Abstract
Background
The luxS/AI-2 signaling pathway has been reported to interfere with important physiological and pathogenic functions in a variety of bacteria. In the present study, we investigated the functional role of the streptococcal luxS/AI-2 system in metabolism and diverse aspects of pathogenicity including the adaptation of the organism to stress conditions using two serotypes of Streptococcus pyogenes, M1 and M19.Results
Exposing wild-type and isogenic luxS-deficient strains to sulfur-limited media suggested a limited role for luxS in streptococcal activated methyl cycle metabolism. Interestingly, loss of luxS led to an increased acid tolerance in both serotypes. Accordingly, luxS expression and AI-2 production were reduced at lower pH, thus linking the luxS/AI-2 system to stress adaptation in S. pyogenes. luxS expression and AI-2 production also decreased when cells were grown in RPMI medium supplemented with 10% serum, considered to be a host environment-mimicking medium. Furthermore, interaction analysis with epithelial cells and macrophages showed a clear advantage of the luxS-deficient mutants to be internalized and survive intracellularly in the host cells compared to the wild-type parents. In addition, our data revealed that luxS influences the expression of two virulence-associated factors, the fasX regulatory RNA and the virulence gene sibA (psp).Conclusion
Here, we suggest that the group A streptococcal luxS/AI-2 system is not only involved in the regulation of virulence factor expression but in addition low level of luxS expression seems to provide an advantage for bacterial survival in conditions that can be encountered during infections.Free full text

Functional analysis of the group A streptococcal luxS/AI-2 system in metabolism, adaptation to stress and interaction with host cells
Abstract
Background
The luxS/AI-2 signaling pathway has been reported to interfere with important physiological and pathogenic functions in a variety of bacteria. In the present study, we investigated the functional role of the streptococcal luxS/AI-2 system in metabolism and diverse aspects of pathogenicity including the adaptation of the organism to stress conditions using two serotypes of Streptococcus pyogenes, M1 and M19.
Results
Exposing wild-type and isogenic luxS-deficient strains to sulfur-limited media suggested a limited role for luxS in streptococcal activated methyl cycle metabolism. Interestingly, loss of luxS led to an increased acid tolerance in both serotypes. Accordingly, luxS expression and AI-2 production were reduced at lower pH, thus linking the luxS/AI-2 system to stress adaptation in S. pyogenes. luxS expression and AI-2 production also decreased when cells were grown in RPMI medium supplemented with 10% serum, considered to be a host environment-mimicking medium. Furthermore, interaction analysis with epithelial cells and macrophages showed a clear advantage of the luxS-deficient mutants to be internalized and survive intracellularly in the host cells compared to the wild-type parents. In addition, our data revealed that luxS influences the expression of two virulence-associated factors, the fasX regulatory RNA and the virulence gene sibA (psp).
Conclusion
Here, we suggest that the group A streptococcal luxS/AI-2 system is not only involved in the regulation of virulence factor expression but in addition low level of luxS expression seems to provide an advantage for bacterial survival in conditions that can be encountered during infections.
Background
Bacterial cell density-dependent signaling, also termed "quorum sensing", is used by bacteria to collectively modulate gene expression in response to changes in the population density [1-6]. Although signaling can be achieved through a variety of regulatory mechanisms, all systems described to date involve the production, secretion and detection of extracellular low-molecular-weight signaling molecules called "autoinducers" [1,3,7,8]. Due to the specificity of their respective sensors, recognition of acylated homoserine lactones (AHLs) (gram-negative bacteria) and peptide autoinducers (gram-positive bacteria) is restricted for communication within the same species. More recently, a novel quorum sensing system involving a furanone-like signaling molecule termed "autoinducer 2" (AI-2) was described to regulate bioluminescence in Vibrio harveyi [9,10]. AI-2 is synthesized by the luxS gene product, which has been identified in the genome of over 55 gram-negative and gram-positive bacterial species [1,11,12]. Since culture supernatants of several bacterial species had a complementary effect on luxS-deficient V. harveyi, AI-2 has been proposed to function as a "universal" signaling molecule for interspecies communication [1,9,10]. In addition to controlling bioluminescence, recent studies show that the luxS/AI-2 signaling is involved in the regulation of pathogenicity in several organisms [5,12].
