmp-4 at this stage [64], but the additional activation of Smad1 that we report in the endoderm suggests that either diffusion of Bmp-4 from the mesoderm induces Smad1/5/8 phosphorylations in the endoderm, or that additional BMPs are expressed in the endoderm. Regional differences in BMP pathway activation also are present in the AP axis. Smad1/5/8 phosphorylations are present in the midgut endoderm but not in the hindgut endoderm. The function of the BMP pathway in early gut endoderm is still unknown. Later in gut development, some roles for the pathway have been suggested (see below).
Pattern of the adult intestinal and colonic epithelium
The intestinal endoderm layer forms the intestinal characterized in the RAD axis with the establishment of the villus-crypt axis. The pseudostratified endoderm formed of undifferentiated cells undergoes a columnar transformation accompanied with a mesodermal outgrowth. This process results in the development of structures termed villi, which form along a cranial to caudal wave (fig. 1). AP axis influences the RAD axis in morphologic and epithelial cellular differentiation. In late fetal life, small intestine epithelium is characterized by long and thin villi, whereas colon epithelium shows wide and flat villi. These villi are separated by a proliferating intervillus epithelium (fig. 1). As the gut develops the intervillus epithelium is reshaped downward forming crypts. The crypt villous unit allows for a great increase in surface area for absorption. Small intestines conserve their villus-crypt unit throughout life (fig. 1). In many species, (including human but not in chick) the embryonic villi will be lost in adult colonic epithelium. Human colon has a relatively flat epithelium separated regularly by crypts (fig. 1). The formation of these crypt-villous structures and epithelial cellular differentiation relies on reciprocal signaling between the endoderm and mesoderm (EM) (for review see [68]).
The function of small intestine epithelium is digestion and absorption of nutrient. Therefore, the epithelial cells are highly specialized and metabolically active. These cells undergo a relatively rapid generation and death continuously throughout the life of the organism. The cells are derived from a stem cell located in the middle of the crypt. Asymmetric division is essential to insure maintenance of stem cell number and final homeostasis of the intestinal epithelium. The stem cells have a high proliferative rate with embryonic cell like features. They can be morphologically identified by a large nucleus compartment with diffuse chromatin and scant cytoplasm with few small organelles. The number of stem cells in the small intestine is estimated at around 4–6 per crypt. This cell produces progenitors, which appear undifferentiated in the crypt but eventually produce four cell types: enterocytes, enteroendocrine cells, Paneth cells, and goblet cells. Cellular position along the crypt-villous unit (in the RAD axis) is different following the differentiation state of its cells (bottom for undifferentiated cells, and top for more differentiated cells), with exception of Paneth cells that always are located at the bottom of the crypts. Morphological changes are achieved during migration from the stem cell to the crypt-villous junction. When these cells reach the crypt-villous junction, their differentiation is complete. Enterocytes are the most abundant intestinal epithelial cells (up to 80% of all epithelial cells).
Enterocytes are columnar cells with apical microvilli, that greatly increase the absorptive surface, and lateral junctions with neighbor cells. The enterocytes have hydrolytic and absorptive functions and are responsible for degradation of nutrients. The turnover of enterocytes is estimated in mouse at around 3 days. The enterocytes are characteristic of the small intestine and are the main absorptive cell of the intestine. Goblet cells are scattered from the middle of the crypt to the tip of the villus. They represent 5 % of the small intestine epithelial cells. They are characterized by specific mucous granules found in the cytoplasm. The mucus constitutes a barrier against the intestinal contents. Goblet cell turnover is quick, around 3 days. Enteroendocrine cells represent a small percentage of the small intestine epithelial cells. They produce numerous hormones that assist in regulating gastrointestinal motility. Paneth cells, in contrast of the three other intestinal epithelial cell types, have a longer turnover period of about 20 days. Mature Paneth cells are columnar epithelial cells with apical cytoplasmic granules. Around 10 Paneth cells are present per crypt. Paneth function is mostly associated with the antimicrobial defense of the intestine.
The pattern of the different cell types in the small intestine in the RAD axis is such that the mid-crypt position of the stem cell produces progenitor cells, rapidly dividing, committed but undifferentiated, that “move” lumenally to the villous. At the crypt-villous junction, these cells differentiate. Their luminal migration is both passive due to being “pushed” by newly “born” progenitors from the crypts, apoptotic loss of cells from the villous tip, and from newly described epithelial cell-cell and EM signals (described below). In the small intestine, the Paneth cell is the only cell type that apparently disregards this rule, as it is uniquely located at the base of the crypts, apparently migrating “downward”.
