Abstract
Free full text

Cyp7b, a novel brain cytochrome P450, catalyzes the synthesis of neurosteroids 7α-hydroxy dehydroepiandrosterone and 7α-hydroxy pregnenolone
7α-hydroxy pregnenolone
Abstract
Steroids produced locally in brain (neurosteroids), including dehydroepiandrosterone (DHEA), influence cognition and behavior. We previously described a novel cytochrome P450, Cyp7b, strongly expressed in rat and mouse brain, particularly in hippocampus. Cyp7b is most similar to steroidogenic P450s and potentially could play a role in neurosteroid metabolism. To examine the catalytic activity of the enzyme mouse Cyp7b cDNA was introduced into a vaccinia virus vector. Extracts from cells infected with the recombinant showed NADPH-dependent conversion of DHEA (Km, 13.6 μM) and pregnenolone (Km, 4.0 μM) to slower migrating forms on thin layer chromatography. The expressed enzyme was less active against 25-hydroxycholesterol, 17β-estradiol and 5α-androstane-3β,17β-diol, with low to undetectable activity against progesterone, corticosterone, and testosterone. On gas chromatography and mass spectrometry of the Cyp7b metabolite of DHEA the retention time and fragmentation patterns were identical to those obtained with authentic 7α-hydroxy DHEA. The reaction product also comigrated on thin layer chromatography with 7α-hydroxy DHEA but not with 7β-hydroxy DHEA; when [7α-3H]pregnenolone was incubated with Cyp7b extracts the extent of release of radioactivity into the medium suggested that hydroxylation was preferentially at the 7α position. Brain extracts also efficiently liberated tritium from [7α-3H]pregnenolone and converted DHEA to a product with a chromatographic mobility indistinguishable from 7α-hydroxy DHEA. We conclude that Cyp7b is a 7α-hydroxylase participating in the synthesis, in brain, of neurosteroids 7α-hydroxy DHEA, and 7α-hydroxy pregnenolone.
Steroid hormones regulate a variety of biological functions, including reproductive and adaptive responses. Though principally synthesized by the adrenal gland, gonads, liver, and placenta, the brain is a further site of steroid synthesis and metabolism. Dehydroepiandrosterone (DHEA) and pregnenolone (1), as well as progesterone (2), are reported to accumulate in the brain despite adrenalectomy and gonadectomy.
Such brain-derived steroids have been termed neurosteroids (reviewed in ref. 3) and a growing body of evidence indicates that these can influence neuronal activity and behavior. Both DHEA and pregnenolone improve posttraining memory when injected into limbic structures of the mouse brain (4–6); other effects on cognitive processes have been noted (2, 7–9). Pregnenolone and related molecules interact principally with γ-aminobutyric acid receptors (10–17), though modulation of N-methyl-d-aspartate (18–24), σ-opioid (24, 25), and progesterone (26) receptors has also been suggested. However, it is so far unknown whether the documented in vivo effects are mediated directly by these steroids or by the products of their metabolism in brain and other tissues.
These advances have focused intense current interest on the pathways of steroid synthesis and metabolism in brain. Primary rat glial cells can synthesize pregnenolone and progesterone from cholesterol (27), while neurosteroidogenesis has been reported in isolated rat retina (28) and brain (29). In addition to the production of pregnenolone and DHEA from cholesterol, a variety of novel steroids are made in brain extracts or cultured brain cells, including 20α-dehydropregnenolone, 7α-hydroxy derivatives of pregnenolone and DHEA, progesterone, and both 3α- and 3β-hydroxy-5α-pregnan-20-one (reviewed in ref. 3). Androgens are also modified, particularly through the action of aromatase and a 5α-reductase (reviewed in ref. 30). However, the specific enzymes responsible for these and other transformations in the central nervous system have not been well characterized.
