Abstract
Free full text

A safe packaging line for gene transfer: separating viral genes on two different plasmids.
Abstract
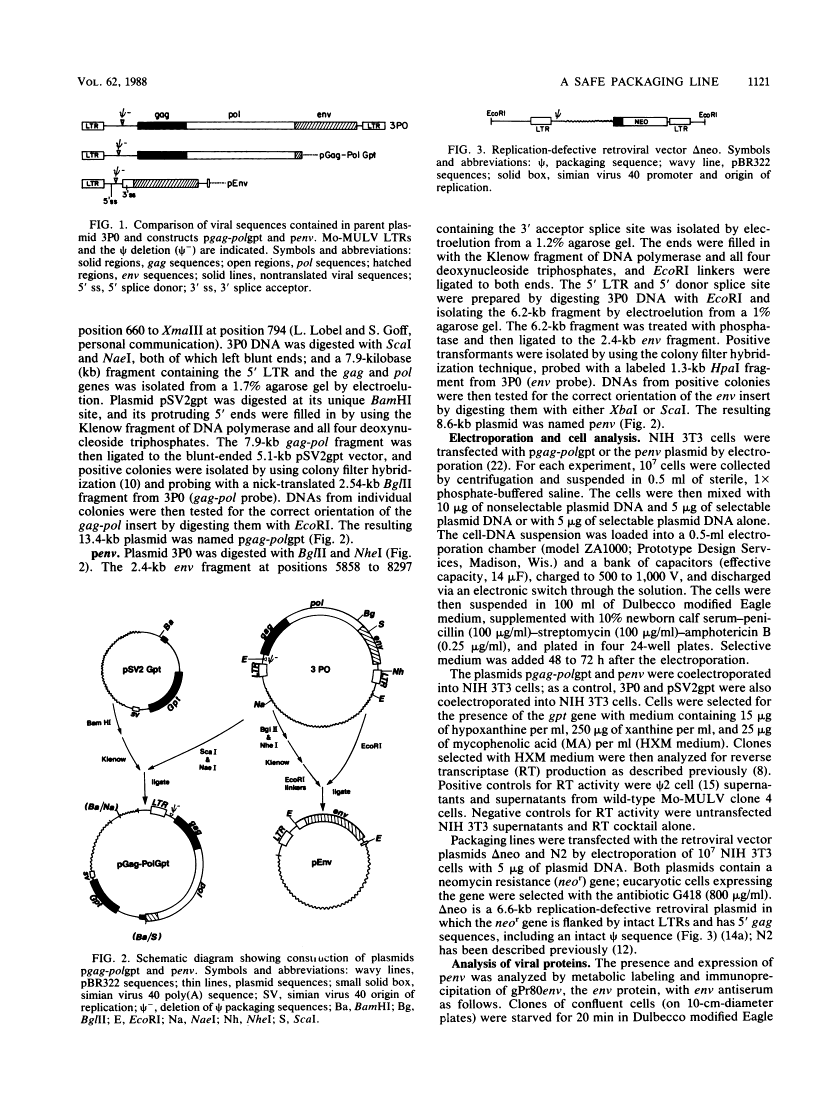
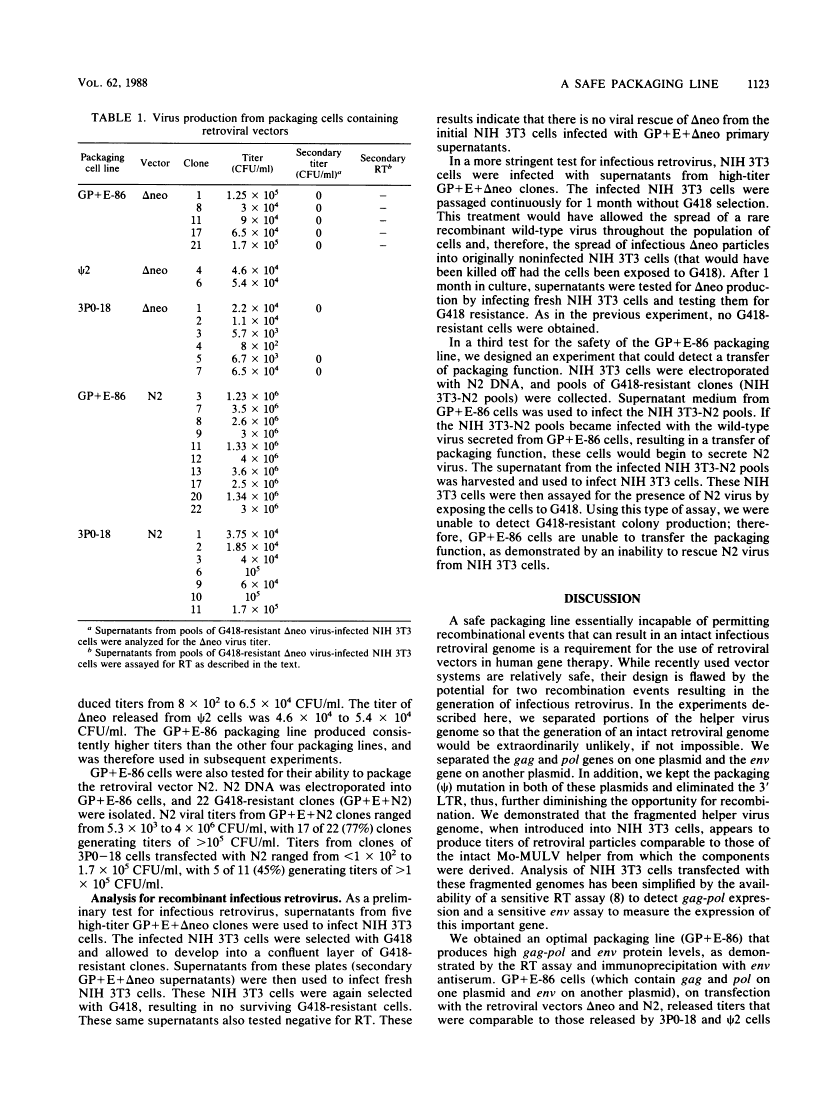
A retrovirus packaging cell line was constructed by using portions of the Moloney murine leukemia virus in which the gag, pol, and env genes of the helper virus were separated onto two different plasmids and in which the psi packaging signal and 3' long terminal repeat were removed. The plasmid containing the gag and pol genes and the plasmid containing the env gene were cotransfected into NIH 3T3 cells. Clones that produced high levels of reverse transcriptase and env protein were tested for their ability to package the replication-defective retrovirus vectors delta neo and N2. One of the gag-pol and env clones (GP+E-86) was able to transfer G418 resistance to recipient cells at a titer of as high as 1.7 X 10(5) when it was used to package delta neo and as high as 4 X 10(6) when it was used to package N2. Supernatants of clones transfected with the intact parent gag-pol-env plasmid 3P0 had comparable titers (as high as 6.5 X 10(4) with delta neo; as high as 1.7 X 10(5) with N2). Tests for recombination events that might result in intact retrovirus showed no evidence for the generation of replication-competent virus. These results suggest that gag, pol, and env, when present on different plasmids, may provide an efficient and safe packaging line for use in retroviral gene transfer.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.1M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson WF. Prospects for human gene therapy. Science. 1984 Oct 26;226(4673):401–409. [Abstract] [Google Scholar]
- Bosselman RA, Hsu RY, Bruszewski J, Hu S, Martin F, Nicolson M. Replication-defective chimeric helper proviruses and factors affecting generation of competent virus: expression of Moloney murine leukemia virus structural genes via the metallothionein promoter. Mol Cell Biol. 1987 May;7(5):1797–1806. [Europe PMC free article] [Abstract] [Google Scholar]
- Cline MJ, Stang H, Mercola K, Morse L, Ruprecht R, Brown J, Salser W. Gene transfer in intact animals. Nature. 1980 Apr 3;284(5755):422–425. [Abstract] [Google Scholar]
- Cone RD, Mulligan RC. High-efficiency gene transfer into mammalian cells: generation of helper-free recombinant retrovirus with broad mammalian host range. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6349–6353. [Europe PMC free article] [Abstract] [Google Scholar]