Apart from its role in quorum sensing, the enzyme LuxS is tightly coupled to the S-adenosylmethionine (SAM) utilization pathway [11-13]. SAM is an essential donor of methyl groups for DNA, RNA and other methylation reactions. Its utilization by methyl transferases yields S-adenosylhomocysteine (SAH), which is toxic for the cells and is eliminated through hydrolysis by the nucleosidase Pfs to produce S-ribosylhomocysteine (SRH) and adenine. Finally, LuxS cleaves SRH into homocysteine and 4,5-dihydroxy-2,3-pentanedione (DPD). DPD spontaneously forms the cyclic pro-AI-2 molecule, which in V. harveyi reacts with borate to form a stable cyclic furanosyl borate diester [1,11,13].
Knowledge about quorum-sensing systems in the gram-positive human pathogen Streptococcus pyogenes is rather limited. S. pyogenes (Group A Streptococcus, GAS) is responsible for a broad range of diseases including mild illnesses such as pharyngitis, impetigo and scarlet fever and more severe invasive infections such as necrotizing fasciitis, streptococcal toxic shock syndrome and post-infectious rheumatic fever [14,15]. In GAS, like in other bacteria, pathogenicity is multifactorial and requires the coordinated temporal regulation of virulence factor expression in response to changing environmental conditions and cell population density [14,16,17]. Studies have confirmed that expression of several GAS virulence genes is temporal and dependent on growth phase [16-20]. Furthermore, a number of specific regulators (e.g. two-component systems and response regulators) modulate virulence gene expression in a growth phase dependent manner [16,21-24]. Based on similarities with previously described peptide signaling regulons of S. pneumoniae (com system) and S. aureus (agr system), two putative quorum-sensing systems, sil and fasBCA, have been reported in GAS [21,25]. Although no signaling peptide could be identified within the fasBCA locus, the putative pheromone peptide SilCR from the sil operon was shown to regulate DNA uptake and the ability of GAS to cause invasive infection [21,25]. In addition, the observation of a luxS homologue in the GAS genome suggested that quorum sensing via the luxS/AI-2 signaling could have a relevant function in the pathogenesis of this organism [26,27]. Two recent studies report a role of luxS/AI-2 in the regulation of virulence gene expression in M3 and M6 serotypes [26,27]. However, the role of the luxS/AI-2 in the AMC-related metabolism and adaptation to stress conditions in GAS remains unknown.
Here, we were interested in investigating further the function of the luxS/AI-2 system in GAS serotypes M1 and M19. Expression of luxS in connection with production of AI-2 like activity was analyzed. The metabolic role of luxS in the activated methyl cycle (AMC) in GAS was examined. Wild-type and isogenic luxS-deficient strains were compared in regard to their adaptation to diverse growth and stress conditions as well as diverse aspects of pathogenicity including interaction with epithelial cells and macrophages. In summary, our data suggest an important function of the luxS/AI-2 system in survival and growth of GAS under conditions that are relevant during infections.
Results
Construction of luxS-deficient mutants
As reported previously, GAS possesses a luxS homolog (coding sequence: 483 bp), the predicted translational product of which shares 36% identity with the LuxS protein from V. harveyi [26]. Analysis of the luxS coding sequences in clinical isolates of two different serotypes, RDN29 (M1 type) and RDN02 (M19 type), revealed 100% amino acid sequence identity. In a previous study, a GAS strain of serotype M6 (JRS4) was shown to contain in its genome the insertion element, IS 1239, inserted 111 bp upstream of the luxS translational start codon [26]. This insertion was assumed to have an influence on luxS expression [26]. Further analysis of the sequence up to 300 bp upstream of the luxS coding sequence in RDN29 and RDN02 did not reveal the presence of IS 1239 (data not shown). To analyze the function of the luxS/AI-2 system in GAS, we deleted the luxS gene in RDN29 and RDN02, thus creating EC478 and RDN306, respectively (Table (Table11).
