Abstract
Background
Chronic stressors are known to increase vulnerability to medical illness, but the mechanisms underlying this phenomenon are poorly understood.Methods
To identify transcriptional control pathways that are modified by chronic stress, we conducted genomewide expression microarrays on familial caregivers of brain-cancer patients (n = 11) and matched control subjects (n = 10). Analyses were conducted on peripheral blood monocytes, which are cells that have the ability to initiate and maintain many inflammatory responses. Salivary cortisol was collected over the course of 3 days as volunteers went about normal activities.Results
Caregivers' patterns of cortisol secretion were similar to those of matched control subjects. However, their monocytes showed diminished expression of transcripts bearing response elements for glucocorticoids, and heightened expression of transcripts with response elements for NF-kappaB, a key pro-inflammatory transcription factor. Caregivers also showed relative elevations in the inflammatory markers C-reactive protein and interleukin-1 receptor antagonist.Conclusions
These findings suggest that even in the absence of excess adrenocortical output, stress brings about functional resistance to glucocorticoids in monocytes, which enables activation of pro-inflammatory transcription control pathways. This persistent activation of inflammatory mechanisms may contribute to stress-related morbidity and mortality.Free full text

A Functional Genomic Fingerprint of Chronic Stress in Humans: Blunted Glucocorticoid and Increased NF-κB Signaling
Abstract
Background
Chronic stressors are known to increase vulnerability to medical illness, but the mechanisms underlying this phenomenon are poorly understood.
Methods
To identify transcriptional control pathways that are modified by chronic stress, we conducted genome-wide expression microarrays on familial caregivers of brain-cancer patients (n=11) and matched control subjects (n=10). Analyses were conducted on peripheral blood monocytes, which are cells that have the ability to initiate and maintain many inflammatory responses. Salivary cortisol was collected over the course of three days as volunteers went about normal activities.
Results
Caregivers’ patterns of cortisol secretion were similar to those of matched controls. However, their monocytes showed diminished expression of transcripts bearing response elements for glucocorticoids, and heightened expression of transcripts with response elements for NF-κB, a key pro-inflammatory transcription factor. Caregivers also showed relative elevations in the inflammatory markers C-reactive protein and interleukin-1 receptor antagonist.
Conclusions
These findings suggest that even in the absence of excess adrenocortical output, stress brings about functional resistance to glucocorticoids in monocytes, which enables activation of pro-inflammatory transcription control pathways. This persistent activation of inflammatory mechanisms may contribute to stress-related morbidity and mortality.
Mounting evidence indicates that chronic psychological stressors – such as caring for a demented family member, having a severely troubled marriage, or working in a hostile environment – contribute to the development and progression of medical illnesses (1). Stressed persons are prone to viral infections, more frequent and severe flare-ups of asthma, multiple sclerosis, and arthritis, and to developing premature coronary disease (2–7).
The mechanisms responsible for this phenomenon are not well understood. There has been much speculation regarding the contribution of the hypothalamic-pituitary-adrenocortical (HPA) axis, which releases cortisol into circulation following exposure to many life stressors (8–9). In leukocytes, cortisol ligates cytosolic glucorticoid receptors (GR), and these complexes translocate to the nucleus, where they inhibit activity of several immunoregulatory transcription control pathways, including nuclear-factor kappa-B (NF-κB), activator-protein 1 (AP-1), and JAK-STAT factors (10). Because of cortisol’s ability to inhibit a broad array of cellular immune functions, a prevailing assumption has been that it contributes to stress-evoked disease through immuno-suppressive mechanisms.
However, with increasing recognition that inflammation is a key pathogenic mechanism in many infectious, autoimmune, cardiovascular, and psychiatric diseases (11–13), the adequacy of this explanation has been called into question (14–15). This is because when taken to its logical end, this hypothesis suggests a paradoxical and inaccurate conclusion: that in boosting cortisol output and slowing immune activity, chronic stressors should ameliorate the symptoms of inflammation-related diseases. Of course, this conclusion is at odds with the excess morbidity and mortality documented in chronically stressed individuals (1).
To resolve this paradox, researchers have advanced an alternative hypothesis focusing on cellular resistance to cortisol-mediated signaling (14,16–18). It specifies that chronic stressors elicit sustained elevations in cortisol which, over time, prompt immune cells to undergo a compensatory downregulation of GR activity. This adaptively limits cortisol’s ability to further dampen immune responses. However, in cells like monocytes that are tightly regulated by cortisol, this dynamic also diminishes the potency of an important hormonal constraint, which acts to tonically inhibit NF-κB, AP-1, and other pro-inflammatory transcriptional control pathways (10). The long-term result of this process is mild, low-grade inflammation, fostered by monocytes that have acquired resistance to cortisol. The resulting persistent inflammation is hypothesized to contribute to the infectious, autoimmune, and cardiac diseases to which stress is linked.
Support for this account has accrued in studies of humans and animals (14,17,19,20), where chronic stressors have been shown to diminish the capacity of glucocorticoids to suppress endotoxin-stimulated cytokine production. While these findings provide encouraging support for the glucocorticoid-resistance hypothesis, it is difficult to draw definitive conclusions from them, because they rely on ex vivo methods, synthetic analogues of cortisol, and/or high doses of endotoxin to activate monocytes. A further problem is that existing research has relied upon culture systems that interrogate only a single activation pathway, which involves toll-like receptor 4 and the MyD88 adaptor molecule (21). Glucocorticoids regulate monocyte behavior through modulation of multiple signaling pathways (22), so a thorough evaluation of the resistance hypothesis requires a model system that fully captures these dynamics in vivo.