- Cone RD, Weber-Benarous A, Baorto D, Mulligan RC. Regulated expression of a complete human beta-globin gene encoded by a transmissible retrovirus vector. Mol Cell Biol. 1987 Feb;7(2):887–897. [Europe PMC free article] [Abstract] [Google Scholar]
- Dick JE, Magli MC, Huszar D, Phillips RA, Bernstein A. Introduction of a selectable gene into primitive stem cells capable of long-term reconstitution of the hemopoietic system of W/Wv mice. Cell. 1985 Aug;42(1):71–79. [Abstract] [Google Scholar]
- Eglitis MA, Kantoff P, Gilboa E, Anderson WF. Gene expression in mice after high efficiency retroviral-mediated gene transfer. Science. 1985 Dec 20;230(4732):1395–1398. [Abstract] [Google Scholar]
- Goff S, Traktman P, Baltimore D. Isolation and properties of Moloney murine leukemia virus mutants: use of a rapid assay for release of virion reverse transcriptase. J Virol. 1981 Apr;38(1):239–248. [Europe PMC free article] [Abstract] [Google Scholar]
- Gruber HE, Finley KD, Hershberg RM, Katzman SS, Laikind PK, Seegmiller JE, Friedmann T, Yee JK, Jolly DJ. Retroviral vector-mediated gene transfer into human hematopoietic progenitor cells. Science. 1985 Nov 29;230(4729):1057–1061. [Abstract] [Google Scholar]
- Grunstein M, Hogness DS. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. [Europe PMC free article] [Abstract] [Google Scholar]
- Hock RA, Miller AD. Retrovirus-mediated transfer and expression of drug resistance genes in human haematopoietic progenitor cells. Nature. 1986 Mar 20;320(6059):275–277. [Abstract] [Google Scholar]
- Keller G, Paige C, Gilboa E, Wagner EF. Expression of a foreign gene in myeloid and lymphoid cells derived from multipotent haematopoietic precursors. Nature. 1985 Nov 14;318(6042):149–154. [Abstract] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. [Abstract] [Google Scholar]
- Lemischka IR, Raulet DH, Mulligan RC. Developmental potential and dynamic behavior of hematopoietic stem cells. Cell. 1986 Jun 20;45(6):917–927. [Abstract] [Google Scholar]
- Lerner N, Brigham S, Goff S, Bank A. Human beta-globin gene expression after gene transfer using retroviral vectors. DNA. 1987 Dec;6(6):573–582. [Abstract] [Google Scholar]
- Mann R, Mulligan RC, Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. [Abstract] [Google Scholar]
- Miller AD, Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986 Aug;6(8):2895–2902. [Europe PMC free article] [Abstract] [Google Scholar]