The principal function of the adult colon epithelium is to absorb water and salt. Transient formation of colonic villi is present in embryonic proximal intestine, but in human these villi are flattened by birth. The mature colon epithelium has mainly two differentiated cell types: the enterocyte and goblet cell. The colon also has endocrine cells. The goblet cells are mainly found in the midcrypt whereas the absorptive enterocytes (or colonocytes) are found at the surface (or top of the crypt), the surface between the crypt is called the “intercrypt table” and consists mainly of enterocytes. Endocrine cells are found in highest numbers at the base of the crypt [69]. The stem cell and proliferating compartment in the colonic epithelium resides at the base of the crypt. All cellular “movements” are towards the lumen.
Genetic control of intestinal and colonic epithelial pattern
The fundamental pattern in adult gut epithelium is the RAD axis, with the progenitor/proliferative cells being deep to the differentiated/functional/and finally apoptotic cells being luminal [70]. The formation of this pattern occurs embryologically but it is maintained in the adult organ. Disruption of this pattern results in dysfunctional bowel and can lead to malignant growth. Many new insights into the molecular controls of this pattern have been recently described and will be summarized here. These involve cellular interactions from the mesoderm to the epithelium and between epithelial cells. Many of the same factors shown to be important in embryologic pattern formation of the gut continue in their importance in pattern formation of the adult organ. We respectively will comments the different pathways and factors involved in maintenance of the crypts – the proliferative/progenitor region and follow through control of cell fate decisions, cellular differentiation and finally apoptosis.
Interventions of the WNT and BMP signaling pathways in adult intestinal and colonic epithelium
WNT genes encode secreted proteins, which control numerous developmental processes. WNT genes are included on the same family but can be distinguished in two different functional groups: the WNT/β-catenin signaling pathway and the WNT/Ca2+ signaling pathway [71]. In the first group, WNT expression leads to the nuclear translocation of β-catenin and its association with T-cell factor (TCF) family members (HMG box-containing DNA-binding proteins). These β-catenin/TCFs complexes mediate WNT signaling pathway by transcriptional activation of WNT target genes. This pathway is important in gut epithelial development. New data has implicated the Wnt/β-catenin/Tcf4 pathway as critically important in maintaining the proliferative compartment of the adult gut epithelium (fig. 2).
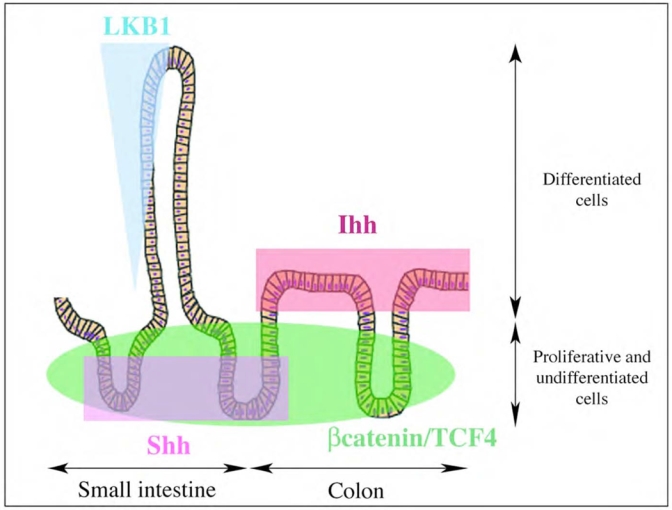
Pathways involved in cell differentiation, homeostasis and apoptosis in the adult intestinal epithelium, β-catenin and TCF4 are expressed in the proliferative compartment, where are located the intestinal epithelial stem cells. The β-catenin/TCF4 complex mediates WNT signaling pathway by transcriptional activation of target genes (C-MYC, BMP4, EphB2, EphB3; other genes are reviewed in www.stanford.edu/~rnusse/wntwindow.html). β-catenin/TCF4 pathway is important in maintaining the proliferative compartment of the adult intestinal epithelium. Two members of the hedgehog family are expressed in the adult intestinal epithelium. Shh is expressed in the base of the small intestinal crypt and could positively regulate precursor cell proliferation. Ihh is expressed in the differentiated colonic cells and could regulate the maturation of the enterocytes. LKB1, a Ser/Thr kinase, regulates specific p53-dependent apoptosis pathways in the intestinal epithelium. Its gradient expression pattern along the villus axis highly suggests a function of LKB1 in the natural apoptosis of the intestinal epithelial cells.