Table 1
Bacterial strains and plasmids used in this study
| Strain/plasmid | Relevant characteristics | Source |
| Streptococcus pyogenes | ||
 RDN29 RDN29 | M1 type | ATCC 700294 |
 RDN02 RDN02 | M19 type | Lab strain collection |
 EC478 EC478 | Isogenic luxS-deficient mutant of RDN29 | This study |
 RDN306 RDN306 | Isogenic luxS-deficient mutant of RDN02 | This study |
 EC460 EC460 | RDN29 (pEC82) | This study |
 EC489 EC489 | EC478 (pEC82) | This study |
 EC490 EC490 | EC478 (pEC83) | This study |
 EC480 EC480 | RDN02 (pEC82) | This study |
 EC481 EC481 | RDN306 (pEC82) | This study |
 EC482 EC482 | RDN306 (pEC83) | This study |
| Escherichia coli | ||
 DH5α DH5α | Host for cloning; AI-2 deficient (contains a frame-shift mutation in luxS) | Lab strain collection |
 TOP10 TOP10 | Host for cloning | Invitrogen |
 EC467 EC467 | DH5α (pEC82) | This study |
 EC464 EC464 | DH5α (pEC83) | This study |
| Vibrio harveyi | ||
 BB170 BB170 | AI-1 sensor deficient, AI-2 sensor positive | [9] |
| Plasmids | ||
 pAT21 pAT21 | Apr Kmr; pBR322Ω 1.5-kb pJH1 ClaI (aphIII) | [61] |
 pUC19 pUC19 | ColE1ori, Ampr, lacZ | New England Biolabs |
 pEC131 pEC131 | pUC19ΩluxSup-aphIII-luxSdown | This study |
 pEC82 pEC82 | repDEG-pAMβ1, ermAM/B, ColE1ori | This study |
 pEC83 pEC83 | pEC82ΩPluxS-luxS-TT | This study |
Temporal luxS expression and production of AI-2 like activity
First, we investigated luxS expression at the transcriptional level. Northern blot analysis of RNA extracts from wild-type strains revealed a monocistronic luxS-specific transcript of approximately 650 bases in size (Fig. (Fig.1A).1A). In both strains, the luxS transcript levels were abundant from lag to early-logarithmic phase, after which gradual decrease of the signal intensity towards stationary phase was observed (Fig. (Fig.1A).1A). Primer extension analysis further identified the transcription initiation site (+1) as a guanine located 20 nucleotides upstream of the translation start codon (ATG) (Fig. (Fig.1B).1B). Then, we investigated whether the growth phase-dependent expression of luxS transcripts correlated with production of AI-2 like activity. Analysis of conditioned media (CM) from the luxS-deficient mutants demonstrated a loss of AI-2 like activity production in a bioluminescence reporter assay described earlier [9]. In contrast, CM from the wild-type parental strains induced luminescence in the AI-1 sensor defective reporter V. harveyi strain BB170 with AI-2 like activity peaking at late-logarithmic phase (Fig. 2A, B). CM prepared from the complemented luxS-deficient strains restored luminescence production in the reporter V. harveyi strain to a level comparable to that of wild-type CM (Fig. (Fig.2A).2A). Furthermore, we showed that the S. pyogenes luxS gene complemented successfully the lack of AI-2 production in E. coli DH5-α (data not shown), thus confirming that the luxS homologue in both M1 and M19 strains is required for production of AI-2 like activity. We then addressed the question whether CM with AI-2 like activity has an auto-regulatory effect on luxS expression in S. pyogenes. CM from wild-type and luxS-deficient cultures grown to different phases was added to early-logarithmic wild-type cultures. No significant differences in the intensity of the luxS transcript signal were observed by Northern blot analysis when comparing induction with CM from both wild-type and mutant strains and control media (data not shown). Taken together, our data demonstrate that luxS transcription and production of AI-2 like activity are shifted out of phase. AI-2 like activity peaked at a time point – late-logarithmic phase – when luxS transcript levels rapidly decreased. Importantly, an auto-regulatory function of AI-2 like activity containing CM on luxS expression could not be observed in GAS.