Oxidative interconversions and metabolism of cholesterol and its steroid derivatives are principally performed by cytochrome P450 (Cyp) enzymes, a family of heme-containing mono-oxygenases located on intracellular membranes. Several Cyps are present in the central nervous system (31–42). Activities or mRNAs corresponding to key steroidogenic enzymes Cyp11a1 (side-chain cleavage, scc), Cyp17 (17-hydroxylase/17,20 lyase), and Cyp11b1 (11β-hydroxylase) can be detected in rat brain (43–45), in addition to Cyp19 (aromatase) (46). Furthermore, mRNAs encoding the non-Cyp hydroxysteroid dehydrogenases (HSD) 3α-HSD, 3β-HSD, and 11β-HSD have been reported in the central nervous system (45, 47–49).
We recently reported the molecular cloning of cDNAs from rat and mouse brain corresponding to a novel Cyp designated Cyp7b (50). This enzyme shares 39% sequence identity to hepatic cholesterol 7α-hydroxylase (Cyp7a) and lesser but significant homology with other steroidogenic Cyps. The postulated steroidogenic domain (51, 52), found in many of these enzymes, is present in both Cyp7a and Cyp7b. Cyp7b mRNA is predominantly expressed in rodent brain, particularly in the hippocampus (50), unlike Cyp7a, which is liver-specific (52–54). In the current study we report the substrate specificity of this new brain enzyme and show that the enzyme selectively modifies neurosteroids pregnenolone and DHEA.
EXPERIMENTAL PROCEDURES
Expression of Cyp7b in Mammalian Cells.
To express Cyp7b from vaccinia virus (VV) the sequence flanking the postulated mouse cDNA translation initiation codon (TCGGGATGC; ref. 50) was altered to the consensus (CCACCATGR; refs. 55 and 56) for translation initiation in mammalian cells. Oligonucleotide-directed mutagenesis was by PCR amplification; oligonucleotides employed were 5′d-GGCCCTCGAGCCACCATGCAGGGGGCCACG-3′ and 5′-dGGCCGAATTCTCAGCTTCTCCAAGAT-3′. Five cycles of amplification were performed, the products separated by agarose gel electrophoresis, digested with XhoI and EcoRI, and inserted between the SalI and EcoRI sites of plasmid pTG186-poly (57, 58), downstream of an early VV promoter, generating the construct pVV-Cyp7b. Transfer to the VV genome by homologous recombination in vivo was according to standard procedures (57, 58).
Preparation of Cell and Tissue Extracts.
HeLa cells were grown to semi-confluence (106 cells/5 cm dish; 5 ml medium) and infected (0.1 plaque-forming units/cell) with recombinant (VV-Cyp7b) or control viruses (VV-Copenhagen strain or a thymidine kinase negative Copenhagen derivative). After incubation (16 h, 37°C) cells were washed, resuspended in 1/10 original volume (500 μl) of W (Waxman’s) buffer (0.1 M KPO4/1 mM EDTA/20% glycerol, pH 7.5; ref. 59) and pelleted by centrifugation. For whole cell extracts, cells were resuspended into 1/100th volume W buffer and stored frozen at −70°C. For microsome preparations, cell suspensions were sonicated (six times for 5 sec at 0°C), unbroken cells removed by centrifugation, and the microsomal fraction pelleted (100,000 × g, 45 min, 4°C) as described (59). Microsomes were resuspended (Potter homogenizer) in 1/50th volume W buffer and stored at −70°C. Liver and brain extracts were prepared from rat (Fischer, male). Frozen tissue was homogenized (Dounce) in W buffer (2.5 ml/g). The homogenate was centrifuged (4,000 × g, 5 min, 4°C) and the aliquotted supernatant stored at −70°C.
Assay for Steroid Conversions.
Unlabeled steroids were from Sigma; reference hydroxylated steroids were received from H. Lardy (University of Wisconsin) (7α-hydroxy DHEA) and were also available locally (I.B.; 7α-hydroxy DHEA; 7β-hydroxy DHEA). [14C]- or [3H]-labeled steroids were from DuPont/NEN (45–60 mCi/mmol and 20–95 Ci/mmol, respectively; 1 Ci = 37 GBq). Cell or tissue extracts (typically 50 μl) were added to labeled steroid (lyophilized, typically 1 nmol) and diluted to a volume of 175 μl with W buffer. Then 25 μl 8 mM NADPH was added, the reactions incubated at 37°C (typically for 20 min), and steroids extracted (2 × 500 μl ethyl acetate). For thin layer chromatography (TLC) the organic phase was dried down and resuspended in 10–20 μl ethyl acetate. Tritium release assays employed [7α-3H]pregnenolone in a standard reaction except that 25 pmol of substrate was employed. For liquid scintillation counting 3 ml Ultima Gold scintillation fluid (Packard) was added.