Expression of Wnt genes during mouse and chick gut development suggested possible roles in gut patterning [10, 42, 72]. These publications have shown Wnt expression in the developing gut mesoderm and suggest a role in controlling AP boundaries. Although no published data has shown specific WNT factor expression in the adult gut, significant evidence documents that its pathway is important at the crypt. WNT proteins signal via complexes formed with β-catenin. After expression of WNT morphogens, WNT binds to its membranous receptor Frizzled and activates its. These stimulation leads to the inhibition of GSK-3β activity and to cytoplasmic and nuclear (β-catenin accumulations. The consensual model states that nuclear (β-catenin interacts and binds to TCF factors to activate Wnt target genes (for review, see [73] and http://www.stanford.edu/~rnusse/wntwindow.html). Recently this model was discussed by new data that demonstrated activation of the WNT pathway with membrane targeted β-catenin forms [74]. In the digestive epithelium, β-catenin is present in all membranes along the crypt-villus unit, but nuclear accumulation of β-catenin is specifically found in the epithelial cells located from the bottom third of the small intestine crypt to the bottom and at the bottom of the colonic crypt [2, 3]. The effector of the WNT/β-catenin signaling pathway, Tcf4 is found expressed in the gut epithelium throughout life [75, 76]. Tcf4 is expressed in a gradient highest in the cells at the base of the crypt [77, 78]. Tcf4 knockout mice appear to lose the intestinal epithelial progenitor and stem cell population and die before crypt formation is evident [76]. These data suggest that TCF4 functions in intestinal epithelial stem-cell maintenance. The experiments identified new signaling pathways involved in the intestinal epithelium and demonstrate a central role of β-catenin/TCF4 in the maintenance of the crypt progenitor cells [2, 3,79, 80]. A direct target of the β-catenin/TCF4 is the Cdx1 gene, one of the mouse Drosophila caudal homologues [81]. Cdx1 is expressed in the developing intestine endoderm [82] and its product finally localizes in the proliferative crypt compartment during differentiation [83]. After WNT stimulation, it was demonstrated that Cdx1 expression is stimulated and let us hypothesize that its homeobox gene is one of the WNT signaling pathway effector involved in the maintenance of the proliferative intestinal compartment
The BMP signaling pathway is involved in the early steps of AIP formation and gut development (as reviewed upper). However, human genetic data has demonstrated that this pathway plays an important role in intestinal epithelial homeostasis. Recently, mutations in different members of the BMP signaling pathway were found associated with the human pre-cancerous Juvenile Polyposis Syndrome (JPS). A specific SMAD4 mutation is found in some IPS patients and results in a truncated protein [84, 85]. The Smad4 protein is the common shuttle of both TGF-β (Transforming Growth Factor-β) and BMP(s) signaling pathways. SMAD4 mutations associated with IPS leads to the hypothesis that other members of TGF-β or BMP signaling pathways may be involved in the IPS patients without the specific SMAD4 mutation. In fact, in some germline nonsense mutations were found in the bone morphogenetic protein receptor 1A (BMPR1A) gene [86]. These mutations resulted in protein truncation due to a deletion of the intracellular serine-threonine kinase domain necessary for Smad protein phosphorylation and signal transduction. These genetic studies show that perturbation of the BMP signaling pathway is associated with intestinal hamartomous polyps. This suggests that BMP signaling is involved in normal epithelial differentiation and homeostasis in the gut. Expressions of phosphorylated forms of Smad1/5/8 proteins are found in the intestinal epithelial cells and in the lamina propria stromal cells of the adult human small intestine and colon (D.J. Roberts and P. de Santa Barbara, unpublished data). Recently, different investigators found that intestinal epithelial BMP4 expression is under the control of the β-catenin/TCF4 activity [2, 80]. Precise actions of BMP pathway in the differentiated intestinal epithelium and the defects involving mutations in the BMP pathway compounds need to be further investigated.