Here we address these problems by conducting genome-wide transcriptional surveys on the monocytes of two groups of volunteers: those in the midst of a severe chronic stressor – acting as caregiver for a family member with malignant brain cancer – and a matched sample of healthy controls. When used in concert with promoter-based bioinformatics techniques (23), these genome-wide transcriptional profiles reveal how strongly cortisol signals are being registered across the entire transcriptome. On the basis of the glucocorticoid-resistance hypothesis, we expected that the stress of caregiving would diminish glucocorticoid-mediated transcription in monocytes, and at the same time enhance transcription of pro-inflammatory mRNAs. The latter outcome was expected to be especially pronounced for genes controlled by NF-κB, which is subject to potent counter-regulation by GR-dependent mechanisms (10). Because monocytes initiate and maintain inflammatory responses to many pathogenic stimuli, we also expected these stress-related dynamics to be accompanied by higher systemic concentrations of inflammatory molecules like C-reactive protein and interleukin-6.
METHODS
Subjects
The subjects were from a larger project exploring the psychological and immunologic consequences of caregiving that ran from January, 2005 to December, 2007. This report focuses on a subgroup of volunteers who participated between November 2005 and August 2006. The caregivers were recruited from the CNS tumor clinics at the British Columbia Cancer Agency, Vancouver Centre. All were primary familial caregivers for patients being treated for glioblastoma multiforme, the most common and aggressive primary brain tumor, with 5-year survival rates of approximately 10–20 percent (24) Controls were recruited from the broader Vancouver, B.C. community using advertisements in newspapers. To be eligible, they had to (a) match an enrolled caregiver on age, gender, ethnicity, and marital status, and (b) be free of major stressors such as divorce, bereavement, unemployment, and family illness during the past year. The project was approved by the Research Ethics Boards of the University of British Columbia and the British Columbia Cancer Agency, and all subjects provided written informed consent before participating.
Psychological Distress
Distress was assessed with the Perceived Stress Scale (25), the Satisfaction With Life Scale (26), and a modified version of the Profile of Mood States (27), which focused on feelings of anxiety, anger, guilt, vigor, contentment, and joy. These instruments have been extensively validated and showed excellent psychometrics in our sample, with Cronbach’s alpha‘s > .76.
Monocyte Gene Expression
To conduct genome-wide expression microarrays, 20-ml of blood was drawn by antecubital venipuncture into Vacutainer Cell Preparation Tubes (Becton-Dickinson; Oakville, Ontario). After isolation of mononuclear cells through density-gradient centrifugation, monocytes were captured via immuno-magnetic positive selection with antibodies against CD14 (Miltenyi Biotec; Auburn, California). RNA was subsequently extracted using RNAlater/RNeasy (Qiagen, Valencia California). 5 mg of the resulting RNA was the assayed using Affymetrix U133A high-density oligonucleotide arrays (28) in the UCLA DNA Microarray Core as previously described (29,30). Robust Multiarray Averaging (31) was applied to quantify expression of the 22,283 assayed transcripts, and differentially expressed genes were identified as those showing ≥50% difference in mean expression levels between caregivers and controls (corresponding to a false discovery rate of 10%; 32). The raw data are deposited in the Gene Expression Omnibus; Accession number: GSE7893.
To identify upstream signal transduction pathways that drive differential gene expression in leukocytes from stressed vs. control individuals, we used a 2-sample variant of the Transcription Element Listening System (TELiS; 23) (http://www.telis.ucla.edu). TELiS analyzes differential gene expression data in terms of the prevalence of transcription factor-binding motifs (TFBMs) within the promoters of differentially expressed genes. This approach can accurately identify the activation of specific hormone or cytokine signaling pathways based on the resulting pattern of gene induction which occurs selectively in genes bearing TFBMs responsive to transcription factors activated through that pathway (23). The present analyses assessed glucocorticoid receptor activity using the TRANSFAC V$GR_Q6 DNA motif, and NF-κB/Rel transcription factor activity using the V$CREL_01 motif (which was characterized by binding of the p50/p65 cRel heterodimer, but can also bind RelB and other NF-κB/Rel family proteins; 33). p-values were calculated using an independent sample t-test with Welch’s correction for heteroscedasticity (34). Primary analyses utilized default parameter settings shown to be optimal in previous studies (analysis of −600 bp sequence upstream of transcription start site, with a .90 MatInspector match stringency; 23).
Confirmation by RT-PCR
A subset of transcripts identified as differentially expressed in microarray analyses were independently assayed by quantitative real-time RT-PCR using TaqMan Gene Expression Assays (Applied Biosystems, Foster City, CA, USA). Eleven genes involved with immune response were chosen for analysis. Assays for each sample were carried out in triplicate using an iCycler instrument (Biorad, Hercules, CA, USA), Quantitect Probe RT-PCR enzymes (Qiagen), and the manufacturer’s recommended 1-step thermal cycling protocol. Threshold cycle numbers for each analyte were normalized to GAPDH for analysis. A general linear model with sample (i.e., replicate) nested within persons was used to evaluate differential expression of each individual transcript.