Determinations of Apparent Km and Vmax.
Reactions were performed using substrate concentrations in the range 5–20 μM with aliquots being removed at 0, 2, 5 and 10 min. After separation by TLC and quantification by liquid scintillation counting, initial reaction velocities were expressed in pmol/min per mg protein. Values for Vmax and Km for each substrate were calculated by linear regression analysis (Lineweaver–Burk plot).
TLC.
Reaction products were applied to silica gel TLC sheets (Merck) and developed in ethyl acetate/n-hexane/acetic acid (16:8:1) as described (buffer system N in ref. 60). [14C]Steroids were visualized by autoradiography; for [3H]steroids chromatograms were treated first with EN3HANCE spray (DuPont). On preparative chromatograms steroids were localized under UV light (UVM57 Chromato-vue monitor, UltraViolet Products, San Gabriel, CA). Quantification of chromatograms was by liquid scintillation counting of excised products or by phosphorimaging. For comparative TLC with reference α- and β-hydroxy steroids buffer system A (dichloromethane/acetone, 4:1; ref. 60) was also employed.
Mass Spectrometry.
A ×10 scaled up reaction was employed using 95% unlabeled DHEA (Sigma) and 5% [14C]DHEA (final specific activity, 2.25–3 mCi/mmol); the reaction time was extended to 1 h. Product was purified by TLC, excised, and extracted with ethyl acetate before drying down. The dried residue and authentic 7-hydroxy DHEA (7HD; 50 μg) were converted to their methoxime-trimethylsilyl derivatives as described (61). Analysis of these products was performed using a Trio 1000 mass spectrometer operating in electron impact mode, linked to a Hewlett–Packard model HP5890 gas chromatograph fitted with an HP-1 crosslinked methyl siloxane column (25 m, i.d. 0.25 mm, 0.17 μm film) under the following conditions: electron energy 70 eV, source temperature 200°C, interface temperature 280°C, oven temperature 50°C increasing at 30°C per minute to 200°C, and then at 10°C per minute to 300°C, injection temperature 280°C.
RESULTS
Expression of Cyp7b from Recombinant Vaccinia.
To produce active Cyp7b enzyme the complete mouse cDNA (50) was first modified to flank the open reading frame with appropriate restriction sites and a translation initiation consensus. This was introduced into a transfer plasmid under the control of the VV 7.5K early gene promoter, within the body of a cloned VV thymidine kinase (TK) gene. Recombinational exchange in vivo was used to transfer the expression cassette to the VV genome; TK-negative recombinants (VV-Cyp7b) were selected. Cultured HeLa cells were infected with recombinant viruses and harvested for biochemical assay.
Recombinant Cyp7b Modifies 3β-Hydroxy Steroids.
Previously we argued (50) that Cyp7b may catalyze transformation of cholesterol or its steroid derivatives in view of significant homology to hepatic cholesterol 7α-hydroxylase. Accordingly, cell extracts were incubated with a selection of radiolabeled steroids; products were subjected to TLC and visualized by autoradiography. DHEA was efficiently converted (≈95%) to a slower migrating product, consistent with hydroxylation of the steroid molecule (Fig. (Fig.11a). Significant conversion was also observed with pregnenolone; some activity was recorded with 25-hydroxycholesterol, 17β-estradiol, and 5α-androstane-3β,17β-diol. Other substrates tested including progesterone, testosterone, and corticosterone were not efficiently metabolized, though minor conversion products were observed (<3% substrate conversion), indicating that Cyp7b shows a strong preference for 3β-hydroxy steroids.