Genetic control of cellular patterning in the intestinal and colonic epithelium
Paneth and enterocyte cell fate choiced must involve the Rho GTPase family members. These factors play a central role in all eukaryotic cells by controlling the organization of the actin cytoskeleton [87]. These proteins integrate information from different signaling pathways and act as effectors to mediate effects on migration, proliferation and differentiation [88]. Rac1 is a member of the Rho family of GTP-binding proteins that can activate the Jun N-terminal Kinase (INK) and p38 Mitogen-Activated-Protein (MAP) kinase pathways [89]. Expressions of either constitutively active and/or dominant-negative Rac1 forms in mice result in perturbation of cell differentiation in the intestinal epithelium [4]. Sustained Rac1 activation leads to an early differentiation of Paneth and enterocyte cells within the small intestine intervillus epithelium in late fetal mice, but no impact was observed on Goblet and enteroendocrine cells. In adult, forced Rac1 activation increases cell proliferation in intestinal crypts and leads to unusually wide villi [5]. Activated Rac1 specifically increases phosphorylation of INK in both intervillus and villus epithelial cells and alters the actin cytoskeleton [5]. The cytoskeleton is also involved in cell migration. The position of cells along the crypt-villous axis (RAD) is one of the important factors thought to play a role in cellular differentiation [90].
A fundamental pathway utilized by many systems to direct cellular differentiation is the Notch-Delta receptor-ligand signaling system [91]. Adult gut epithelial cells use this system to affect cell fate in the proliferative zone of the crypt-villous unit. The Notch pathway affects cell fate decisions by using lateral inhibition in cell-cell interactions with its cell membrane based receptor Delta [92]. Feedback amplification of relative differences in Notch and Delta results in subsets of cells with high levels of Notch and others with high Delta levels. Elevated cellular Notch levels induce expression of transcription factors, such as Hes1 [93]. Hes1 is a transcriptional represser, and Hes1 positive cells have been shown to remain in the precursor population [94]. Downstream targets of Hes1 have been recently described to include Math1, a basic helix-loop-helix transcription factor [95]. Math1 expression is present in both developing and mature mouse intestinal epithelium and co-localizes with proliferating markers in the progenitor region of the crypt in small intestinal epithelium. Math1 null mice have increased reporter expression in crypt cells, lack intestinal goblet, Paneth, and enteroendocrine cells, and show no increased apoptosis. These findings suggest that Math1 is involved in early epithelial cell fate decisions. Math1 expression is needed for cells to make their first lineage specifying choice. Math1 non-expressing cells remain in the progenitor pool and can only become enterocytes. These results are informative not only in that Math1 is an early cell fate determining factor but also that there are apparently two progenitor cell types -a Math1 dependent progenitor for goblet, Paneth and enteroendocrine cells – and a Math1 independent progenitor for enterocytes.
During embryonic development, Eph receptors and their ephrin ligands (Eph/ephrin) have been shown to be essential for migration of many cell types [96] and pattern boundaries [97]. Eph receptors constitute a large family of transmembrane tyrosine kinase receptors [98]. Binding and activation of Eph receptors to ephrin ligands require cell-cell interaction [99]. Eph-ephrin signaling converges to regulate the cytoskeleton [100]. Members of the Eph-ephrin signaling pathway were found expressed in the small intestine epithelium [3]. EphB2 and EphB3 receptors are expressed in the proliferative compartment, whereas their ligand ephrin-B1 is expressed in adjacent differentiated cells. This suggests that the Eph-ephrin system may regulate epithelial cell migration, therefore position in the RAD axis, which is critical in determining cell fate. The neonatal intestinal epithelium of EphB2/EphB3 double mutant mice presents perturbation in the proliferative/differentiated compartment boundary with presence of ectopic proliferative cells along the villus. In adults, EphB3 receptor is restricted in its gut epithelial expression to the crypt base columnar cells, where the Paneth cells reside. EphB3 null mice show abnormalities in the localization of the Paneth cells, with scattered Paneth cells throughout the entire crypt and the base of the villus. These results support the role of the Eph-ephrin system in maintaining the integrity of the epithelial cell pattern in the RAD axis.