Biomarkers of Systemic Inflammation
Systemic immune activation was assessed through serum levels of three widely used protein biomarkers of inflammation: C-reactive protein, interleukin-1 receptor antagonist, and interleukin-6. C-reactive protein was analyzed using a high-sensitivity, chemiluminescent technique on an IMMULITE 2000 (Diagnostic Products Corporation, Los Angeles, California). This assay has an inter-assay coefficient of variation of 2.2% and a lower detection threshold of .20 mg/L. Interleukin-1 receptor antagonist is a molecule released by monocytes to neutralize the pro-inflammatory activities of interleukin-1. It was measured by enzyme-linked immunosorbent assay (ELISA) using a commercially available kit from Biosource International (Burlington, Ontario). This assay has a minimum detection threshold of 4 pg/ml and showed intra- and inter-assay coefficients of variation < 5%. Interleukin-6 was assayed using a high-sensitivity ELISA kit (Quantikine HS IL-6; R&D Systems; Minneapolis, MO, USA) with a minimum detectable volume of 0.039 pg/ml. It showed intra- and inter-assay variability <10%.
Patterns of Cortisol Output
Diurnal output of cortisol was assessed by having subjects collect saliva as they went about 3 days of normal activities. To facilitate the collection process, we lent them a handheld computer (Palm Zire 21; Sunnyvale, California) which signaled them to collect saliva at waking, and at 1/2, 1, 4, 9, and 14 hours after waking. Collection was done by chewing on a cotton dental roll (Salivette; Sarstedt, Nümbrecht, Germany). To ensure compliance with the protocol, the computer flashed a three digit code each time the alarm sounded. Subjects recorded the codes on collection containers. When the containers were returned to the lab, the codes on them were matched with those displayed by the computer. Samples marked incorrectly were excluded from analysis. The containers were then centrifuged. After saliva had been aspirated, it was frozen at −30 C until assay.
Cortisol was measured utilizing a commercially available chemiluminescent technique (IBL-Hamburg; Hamburg, Germany) at the Technical University of Dresden. This assay has a sensitivity of 0.16 ng/ml and intra- and inter-assay coefficients of variation less than 12%. After cortisol values had been log-transformed, each day’s data were used to create indices of morning response (output over the first hour) and total daily secretion using area-under-the-curve calculations. An index of diurnal rhythm was also computed by simple linear regression of cortisol onto time since waking. Values for each day of sample collection were then averaged. The mean inter-day correlations were .68 for total volume, .46 for diurnal rhythm, and .27 for morning response.
Potential Confounders
There are a number of potential differences between caregivers and controls that could contribute to transcriptional disparities. Through a validated battery of questions (35–37) we solicited information on the most likely demographic (age, gender, ethnicity, and educational background), behavioral (use of cigarettes and alcohol; exercise and sleeping tendencies) and biomedical (body mass index, self-rated health, functional limitations, personal history of major diseases) confounders.
RESULTS
Preliminary Analyses
Table 1 describes the sample’s demographic, behavioral, and biomedical characteristics. The sample consisted of 11 subjects caring for a family member with malignant brain cancer, and 10 controls who were demographically similar but free of major stressors. None of them had a personal history of cancer, autoimmune conditions, liver or kidney disease, HIV/AIDS, or tuberculosis. The groups were similar in terms of age, gender, ethnicity, cigarette and alcohol use, exercise and sleep habits, body mass index, functional limitations, and history of cardiovascular disease (all p’s > .17 by independent samples t-test; Table 1). Caregivers family members’ had received their brain cancer diagnosis about 8 months prior to study entry (mean = 31.5 ± 5.3 weeks).
Table 1
Demographic, behavioral, and biomedical characteristics.
| Caregivers (n = 11) | Controls (n = 10) | |
|---|---|---|
| Mean ± SEM or % | Mean ± SEM or % | |
| Age at Entry, Years | 52.5 ± 4.0 | 55.6 ± 4.6 |
| Gender, % Male/ Female | 37.3 / 72.7 | 50.0 / 50.0 |
| Ethnicity, % Caucasian | 90.9 | 80.8 |
| Education, % University Degree | 45.5 | 50.0 |
| Cigarette Smoking, % Daily Smokers | 27.3 | 10.0 |
| Exercise, Minutes Weekly | 130.9 ± 35.6 | 152.5 ± 41.44 |
| Alcohol Consumption, Drinks Weekly | 7.0 ± 3.5 | 8.3 ± 2.5 |
| Body Mass Index, kg/m2 | 25.8 ± 0.9 | 25.9 ± 1.0 |
| Self-Rated Sleep Quality, Poor (0) – Good (3) | 1.4 ± 0.2 | 1.2 ± 0.2 |
| Activity Limitations, None (1) - Serious (6) | 1.7 ± 0.2 | 1.5 ± 0.2 |
| Personal History Cardiovascular Disease, % | 27.3 | 30.0 |
Figure 1 presents disparities between caregivers and controls in terms of psychological distress. Scores on the Perceived Stress Scale were significantly higher in caregivers, (t = 3.31, p = .003), indicating they found life stressful, overwhelming, and unpredictable. Indeed, their scores were at the 80th percentile of the US population distribution (38). Caregivers also reported decreased satisfaction with their lives (t = −2.23, p = .04), and less frequently experienced positive emotions such as joy, vigor, and contentment (p’s < .004). They did not, however, report a higher frequency of negative emotions like anger, guilt, and anxiety than controls (p’s > .16).