- Miller AD, Eckner RJ, Jolly DJ, Friedmann T, Verma IM. Expression of a retrovirus encoding human HPRT in mice. Science. 1984 Aug 10;225(4662):630–632. [Abstract] [Google Scholar]
- Miller AD, Law MF, Verma IM. Generation of helper-free amphotropic retroviruses that transduce a dominant-acting, methotrexate-resistant dihydrofolate reductase gene. Mol Cell Biol. 1985 Mar;5(3):431–437. [Europe PMC free article] [Abstract] [Google Scholar]
- Mulligan RC, Berg P. Selection for animal cells that express the Escherichia coli gene coding for xanthine-guanine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2072–2076. [Europe PMC free article] [Abstract] [Google Scholar]
- Neel BG, Hayward WS, Robinson HL, Fang J, Astrin SM. Avian leukosis virus-induced tumors have common proviral integration sites and synthesize discrete new RNAs: oncogenesis by promoter insertion. Cell. 1981 Feb;23(2):323–334. [Abstract] [Google Scholar]
- Potter H, Weir L, Leder P. Enhancer-dependent expression of human kappa immunoglobulin genes introduced into mouse pre-B lymphocytes by electroporation. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7161–7165. [Europe PMC free article] [Abstract] [Google Scholar]
- Sorge J, Wright D, Erdman VD, Cutting AE. Amphotropic retrovirus vector system for human cell gene transfer. Mol Cell Biol. 1984 Sep;4(9):1730–1737. [Europe PMC free article] [Abstract] [Google Scholar]
- Varmus HE, Quintrell N, Ortiz S. Retroviruses as mutagens: insertion and excision of a nontransforming provirus alter expression of a resident transforming provirus. Cell. 1981 Jul;25(1):23–36. [Abstract] [Google Scholar]
- Watanabe S, Temin HM. Construction of a helper cell line for avian reticuloendotheliosis virus cloning vectors. Mol Cell Biol. 1983 Dec;3(12):2241–2249. [Europe PMC free article] [Abstract] [Google Scholar]
- Williams DA, Lemischka IR, Nathan DG, Mulligan RC. Introduction of new genetic material into pluripotent haematopoietic stem cells of the mouse. Nature. 1984 Aug 9;310(5977):476–480. [Abstract] [Google Scholar]
- Williams DA, Orkin SH, Mulligan RC. Retrovirus-mediated transfer of human adenosine deaminase gene sequences into cells in culture and into murine hematopoietic cells in vivo. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2566–2570. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from Journal of Virology are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/jvi.62.4.1120-1124.1988