TLC (ascending) of steroid transformations in HeLa cells infected with a vaccinia-Cyp7b recombinant (VV-Cyp7b). (a) Products generated by incubating control vaccinia-infected cell extracts (v); or extracts of cells infected by VV expressing Cyp7b (7b), with steroids 25-hydroxycholesterol (25OHC), pregnenolone (Preg), DHEA, progesterone (Prog), corticosterone (B), cortisol (F), testosterone (Test), estradiol (E2), and 5α,androstane-3β,17β-diol (And); lateral arrowheads indicate the origin. (b) Products obtained with no extract control (o), control vaccinia-infected cells (v), VV-Cyp7b infected cells (7b), rat brain extract (Br), and rat liver extract (Li). (c) Products generated by 0, v, and 7b cell preparations (abbreviations as above) using intact live (vaccinia-infected) cells (ic), purified microsomes (m), or by purified microsomes from VV-Cyp7b infected cells incubated in the absence of NADPH (-N) or with 1 or 10 μM clotrimazole (1C, 10C).
Cyp7b mRNA is expressed abundantly in mouse and rat brain but only at low levels in liver (50). To confirm that Cyp7b activity is also present in brain, primary rat brain and liver extracts were incubated with [14C]DHEA. The rat brain extract generated a major product with an identical mobility on TLC to that produced by VV-Cyp7b extracts, indicating that this activity is present in rat brain (Fig. (Fig.11b). The primary product obtained when radiolabeled DHEA was incubated with mouse brain extracts also displayed a mobility identical to the VV-Cyp7b product (not shown). While the rat liver extract produced a range of products, including some which comigrate with the major VV-Cyp7b and brain product, there was no evidence of selective conversion to the Cyp7b product.
The same conversion was observed with fresh intact cells infected with VV-Cyp7b (tested for DHEA alone), frozen cells (data not shown), and purified microsomal preparations (Fig. (Fig.11c). To confirm that the reaction is catalyzed by a recombinant Cyp, reactions using DHEA as a substrate were performed in the presence or absence of the Cyp reductase cofactor NADPH or with the addition of clotrimazole, a selective inhibitor of Cyp-mediated reactions. No products were observed in the absence of NADPH, while clotrimazole caused a marked reduction in Cyp7b activity at 1 μM and abolished activity at 10 μM (Fig. (Fig.11c).
Mass Spectrometric Analysis of Cyp7b Product.
We surmised that, in view of homology with cholesterol 7α-hydroxylase (50), Cyp7b might catalyze the hydroxylation of DHEA and pregnenolone at the 7 position. Accordingly, mass spectrometry was performed on the Cyp7b conversion product of DHEA in parallel with reference 7HD. A scaled-up reaction was performed employing [14C]DHEA diluted 20-fold with unlabeled DHEA; the reaction product was purified by TLC. This was then derivatized, in parallel with reference 7α-hydroxy DHEA (7HD), and analyzed by gas chromatography and mass spectrometry. The gas chromatograms of the derivatives of the Cyp7b product and reference 7HD each contained one major peak. The retention times were identical for both Cyp7B product and 7HD (13.60 min; data not shown).
The mass spectrum of the major peak obtained for the derivatized Cyp7b product (Fig. (Fig.22a) showed the same fragmentation pattern as that of the derivative of 7HD (Fig. (Fig.22b); the molecular ion (m/z 477) was clearly present in both spectra but at low abundance while the common base peak (m/z 387) resulted from loss of a trimethylsilanol group. The identity of the two patterns confirms that Cyp7b hydroxylates DHEA at the 7 position.

Mass spectrometry of the Cyp7b metabolite of DHEA and reference 7HD. Methoxime-trisilyl derivatives of Cyp7b product (a) and reference 7HD (b) were purified by gas chromatography, and mass spectra were determined.
Gas chromatography of chemically derivatized Cyp7b product and reference 7HD also revealed two minor peaks in addition to the major derivatization product, indicative of partial derivatization of the original purified Cyp7b product and reference 7HD (not shown). Retention times for derivatization side-products were identical for 7HD and Cyp7b product. These were also analyzed by mass spectrometry. Spectra of the side-products were pairwise identical between Cyp7b product and reference 7HD (not shown), further confirming co-identity of the parent molecules.