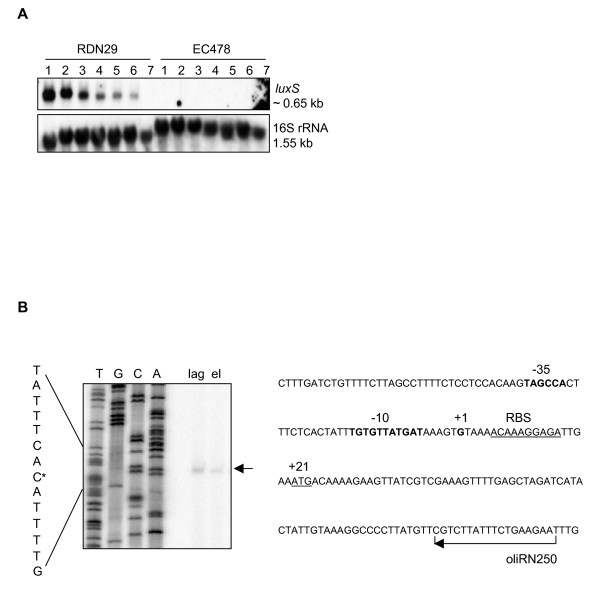
Analysis of the luxS transcript in S. pyogenes. (A) Temporal analysis of luxS expression. Northern blot analysis of total RNA isolated from RDN29 (wild-type M1 strain) and EC478 (isogenic luxS-deficient mutant) cultures at (1) lag, (2) early-logarithmic, (3) mid-logarithmic, (4) mid-late-logarithmic, (5) late-logarithmic, (6) early-stationary and (7) stationary (O/N) phase of growth. Blots were hybridized with probes specific to luxS and rRNA16S (acting as loading control). The estimated sizes of transcripts are indicated. The blots shown are representative of four independent experiments. Identical luxS expression profiles were observed in RDN02 (wild-type M19 strain) and RDN306 (isogenic luxS-deficient mutant). (B) Determination of the transcriptional start site of luxS. Left: Primer extension analysis using total RNA isolated from RDN29 wild-type cultures at lag (lag) and early-logarithmic (el) phase and the γ-32P-radiolabelled reverse primer oliRN250. As a reference ladder, a DNA sequencing reaction using plasmid pEC83 as template and reverse primer oliRN250 is shown (lanes TGCA). The complementary base (C*) of the transcriptional start site (G), located 20 nucleotides upstream of the translational start codon (ATG), is indicated. The picture shown is representative of three independent experiments. Right: Sequence of the 5' part of the luxS locus. The putative -35 and -10 promoter regions and +1 transcriptional start site are represented in bold. The putative ribosomal binding site (RBS) and translational start codon (ATG) are underlined. The arrow indicates the direction of primer oliRN250, which was used for primer extension. An inverted repeat sequence located 97 bp downstream of the translation stop codon could serve as a rho-independent transcriptional terminator (not shown).
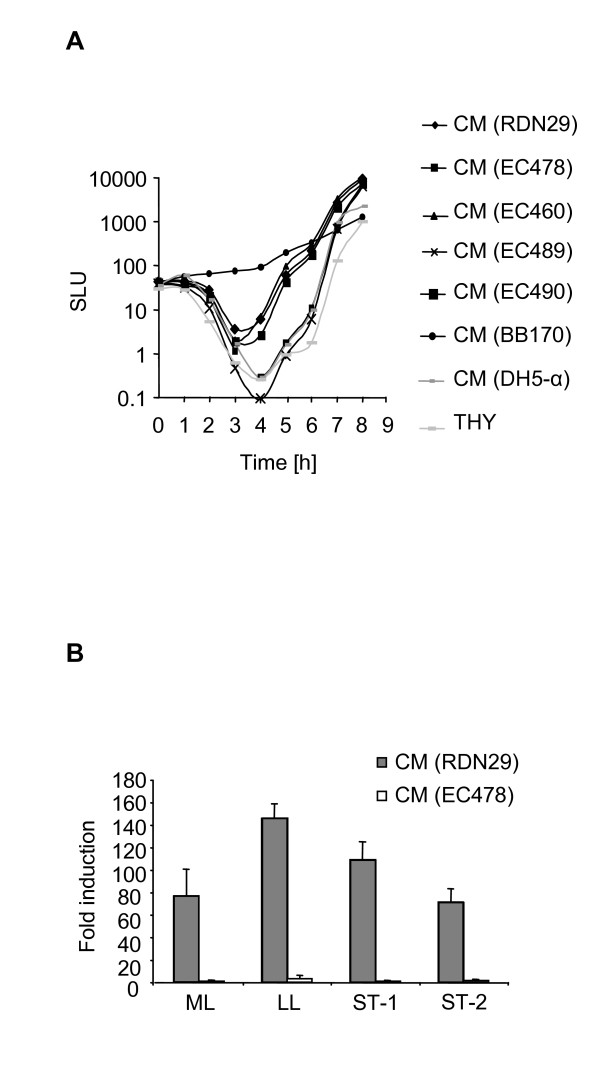
Induction of luminescence in V. harveyi by CM from S. pyogenes. (A) Induction of luminescence in V. harveyi reporter strain BB170 by CM of wild-type and luxS-deficient mutants. RDN29: wild-type M1 strain, EC460: RDN29 (pEC82), EC478: isogenic luxS-deficient mutant, EC489: EC478 (pEC82), EC490: EC478 complemented with pEC83. THY medium and CM of DH5-α (AI-2 deficient) were used as negative controls; CM of BB170 was used as positive control. CM were prepared from cultures at late-logarithmic phase of growth. The luminescence values expressed in specific light units (SLU = RLU (relative light units)/OD620 nm) (y axis) are plotted versus time (h) (x axis). Data shown are representative of three independent experiments. Similar data were obtained with CM of the RDN02 (M19 serotype) derivative strains. (B) Fold induction of V. harveyi BB170 luminescence by CM of RDN29 and EC478. CM was prepared from cultures at mid-logarithmic (ML), late-logarithmic (LL), early-stationary (ST-1) and stationary phase (ST-2) of growth. Fold induction corresponds to the SLU values obtained with the CM of the analyzed strains versus those obtained with the CM of the negative control THY. Fold induction was calculated after ~4 h of induction when the cell density begins to rise. Induction of luminescence with late-logarithmic CM from the luxS-deficient mutant was ~60 fold lower compared to CM from the parent wild-type strain. The average values and standard deviations of three independent experiments are shown. Similar data were obtained with CM of the RDN02 derivative strains.
Role of luxS in growth and metabolism
Growth rates and yields, colony and chain morphology of wild-type and luxS-deficient strains in complex THY medium, THY medium supplemented with 10% fetal bovine serum, C-medium and CDM were similar (data not shown). In addition, wild-type and luxS-deficient strains did not significantly differ in their ability to form primary adhesion to uncoated polystyrene surfaces (initial step in biofilm formation) when grown in THY or CDM (data not shown). To investigate whether inactivation of luxS and therefore disruption of the AMC would lead to a metabolic burden that might influence growth or fitness, we analyzed the growth rates of wild-type and luxS-deficient strains in sulfur-limited CDM (CDM-S1 and CDM-S2). Although overall growth rates and yields were significantly reduced in the restricted media compared to those in CDM, they were similar when wild-type strains were compared to the luxS-deficient mutants (data not shown). These results indicate that luxS does not have an essential AMC-related metabolic role in GAS.
Role of luxS in adaptation to host-induced stress conditions. Further, we investigated whether luxS is involved in the adaptation of S. pyogenes to stress conditions that can be encountered during infection. Oxidative, acid and salt stresses, which have been shown to interfere with virulence factor expression in GAS [28-30], were studied. Challenging wild-type and luxS-deficient strains with hydrogen peroxide (4 mM) and high salt concentrations (150 and 650 mM NaCl) did not induce any significant change in survival rate (data not shown). However, exposure of bacterial cultures to acidic conditions (pH 4) for 2, 4 and 6 h revealed a profound increase in acid tolerance in the luxS-deficient strains (Fig. (Fig.3A).3A). Complementing the luxS-deficient strains with luxS in trans resulted in restoration of acid sensitivity to a wild-type level (Fig. (Fig.3A,3A, shown only for the 6 h time point), clearly linking luxS to the observed phenotype. Importantly, we could show that exposing S. pyogenes cultures to low pH led to reduced expression of luxS (Fig. (Fig.3B),3B), thus suggesting a correlation between reduced luxS expression and survival under acidic conditions. Accordingly, CM from these cultures showed reduced AI-2 like activity (Fig. (Fig.3C3C).