Read article for free, from open access legal sources, via Unpaywall:
https://jvi.asm.org/content/jvi/62/4/1120.full.pdf
Free to read at jvi.asm.org
http://jvi.asm.org/cgi/content/abstract/62/4/1120
Free after 4 months at jvi.asm.org
http://jvi.asm.org/cgi/reprint/62/4/1120
Citations & impact
Impact metrics
Article citations
Targeting endogenous fatty acid synthesis stimulates the migration of ovarian cancer cells to adipocytes and promotes the transport of fatty acids from adipocytes to cancer cells.
Int J Oncol, 64(3):24, 12 Jan 2024
Cited by: 1 article | PMID: 38214315 | PMCID: PMC10807641
Thrombopoietin-independent generation of platelet-like particles from megakaryoblastic cells.
Sci Rep, 13(1):22553, 18 Dec 2023
Cited by: 0 articles | PMID: 38110522 | PMCID: PMC10728061
Primary and hTERT-Transduced Mesothelioma-Associated Fibroblasts but Not Primary or hTERT-Transduced Mesothelial Cells Stimulate Growth of Human Mesothelioma Cells.
Cells, 12(15):2006, 05 Aug 2023
Cited by: 0 articles | PMID: 37566084 | PMCID: PMC10417280
Mesothelioma-associated fibroblasts enhance proliferation and migration of pleural mesothelioma cells via c-Met/PI3K and WNT signaling but do not protect against cisplatin.
J Exp Clin Cancer Res, 42(1):27, 23 Jan 2023
Cited by: 8 articles | PMID: 36683050 | PMCID: PMC9869633
Toward Combined Cell and Gene Therapy for Genodermatoses.
Cold Spring Harb Perspect Biol, 12(5):a035667, 01 May 2020
Cited by: 15 articles | PMID: 31653644 | PMCID: PMC7197428
Review Free full text in Europe PMC
Go to all (591) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Construction and use of a safe and efficient amphotropic packaging cell line.
Virology, 167(2):400-406, 01 Dec 1988
Cited by: 270 articles | PMID: 2462307
A new avian leukosis virus-based packaging cell line that uses two separate transcomplementing helper genomes.
J Virol, 64(3):1070-1078, 01 Mar 1990
Cited by: 32 articles | PMID: 2154593 | PMCID: PMC249219
Bovine leukaemia virus packaging cell line for retrovirus-mediated gene transfer.
J Gen Virol, 70 ( Pt 8):1987-1993, 01 Aug 1989
Cited by: 5 articles | PMID: 2549178
Construction of a safe and efficient retrovirus packaging cell line.
Adv Exp Med Biol, 241:35-40, 01 Jan 1988
Cited by: 8 articles | PMID: 3223411
Funding
Funders who supported this work.
NHLBI NIH HHS (2)
Grant ID: HL-37069
Grant ID: HL-07230
NIDDK NIH HHS (1)
Grant ID: DK-25274