Cyp7b Hydroxylates DHEA and Pregnenolone at the 7α Position.
While these results demonstrate 7-hydroxylation of DHEA by Cyp7b, and argue that 7α- (rather than 7β-) hydroxylation is most likely, the α/β configuration was not rigorously assigned. We used two methods to confirm the stereochemistry of the reaction.
First, one radiolabeled substrate, [3H]pregnenolone, was predominantly labeled at the 7α position; release of radioactivity from this molecule would indicate α rather than β substitution of H by OH during hydroxylation at the 7 position. Accordingly, this molecule was incubated with extracts of cells expressing Cyp7b. The reaction was extracted with solvent: radioactivity liberated from the solvent-soluble steroid into the aqueous phase was measured.
Cyp7b extracts were found to selectively liberate radioactivity from the steroid molecule (Fig. (Fig.3).3). The extent of release was determined; extracts liberated 35% of the label in a reaction where label present in residual substrate and product were 16% and 49%, respectively, of total starting material (data not shown), which is equivalent to 42% release of radioactivity in a reaction proceeding to completion. The majority (48–68%) of the label is at the 7α position, 25–27% at the 7β position, with minor contributions from the four (15–23%) and other (0–2%) positions (information supplied by DuPont/NEN Technical Division). The 42% release is inconsistent with exclusive 7β-hydroxylation (which could not release more than 27% of the radioactivity), arguing that the 7α position is modified by Cyp7b. Furthermore, hydroxylation of tritium-substituted sites is slower than of unsubstituted sites (62) and the release of radioactivity underestimates the actual extent of modification at the site. We infer that Cyp7b predominantly mediates 7α-hydroxylation of pregnenolone.
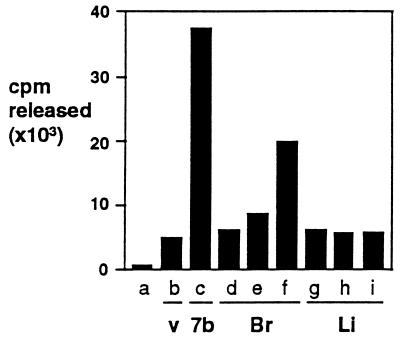
Release of radioactivity from [7α-3H]pregnenolone by Cyp7b and brain extracts. The set of results presented is representative of three independent sets of experiments performed under slightly different conditions but which yielded comparable values. Samples were buffer only (column a); control vaccinia (v) extract (column b); VV-Cyp7b extract (7b) (column c); 10, 20, and 50 μl rat brain (Br) extract (columns d–f); and 10, 20, and 50 μl rat liver (Li) extract (columns g–i). For reaction conditions see Experimental Procedures.
Adult brain extracts also liberated radioactivity from this substrate, while specific release was not detected in control extracts of HeLa cells and liver extracts (Fig. (Fig.3),3), consistent with the known distribution of Cyp7b messenger RNA (50). The apparent low-level release with control and liver extracts was due to nonspecific solubilization of steroid by extract components, while the absence of specific release by liver extracts confirms the finding (63) that pregnenolone is not a substrate for the hepatic cholesterol 7α-hydroxylase, Cyp7a.
Second, α- and β-hydroxy steroids differ in their migration on TLC (60). Accordingly, the radiolabeled Cyp7b DHEA metabolite was mixed with excess unlabeled reference 7α-hydroxy DHEA or 7β-hydroxy DHEA and separated by TLC in two different solvent systems (systems N and A in ref. 60). The unlabeled reference 7α-hydroxy DHEA (localized under UV light) comigrated precisely with Cyp7b product (localized by autoradiography) in both systems, while the 7β species migrated differently. Mobilities relative to DHEA were as follows: 7α-hydroxy DHEA, 0.32 and 0.20; Cyp7b product, 0.32 and 0.20; 7β-hydroxy DHEA, 0.50 and 0.49, respectively, in the two solvent systems (data not shown).
Taken together these results demonstrate that Cyp7b predominantly catalyzes the hydroxylation of 3β-hydroxysteroids DHEA and pregnenolone at the 7α position. However, one minor product of the Cyp7b reaction (≈1% of the yield of 7α-hydroxy DHEA) comigrated with 7β-hydroxy DHEA on TLC (data not shown), consistent with some weak 7β-hydroxylation activity.