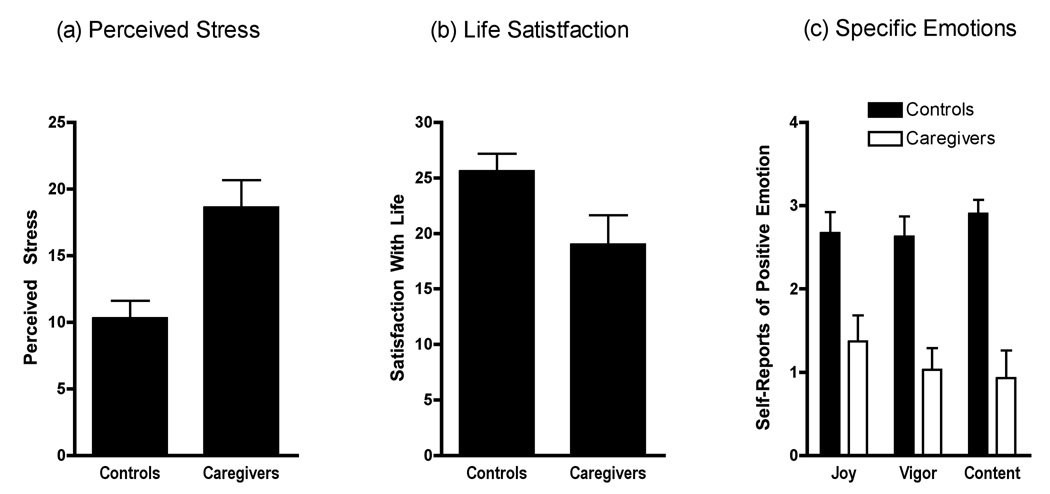
Self-reports of well-being were collected from 11 adults facing a severe chronic stressor (primary caregiver for family member with brain cancer) and 10 demographically-matched nonstressed controls. Caregivers showed (a) higher levels of stress (p = .003), (b) decreased life satisfaction (p = .04), and (c) decreased positive emotions (p’s < .004).
Chronic Stress and Transcriptional Control
Figure 2 presents the “transcriptional fingerprint” of chronic stress in monocytes, with red intensity indicating the magnitude of a gene’s relative over-expression in caregivers versus controls, and green intensity denoting the magnitude of under-expression. A total of 614 transcripts were differentially expressed (Table S1 in supporting information), representing 542 distinct named human genes. 127 (21%) were over-expressed in caregivers, and 488 (79%) were under-expressed, reflecting a net repressive effect of chronic stress (p < .0001 by binomial test).
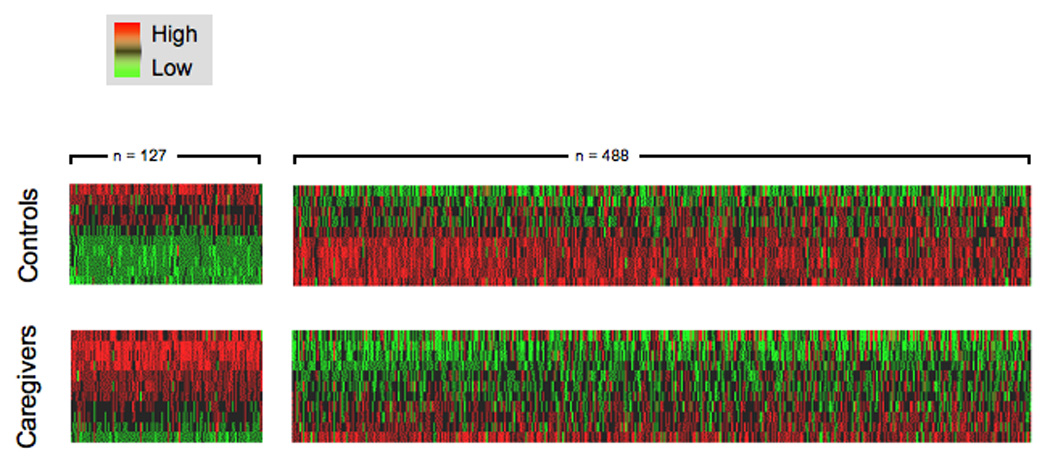
Microarray analysis of gene expression in peripheral blood monocytes identified 614 transcripts showing > 50% difference in mean expression levels across groups (green = under-expression in chronic stress, red = over-expression).
We used the TELiS bioinformatics analysis to quantify the prevalence of transcription factor-binding motifs (TFBMs) in the promoters of differentially expressed genes. Results indicated that among caregivers versus controls, there was a relative downregulation of genes bearing one or more glucocorticoid response elements. Specifically, glucocorticoid receptor TFBMs occurred at 23.3% lower prevalence in regulatory sequences of genes over-expressed by caregivers versus those over-expressed by controls (TRANSFAC V$GR_Q6 motif: 2.13 ± .21 versus 2.77 ± .11 sites/promoter for caregivers and controls; p = .007 by independent-samples t-test). These findings suggest a stress-linked diminution of GR-mediated transcription (Figure 3a).