Functions of the Hedgehog signaling pathways in adult intestinal and colonic epithelium
The hedgehog (Hh) family of morphogens includes three members in most vertebrates, Sonic Hedgehog (Shh), Indian Hedgehog (Ihh) and Desert Hedgehog. All hedgehogs can bind two common homologous receptors: Patched (Ptc)-1 and –2. In the unbound state these receptors negatively regulate the activity of a seven-pass transmembrane receptor Smoothened (Smo) by a so far unresolved mechanism [101]. Upon binding of Ptc by Hh, the suppression of Smo is relieved and pathway activation through the Gli family of transcription factors ensues. Both Shh and Ihh are important endodermal signals in gut tube differentiation and are involved in patterning events along all four axes of its development. Shh plays an essential role in gross morphological patterning of the foregut [102] and both Shh and Ihh are expressed in the developing stomach. A remarkable gastric phenotype has been described in the Shh null mouse that showed hyperplastic epithelium in the stomach with intestinal transformation [1]. A role for Shh is maintained in the regulation of gastric gland homeostasis in the adult stomach [103] and the observed loss of this expression in intestinal metaplasia of the adult stomach may suggest that Shh plays a role in maintenance of the gastric epithelial differentiation program [104]. Both Shh and Ihh are expressed in the developing small intestine where they seem to have both opposing and overlapping functions. The Shh null mouse shows overgrowth of villi that are abnormally innervated and clog the lumen of the duodenum whereas growth of villi in the Ihh mouse is strongly diminished and often lacks innervation [1]. Both the Shh and Ihh null mutant display a reduction in the thickness of the circular smooth muscle layer [1]. Shh expression is down regulated in the developing small intestine in two phases. Initially Shh expression is lost in the prospective pancreatic endoderm and this loss is critical for normal pancreas formation [105]; in a second phase Shh expression is downregulated along the length of the small intestine, experiments in Xenopus suggest that this phase may be critical to normal small intestinal epithelial differentiation [106]. In the adult small intestine Shh mRNA is detected at the base of the crypts around the presumed location of the small intestinal stem cell [104]. Although we have so far not been able to detect Shh protein in the crypts, experiments with the Hh inhibitor cyclopamine suggest that Shh positively regulates precursor cell proliferation [103].
Both Ihh and Shh are expressed in the colon during development and may have partially overlapping functions (fig. 2). The Ihh null mouse has a colonic phenotype that is reminiscent of Hirschprung’s disease with dilatation of parts of the colon and a thin wall with a reduced small muscle layer that lacks innervation at the sites of dilatation [1]. The Shh null mutant and several mutants of the Gli family of transcription factors show a spectrum of anorectal malformations [107]. In the adult colon Shh mRNA can be detected in a few cells at the base of the colonic crypts, as in the small intestine, we fail to detect Shh protein in the epithelium with immunohistochemical techniques, which may indicate that Shh is expressed at very low levels [104]. In the adult, Ihh is expressed by the differentiated colonic enterocytes and seems to be involved in their maturation (G.R. van den Brink, unpublished data).
Genetic control of apoptosis in adult intestinal epithelium
Homeostasis of the intestinal epithelium requires tight control and balance of the different processes of proliferation, differentiation, migration and apoptosis. This coordination requires intervention of numerous and well timed pathways. Perturbations of the balance between proliferation and apoptosis could be the base of cancer predisposition and development [108]. Recently, new data showed involvement of the LKB1 gene, a Serine/Threonine kinase mutated in Peutz-Jegher syndrome [109, 110], in the natural apoptosis of the gut epithelium [6]. The cytoplasmic expression of LBK1 shows a gradient pattern along the villus. LKB1 expression is higher in older epithelial cells (located near the top of the villus) compare to the newly differentiated epithelial cells. LKB1 has been shown to regulate the specific p53-dependent cell death pathway in the intestinal epithelium (fig. 2).
Conclusion and perspectives
Understanding of molecular pathways involved in the gut development requires a thorough grasp of the relevance that the four axes have in gut patterning. The spatial relationships of the tissues in the gut are critical in how the organ as a whole develops. Cell-cell interactions are essential in epithelial patterning that starts at somitic stages of development and continues throughout the life of the organism. EM interactions control endoderm differentiation from the earliest point of development and continue to affect epithelial cells in their proliferation and differentiation (the Wnt/β catenin/Tcf4 system and the Hh signaling pathway) and probably in apoptosis (LKB1 pathway). Epithelial cell to epithelial cell (EE) interactions are critical in cell fate decisions (the Notch-Delta-Hes1-Math1 system). Hox genes appear to affect how these systems are altered dependant upon the AP region of the gut that the epithelium resides, at least in embryonic stages. These transcription factors may work both via EM and EE interactions. Many of these systems are critically important from embryonic stages to adult stages retaining and refining many of their embryonic functions into adult tissues.