Km and Vmax for Cyp7b.
While Cyp7b hydroxylates 3β-hydroxysteroids at the 7α position, the extent and relevance of the conversion will be dependent on kinetic parameters of the enzyme in addition to substrate availability. Km and apparent Vmax values were determined for DHEA, pregnenolone, and 17β-estradiol by varying the substrate concentration (either by altering the radiolabeled substrate added to the reaction or by the addition of further unlabeled substrate) and monitoring the extent of conversion by TLC. Graphically determined initial reaction velocities (vo) were plotted against substrate concentration [S] (double-reciprocal Lineweaver–Burk plot; 1/vo vs. 1/[S]) to determine apparent Km and Vmax values (data not shown; data on request).
The substrate specificity of Cyp7b is summarized in Table Table1.1. Km values for DHEA, pregnenolone, and estradiol were all in the range 3–15 μM, demonstrating high affinity for these substrates. The apparent Vmax value for DHEA was 8-fold higher than for pregnenolone, while the Vmax for 17β-estradiol was more than 10-fold less than for pregnenolone.
Table 1
Substrate specificity of Cyp7b
Cyp7b
| Substrate | % conversion*
| Km†; Vmax‡ | |
|---|---|---|---|
| Major product | Minor product(s) | ||
| DHEA | 95 | 2; 1§ | 13.6; 303‖ |
| Pregnenolone | 70 | 3; <1 | 4.0; 35.9‖ |
| 25-Hydroxycholesterol | 30 | <0.1¶ | ND |
| 5α,Androstane-3β,17β-diol | 11 | 9.1 | ND |
| 17β-Estradiol | 5 | 1 | 7.5; 2.4** |
| Testosterone | 1.2 | 0.4; 0.3; 0.3; 0.2 | ND |
| Progesterone | 0.6 | 0.2 | ND |
| Corticosterone | <0.5 | ||
| Cortisol | <0.5 | ||
| Androstenedione | <0.5 | ||
| Dihydrotestosterone | <0.5 | ||
ND, not determined.
DISCUSSION
We previously reported a new Cyp, Cyp7b, primarily expressed in brain (50), with significant homology to hepatic cholesterol 7α-hydroxylase (Cyp7a) and other steroidogenic Cyps. We report here that Cyp7b preferentially catalyzes the hydroxylation of the neurosteroid DHEA, with significant activity against pregnenolone. Other steroids tested were less effectively converted, though some metabolism of 25-hydroxycholesterol, 17β-estradiol, and 5α-androstane-3β,17β-diol was observed, arguing that Cyp7b selectively metabolizes 3β-hydroxylated steroids (Table (Table1,1, Fig. Fig.4).4). On the basis of chromatographic, tritium release and mass spectrometric data we conclude that hydroxylation of 3β-hydroxy steroids is at the 7α position. Some minor reaction products were also observed (<2% of major product yield). One such product obtained with DHEA comigrated with 7β-hydroxy DHEA while in other experiments (to be reported elsewhere) we have shown that Cyp7b can also catalyze further 2-hydroxylation of the 7α-hydroxy product obtained with 25-hydroxycholesterol; however, the in vitro reaction rate was slower by two orders of magnitude.
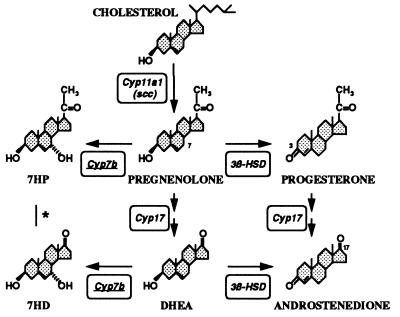
Alternative metabolism of the cholesterol-derived steroids pregnenolone and DHEA to either Cyp7b products or 3β-hydroxysteroid dehydrogenase (3β-HSD) products progesterone and androstenedione (that are not effective substrates for Cyp7b; Table Table1).1).  , It is so far unknown whether Cyp17 (steroid 17-hydroxylase/17,20 lyase) catalyzes the conversion of 7HP to 7HD. Cyp11a1, side-chain cleavage enzyme (scc); 7HD, 7α-hydroxy DHEA; 7HP, 7α-hydroxy pregnenolone.