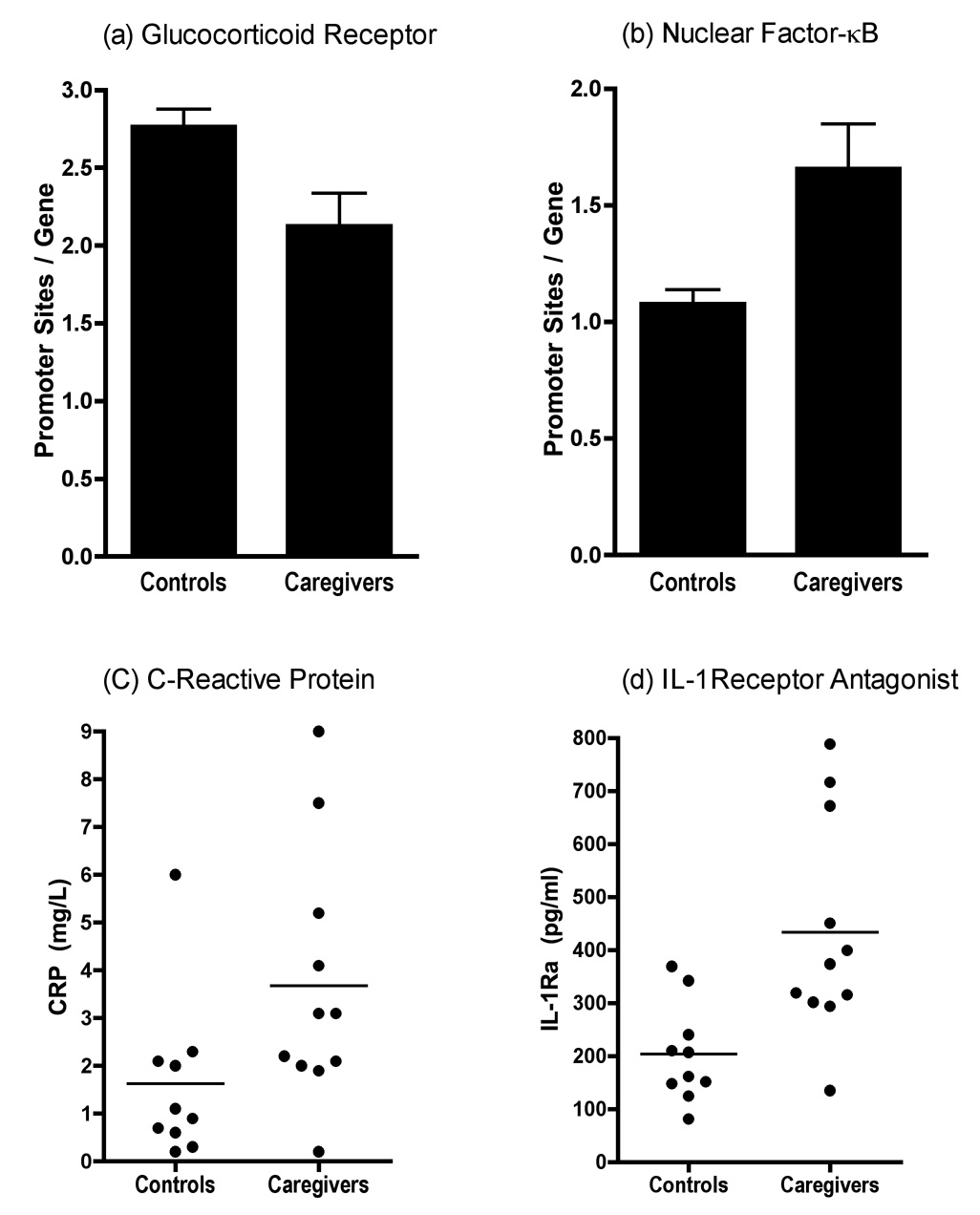
In TELiS bioinformatics analysis of response element prevalence in promoters of differentially expressed genes, (a) GR response elements are under-represented in genes up-regulated in stressed caregivers, whereas (b) transcripts bearing response elements for NF-κB are over-represented. In serum caregivers display significantly higher concentrations of the inflammatory biomarkers (c) C-reactive protein and (d) interleukin-1 receptor antagonist.
Consistent with expectations about increased inflammatory signaling, TELiS identified a parallel upregulation of genes bearing NF-κB response elements among caregivers. There was a 1.54-fold greater prevalence of NF-κB/Rel TFBMs in promoters of genes over-expressed by caregivers relative to those over-expressed by controls (TRANSFAC V$CREL_01 motif; 1.66 ± 0.19 vs. 1.08 ± 0.06 sites/promoter for caregivers and controls; p = .005; Figure 3b). The coupling of increased NF-κB/Rel activity (1.54-fold change) and decreased GR activity (0.77-fold change) resulted in a net 2.01-fold skew in the structure of promoter TFBMs distributions across genes over-expressed in caregivers versus controls.