Some fundamental questions remain to be answered. For example: How do these well described but disparate systems inter-relate and what are the controls that continue to define the AP boundaries respected in epithelial differentiation throughout the organisms’ life?
Gut epithelium is a rich model to study many different developmental questions. The future promises rapid advancement in the coordination of research efforts to fully understand gut development. Molecular developmental biologists using in vivo and in vitro studies with animal model systems provide insight into global patterning events and often uncover novel candidate factors in gut development. Cell and molecular biologists dissect these factors often using tissue culture studies to place molecules into pathways and describe tissue specific roles for molecules. Geneticists and pathologists using human tissues and families can identify molecules responsible for, or candidate factors associated with, diseases and syndromes that feed back into the loop of bench study. These medical investigators can provide insights from well studied factors and identify new roles for them by studying their expression or association in human diseases and disorders. Collaborative efforts will only enrich the field.
Acknowledgments
The authors thank the members of Roberts, Berta and Peppelenbosch laboratories for fruitful discussions and helpful comments on the manuscript. The authors are grateful to H. Clevers for allowing access to data before publication. The authors thank S. Faure and M. Whitman for sharing data before publication. Unpublished data from authors were supported by grants the NICHD to DJ.R. P.d.S.B. is supported by the Association pour la Recherche sur le Cancer (ARC).
Contributor Information
Pascal De Santa Barbara, IGH, Institut de génétique humaine CNRS : UPR1142, 141 rue de la Cardonille, 34396 Montpellier, FR.
Gijs R. Van Den Brink, Laboratory for Experimental Internal Medecine Academic Medical Center, Meibergdreef 9,1105 AZ, AmsterdamNL.
Drucilla J. Roberts, Department of Pathology Massachusetts General Hospital, Harvard Medical School, Department of Pathology, Division of Women’s and Perinatal Pathology, Brigham and Women’s Hospital, Boston, Massachusetts 02114 (USA)US.
References
Full text links
Read article at publisher's site: https://doi.org/10.1007/s00018-003-2289-3
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2435618
HAL Open Archive
http://www.hal.inserm.fr/inserm-00287641
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1007/s00018-003-2289-3
Article citations
Understanding the cellular dynamics, engineering perspectives and translation prospects in bioprinting epithelial tissues.
Bioact Mater, 43:195-224, 24 Sep 2024
Cited by: 0 articles | PMID: 39386221 | PMCID: PMC11462153
Review Free full text in Europe PMC
Standardization and quality assessment for human intestinal organoids.
Front Cell Dev Biol, 12:1383893, 12 Sep 2024
Cited by: 0 articles | PMID: 39329062 | PMCID: PMC11424408
Inflammation-Associated Stem Cells in Gastrointestinal Cancers: Their Utility as Prognostic Biomarkers and Therapeutic Targets.
Cancers (Basel), 16(18):3134, 12 Sep 2024
Cited by: 0 articles | PMID: 39335106 | PMCID: PMC11429849
Review Free full text in Europe PMC
Influence of postruminal casein infusion and exogenous glucagon-like peptide 2 administration on the jejunal mucosal transcriptome in cattle.
PLoS One, 19(8):e0308983, 15 Aug 2024
Cited by: 0 articles | PMID: 39146343 | PMCID: PMC11326568
The developmental mechanics of divergent buckling patterns in the chick gut.
Proc Natl Acad Sci U S A, 121(28):e2310992121, 05 Jul 2024
Cited by: 2 articles | PMID: 38968105
Go to all (187) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Mesenchymal-epithelial interactions during digestive tract development and epithelial stem cell regeneration.
Cell Mol Life Sci, 72(20):3883-3896, 01 Jul 2015
Cited by: 46 articles | PMID: 26126787 | PMCID: PMC5395663
Review Free full text in Europe PMC
Patterning the gastrointestinal epithelium to confer regional-specific functions.
Dev Biol, 435(2):97-108, 12 Jan 2018
Cited by: 38 articles | PMID: 29339095 | PMCID: PMC6615902
Review Free full text in Europe PMC
The gastrointestinal tract stem cell niche.
Stem Cell Rev, 2(3):203-212, 01 Jan 2006
Cited by: 172 articles | PMID: 17625256
Review
Large intestine embryogenesis: Molecular pathways and related disorders (Review).
Int J Mol Med, 46(1):27-57, 21 Apr 2020
Cited by: 13 articles | PMID: 32319546 | PMCID: PMC7255481
Review Free full text in Europe PMC