, It is so far unknown whether Cyp17 (steroid 17-hydroxylase/17,20 lyase) catalyzes the conversion of 7HP to 7HD. Cyp11a1, side-chain cleavage enzyme (scc); 7HD, 7α-hydroxy DHEA; 7HP, 7α-hydroxy pregnenolone.
Several activities capable of 7-hydroxylating cholesterol and steroids have been reported previously. Cholesterol 7α-hydroxylase (Cyp7a), a liver enzyme that catalyzes the first step in the metabolic degradation of cholesterol and that shares 39% sequence identity with Cyp7b, has not been detected in brain. Furthermore, pregnenolone is a potent inhibitor rather than a substrate for the Cyp7a enzyme (63). Recently, an oxysterol 7α-hydroxylase involved in the biosynthesis of bile acids has been reported (64–70). This enzyme has not yet been isolated either as a purified protein or as a cDNA clone. Cyp7b is moderately active against 25-hydroxycholesterol and may account for some of the oxysterol 7α-hydroxylase activity observed in vivo, though it remains to be determined whether there are several enzymes that catalyze this activity. It is also of note that adult rat testes contain an activity capable of 7α-hydroxylation of testosterone and androstenedione (71); a cDNA clone encoding a testosterone 7α-hydroxylase has been reported (72) but this shares only 11% amino acid sequence identity with Cyp7a and rather less homology with Cyp7b.
Several groups have described 7α-hydroxylation of DHEA and/or pregnenolone in brain (34, 37, 73), prostate (34), spleen and thymus (74) and adipose cells (75, 76). The activities described by Warner et al. (34) and Akwa et al. (37) resemble Cyp7b in some respects. However, there are notable differences. Estradiol was reported to be a potent inhibitor of the enzyme activity described by Akwa et al. (37), a finding at odds with those obtained here but which could possibly be ascribed to differences in enzyme preparations and/or kinetic considerations. The enzyme described by Warner et al. (34) appears to differ from Cyp7b in that it has most activity against 5α-androstane-3β,17β-diol, while Cyp7b is very much more active against DHEA.
Nevertheless, the Km values we determine for the Cyp7b substrates pregnenolone and DHEA (4.0 and 13.6 μM) are very similar to the values recorded for the brain microsomal enzyme (4 and 14 μM; ref. 37); we speculate that Cyp7b is responsible for this activity. However, we do not exclude the possibility that there are also other Cyp activities present in brain able to catalyze neurosteroid hydroxylation.
The biological significance of the Cyp7b reaction is as yet unknown. While there is evidence that pregnenolone and DHEA are locally synthesized in brain (1, 2), there have been few determinations of the concentrations of pregnenolone, DHEA, or their derivatives in the central nervous system. Both pregnenolone and DHEA are reported to be present in rat brain within the broad range of 3–20 ng/g (≈10–70 nM; ref. 1), though exchange with a greater pool of sulfated and esterified derivatives may increase the effective local concentration. In human brain the concentrations are somewhat higher (77). While Cyp7b may convert a significant proportion of these steroids to their 7α-hydroxy derivatives, the relative local concentrations in brain of these molecules and their 7α-hydroxylated derivatives are not yet known.
7α-Hydroxylation of cholesterol in liver is a first step in the metabolic conversion of cholesterols to bile acids. However, it is so far unknown whether the observed 7α-hydroxylation of DHEA and other neurosteroids represents inactivation of the molecule or whether, as we feel more likely, 3β-hydroxy steroids such as DHEA are 7α-hydroxylated by Cyp7b to generate the active hormones. Prohormone activation in target tissues is not unusual: testosterone is locally activated in the brain by aromatase and 5α-reductase, while cortisone is activated by 11β-HSD. Notably, it has been argued that 7-oxygenated derivatives are on a metabolic pathway from DHEA to much more active steroid hormones (78). We surmise that the reaction catalyzed by Cyp7b is likely to be pivotal to the documented in vivo action of neurosteroids such as DHEA on cognition and behavior. This may assume some importance in view of the use of DHEA in attempts to ameliorate age-related cognitive decline (79).