To evaluate the sensitivity of the TELiS analyses to technical variations, we repeated them using parametric variations of promoter length (−300 bp, −600 bp, −1000 to +200 bp) and scan stringency (MatSim = .80, .90, .95). Of the six parametric combinations that were evaluable, chronic stress was associated with a 1.72-fold net skew in the relative prevalence of NF-κB/GRE TFBMs, which was statistically significant at p = .0042. We also used RT-PCR to independently verify microarray analysis results for 11 genes involved in inflammatory and immune processes. The results were concordant with the microarray in 9/11 instances (Figure S1 in supporting information), confirming stress-related upregulation of the RUNX1, PTEGES, VEGF, HIG2, TNF, ADM, and ARL4C genes (all p’s < .001), and stress-regulated downregulation of GBP1, HDAC1, and TNFSF10 (all p’s < .03). Though the groups showed differential expression of STAT1 and IL8 by microarray, their values were similar in RT-PCR analyses (p’s > .35).
Because circulating monocytes can have either “resident” or “inflammatory” phenotypes, we considered the possibility that caregiving-related differences in their distributions could explain our findings. However, microarray results indicated that caregivers and controls expressed similar quantities of mRNA for surface markers that differentiate these phenotypes (e.g., CD14, CD16, CCR1, CCR4, CCR7, p’s > .11). These findings suggest that disparities in the proportion of inflammatory to resident monocytes are not responsible for our findings.
Protein Biomarkers of Inflammation
Consistent with the skew towards stress-related monocyte activation, caregivers had about twice as much of the inflammatory biomarker C-reactive protein in circulation as controls (3.14 ± 0.65 vs. 1.62 ± 0.54 mg/L; t = 2.09, p = .05; Figure 3c). They also had more than twice as much serum interleukin-1 receptor antagonist (433.21 ± 61.87 vs. 203.56 ± 29.19 pg/ml; t = 3.25, p = .005; Figure 3d), a molecule released by monocytes to neutralize the pro-inflammatory activities of interleukin-1. There were no caregiving-related differences in serum interleukin-6 (1.18 + .20 vs. 0.96 + .14 pg/ml in caregivers vs. controls; t = 0.88, p = .39). However, much of the interleukin-6 found in circulation derives from adipose tissue (39), so any stress-related effects on monocytes are likely to have been obscured.
Potential Underlying Mechanisms
To identify mechanisms linking chronic stress and transcriptional control, we compared the diurnal output cortisol of caregivers and controls. Subjects collected saliva 6 times daily for a 3-day period, according to a schedule that captures the hormone’s diurnal rhythm. Figure 4 illustrates that caregivers and controls displayed similar patterns of cortisol secretion over the day. Though caregivers showed higher cortisol than controls 4 hours after waking (t = 4.19, p = .029), there were no significant differences at other times of day, and the groups were similar on global indices such as the diurnal rhythm of secretion and total output over the day (p’s >.59). We also considered whether transcriptional differences were attributable to reduced GR expression in caregivers. However, the groups expressed similar quantities of GR mRNA in monocytes (by microarray, 9.80 ± 0.12 versus 10.05 ± 0.18 log2 relative gene expression units, p = .29; by RT-PCR, 4.88 ± 0.92 versus 4.65 ± 0.66 log2 GAPDH-normalized relative expression units, p = .12).
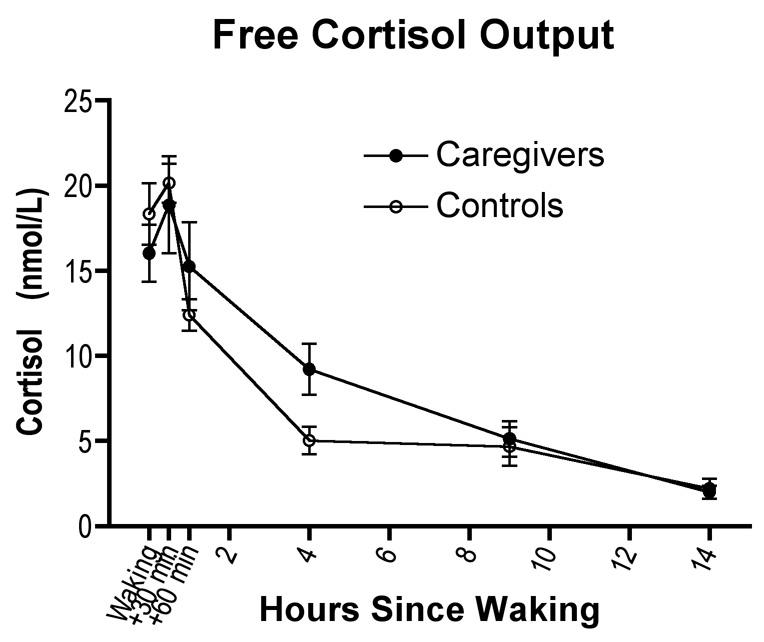
Caregivers showed higher cortisol than controls 4 hours after waking (t = 4.19, p = .029), but did not differ significantly at other times of day, or on global indices such as diurnal rhythm of secretion and total output over the day (p’s >.59).
To evaluate the possibility that demographic, behavioral, and biomedical disparities between caregivers and controls were responsible for the differential transcription patterns, we employed analysis of covariance to remove any variance in gene expression profiles attributable to a potential confounder prior to TFBM analysis (29). Caregivers continued to exhibit higher NF-κB / GRE activity ratios (all p’s ≤ .04) following adjustment for demographic characteristics (age, gender, ethnicity, and educational background), as well as behavioral characteristics (use of cigarettes and alcohol; exercise and sleeping tendencies) and biomedical characteristics (body mass index, self-rated health, functional limitations, personal history of cardiac disease). Group differences in plasma C-reactive protein and interleukin-1 receptor antagonist also persisted following adjustment for these potential confounders. None of the volunteers had a history of other medical conditions (cancers, respiratory conditions, autoimmune disorders, persistent infections) that could bias the findings.
Exploratory Analyses
In addition to the primary hypotheses of altered GR / NF-κB signaling equilibrium, exploratory bioinformatics analyses also evaluated whether other transcription-control pathways were altered under chronic stress. Four patterns consistently emerged across variations in analysis parameters: 1.) caregivers displayed relative upregulation of genes responsive to the EGR1 control pathway (62.9% increase in promoter TFBM prevalence; p = .019), which like NF-κB, heightens expression of transcripts involved with chemotaxis, angiogenesis, and inflammation; 2.) caregivers exhibited diminished expression of genes bearing response elements for interferon regulatory factor 1 (IRF1; 49.6% decline, p = .006), which mediates innate anti-viral responses by activating interferon-responsive genes; 3.) caregivers showed diminished activity of genes bearing response elements for the ELK1 transcription factor mediating MAP kinase-induced transcription (43.2% reduction, p = .002); and, 4.) caregivers showed diminished activity of genes bearing response elements for the Octamer (Oct) family of transcription factors (average 51.7% reduction, p = .012).
To identify common functional characteristics of differentially expressed genes, we conducted additional exploratory Gene Ontology analyses using GOstat (http://gostat.wehi.edu.au). Gene Ontology categories over-represented among genes up-regulated in caregivers included wound healing (e.g., THBS1, EREG; GO:0042060), chemotaxis (e.g., VEGF, IL8; GO:0050918), and angiogenesis (e.g., VEGF, EREG; GO:0001525). Functional characteristics of down-regulated genes included involvement in catabolism (e.g., PSMB5, PRDX3; GO:009056), lytic activity (e.g., ASAHL, LIPA; GO:0000323), and immune defense (e.g., TLR1, HLA-DQA1, GO:006952). These patterns mirror the results of the TELiS analyses in suggesting that chronic stress generally activates pro-inflammatory genes, but may simultaneously inhibit some genes involved in specific microbial-defense operations.
DISCUSSION
Biobehavioral research has long struggled to resolve the paradox that chronic stressors accentuate vulnerability to inflammatory diseases while simultaneously enhancing secretion of immune-dampening glucocorticoid hormones. One hypothesis attempting to reconcile these apparently conflicting observations postulates that chronic stressors bring about functional resistance to cortisol-mediated signaling (14,16–18). Initial support for this proposition has emerged in a series of studies where chronic stressors have been shown to diminish the capacity of glucocorticoids to suppress ex vivo inflammatory cytokine production (14,17,19,20).
Here we build on this work using genome-wide transcriptional profiling and functional bioinformatics techniques to assess GR-mediated gene regulation in vivo. The present results identify an in vivo transcriptional fingerprint of chronic stress in humans, and do so in a cell type that drives inflammatory pathology in many common diseases. This profile suggests a scenario in which long-term stress brings about a functional resistance to glucocortioid signal transduction in monocytes, which reduces inhibition of NF-κB and EGR1, and thereby fosters the kind of pro-inflammatory dynamics that ultimately promote chronic diseases, including diabetes, coronary disease, autoimmune disorders, chronic infections, and some cancers (11–13). Notably, resistance to glucocorticoids and mild, systemic inflammation have also been implicated in the pathogenesis of depression (15,40–42), suggesting that the dynamics observed herein may help explain the affective difficulties often found among caregivers (43).
These findings converge with evidence from studies of rodents, which experimentally manipulate exposure to stressors, and find that it diminishes sensitivity to glucocorticoid-mediated signaling, both in the immune and nervous systems (19,44). They also converge with a recent microarray profile of socially isolated individuals, which documented a similar pattern of diminished GR- and heightened NF-κB-dependent transcription (29). Collectively, these studies suggest that long-term stressor exposure interferes with the transduction of cortisol-mediated signaling and, in doing so, fosters pro-inflammatory dynamics. This may in turn serve as a common biological pathway by which psychosocial risk factors contribute to the development and progression of medical illness (1,45).
The mechanisms responsible for diminished glucocorticoid-mediated transcription in stressed persons remain unclear. We did not observe caregiving-related disparities in the output of cortisol. However, subjects had been caregiving for an average of 8 months, and the lack of difference in cortisol is consistent with evidence that HPA output rebounds to normal (and later below normal) during long-term chronic stress (8). We also considered the possibility that transcriptional disparities were attributable to reduced GR expression in caregivers. However, the groups expressed similar quantities of GR mRNA. Together, these findings suggest that although caregivers are secreting normal volumes of cortisol, and have sufficient GR available to transduce hormone signals, this message is not registered equivalently at the level of monocyte gene transcription. We think it is likely that stressor-induced post-translational modifications to the GR are responsible for this phenomenon (18), but further research is necessary to evaluate this hypothesis.