Acknowledgments
We thank M. Steel, B. Pickard, and B. Davies for their contribution to this work; R. Ambler and I. Mason for lending equipment; M. Warner and J. Å. Gustafsson for providing some reagents; and I. Mason and R. G. M. Morris for helpful discussions. We thank H. Lardy for providing some reference steroids. This work was supported in part by grants from the Medical Research Council (to R.L. and Richard Morris, Centre for Neuroscience) and the National Institutes of Health (Grant HL20948 to D.W.R.); by a program grant and Senior Research Fellowship from the Wellcome Trust (to J.R.S.), and by Biotechnology and Biological Sciences Research Council funding to the Centre for Genome Research.
ABBREVIATIONS
| Cyp | cytochrome P450 (the term Cyp is used throughout to describe cDNAs and protein products irrespective of species of origin) |
| DHEA | dehydroepiandrosterone |
| 7HD | 7-hydroxy DHEA |
| HSD | hydroxysteroid dehydrogenase |
| TLC | thin layer chromatography |
| VV | vaccinia virus |
References
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.94.10.4925
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc24607?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1073/pnas.94.10.4925
Article citations
CYP7B1 deficiency impairs myeloid cell activation in autoimmune disease of the central nervous system.
PNAS Nexus, 3(9):pgae334, 21 Aug 2024
Cited by: 0 articles | PMID: 39262855 | PMCID: PMC11388006
27-Hydroxycholesterol acts on estrogen receptor α expressed by POMC neurons in the arcuate nucleus to modulate feeding behavior.
Sci Adv, 10(28):eadi4746, 12 Jul 2024
Cited by: 0 articles | PMID: 38996023 | PMCID: PMC11244552
Roles of bile acids signaling in neuromodulation under physiological and pathological conditions.
Cell Biosci, 13(1):106, 12 Jun 2023
Cited by: 9 articles | PMID: 37308953 | PMCID: PMC10258966
Review Free full text in Europe PMC
Concentrations of estradiol, progesterone and testosterone in sefrum and cerebrospinal fluid of patients with aneurysmal subarachnoid hemorrhage correlate weakly with transcranial Doppler flow velocities.
BMC Neurosci, 22(1):29, 23 Apr 2021
Cited by: 6 articles | PMID: 33892632 | PMCID: PMC8067654
The Biosynthesis of Enzymatically Oxidized Lipids.
Front Endocrinol (Lausanne), 11:591819, 19 Nov 2020
Cited by: 51 articles | PMID: 33329396 | PMCID: PMC7711093
Review Free full text in Europe PMC
Go to all (135) article citations
Other citations
Wikipedia
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Neurosteroid hydroxylase CYP7B: vivid reporter activity in dentate gyrus of gene-targeted mice and abolition of a widespread pathway of steroid and oxysterol hydroxylation.
J Biol Chem, 276(26):23937-23944, 04 Apr 2001
Cited by: 59 articles | PMID: 11290741
Dehydroepiandrosterone 7-hydroxylase CYP7B: predominant expression in primate hippocampus and reduced expression in Alzheimer's disease.
Neuroscience, 121(2):307-314, 01 Jan 2003
Cited by: 58 articles | PMID: 14521990
Dehydroepiandrosterone 7alpha- and 7beta-hydroxylation in mouse brain microsomes. Effects of cytochrome P450 inhibitors and structure-specific inhibition by steroid hormones.
J Neuroendocrinol, 9(12):923-928, 01 Dec 1997
Cited by: 25 articles | PMID: 9468017
The native anti-glucocorticoid paradigm.
J Steroid Biochem Mol Biol, 100(1-3):95-105, 18 May 2006
Cited by: 30 articles | PMID: 16713254
Review
Funding
Funders who supported this work.
NHLBI NIH HHS (2)
Grant ID: HL20948
Grant ID: P01 HL020948