In addition to providing an explanation for the paradoxical influences of chronic stressors on inflammatory conditions, bioinformatic analyses revealed a broader pattern of diminished IRF1-, ELK-1-, and Oct-mediated transcription in monocytes. These findings suggest that at the same time chronic stress engenders pro-inflammatory activity in monocytes, it may interfere with basic microbial-defense processes involving interferon signaling, cell proliferation and differentiation, and pathogen digestion. These dynamics may help to explain the especially potent influence of chronic stressors in virally-mediated diseases (46).
The principal limitations of this project are its small sample and its cross-sectional design. Although the design precludes inferences about the direction of causal relationships, it is difficult to conceive of plausible reverse-directionality explanations for the findings. Moreover, covariance analyses ruled out a variety of potential demographic, behavioral, and biomedical confounders, and the results converge with experimental studies in animals, wherein the causal influence of stressors on sensitivity to glucocorticoid signaling has been established (19,44). Nonetheless, the findings need to be considered preliminary until they have been substantiated with larger samples, more rigorous prospective designs, additional functional indicators of glucocorticoid sensitivity, and assessments of other hormonal response systems (e.g., the sympathetic nervous system). It also will be important for future studies to determine what role depressive symptoms and other mood states play in mediating the effects of caregiving, and what implications the transcriptional dynamics we identified have for the development and progression of inflammatory diseases. With regard to the latter issue, caregivers’ levels of C-reactive protein averaged 3.14 mg/L, which places them at high-risk for coronary heart disease according to practice guidelines (47). However, it remains unclear whether this inflammation is of sufficient magnitude and duration to bring about clinical illness. But with more research of this nature, scientists and physicians will gain deeper insights into the biological mechanisms through which stressors “get under the skin” to influence disease.
ACKNOWLEDGEMENTS
This work was supported by grants from the Canadian Institutes of Health Research, the National Heart, Lung, and Blood Institute, the National Cancer Institute, the Michael Smith Foundation for Health Research, the Heart and Stroke Foundation of Canada, and the National Alliance for Research on Depression and Schizophrenia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
None of the authors has a biomedical financial interest or a conflict of interest to declare related to this project.
REFERENCES
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.biopsych.2008.03.017
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc2581622?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1016/j.biopsych.2008.03.017
Article citations
Age and Hair Cortisol Levels as Predictors of SARS-CoV-2 Infection.
Int J Environ Res Public Health, 21(9):1166, 02 Sep 2024
Cited by: 0 articles | PMID: 39338049 | PMCID: PMC11430878
Distinct saliva DNA methylation profiles in relation to treatment outcome in youth with posttraumatic stress disorder.
Transl Psychiatry, 14(1):309, 26 Jul 2024
Cited by: 0 articles | PMID: 39060246 | PMCID: PMC11282249
Can social adversity alter the epigenome, trigger oral disease, and affect future generations?
Ir J Med Sci, 193(5):2597-2606, 14 May 2024
Cited by: 0 articles | PMID: 38740675 | PMCID: PMC11450135
Review Free full text in Europe PMC
A Chemical Structure and Machine Learning Approach to Assess the Potential Bioactivity of Endogenous Metabolites and Their Association with Early Childhood Systemic Inflammation.
Metabolites, 14(5):278, 10 May 2024
Cited by: 0 articles | PMID: 38786755 | PMCID: PMC11122766
Bibliometric and visualization analyses of cancer-related fatigue research published worldwide from 2001 to 2023.
Front Oncol, 14:1338325, 30 Apr 2024
Cited by: 0 articles | PMID: 38746672 | PMCID: PMC11091377
Review Free full text in Europe PMC
Go to all (348) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
GEO - Gene Expression Omnibus
- (1 citation) GEO - GSE7893
Quick GO (4)
- (1 citation) GO - GO0042060
- (1 citation) GO - GO0050918
- (1 citation) GO - GO0001525
- (1 citation) GO - GO0000323
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Greater inflammatory activity and blunted glucocorticoid signaling in monocytes of chronically stressed caregivers.
Brain Behav Immun, 41:191-199, 02 Jun 2014
Cited by: 106 articles | PMID: 25242587 | PMCID: PMC4973629
Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling.
Proc Natl Acad Sci U S A, 106(34):14716-14721, 14 Jul 2009
Cited by: 426 articles | PMID: 19617551 | PMCID: PMC2732821
Fatigue and gene expression in human leukocytes: increased NF-κB and decreased glucocorticoid signaling in breast cancer survivors with persistent fatigue.
Brain Behav Immun, 25(1):147-150, 18 Sep 2010
Cited by: 107 articles | PMID: 20854893 | PMCID: PMC3603145
Molecular mechanisms of glucocorticoid receptor signaling.
Medicina (B Aires), 70(5):457-462, 01 Jan 2010
Cited by: 6 articles | PMID: 20920967
Review
Funding
Funders who supported this work.
NHLBI NIH HHS (2)
Grant ID: R01 HL073975
Grant ID: R01 HL073975-01A2