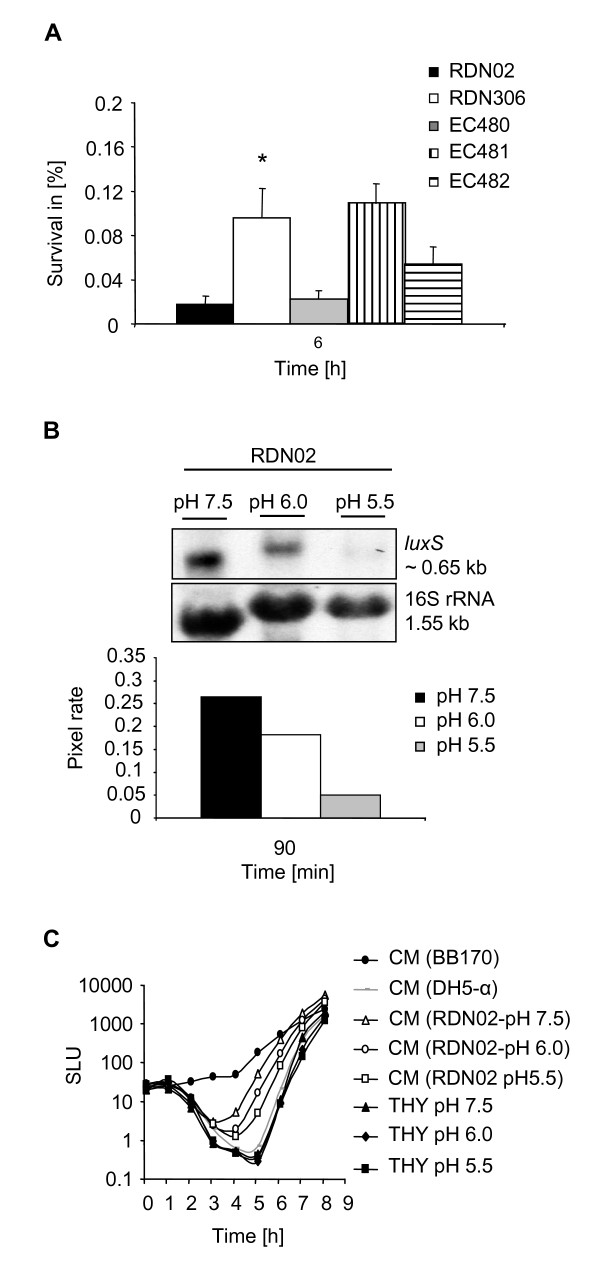
(A) Acid tolerance assay. The ability of RDN02 (wild-type M19 strain), RDN306 (isogenic luxS-deficient mutant), EC480 (RDN02 (pEC82)), EC481 (RDN306 (pEC82)) and EC482 (RDN306 (pEC83)) to survive an acid challenge (pH 4) is shown as survival in percent (y axis) at the 6 h time point. The average % survival values and standard deviations of three independent experiments are shown. The asterisk indicates that the difference in survival rates between the luxS-deficient mutant and the wild-type strain was statistically significant (P < 0.05). Similar data were obtained with the RDN29 (M1 type) derivative strains. (B) Effect of low pH on luxS expression. Northern blot analysis was performed with total RNA isolated from RDN02 cultures grown to early-logarithmic phase and then incubated for 90 min with THY medium at pH 5.5, 6.0 and 7.5. Blots were hybridized with probes specific to luxS and rRNA16S (acting as loading control). The estimated sizes of transcripts are indicated. Pixel rates of signals (pixel counts obtained with the luxS probe versus pixel counts obtained with the rRNA16S probe) are shown. The blot shown is representative of three independent experiments. Similar data were obtained with the RDN29 strain. (C) Induction of luminescence in V. harveyi reporter strain BB170 by CM of RDN02. CM was prepared from RDN02 cultures grown to early-logarithmic phase and then incubated for 90 min with THY medium at pH 5.5 (RDN02-pH 5.5), 6.0 (RDN02-pH 6.0) and 7.5 (RDN02-pH 7.5) for 90 min. THY medium at pH 7.5, 6.0 and 5.5 and CM of DH5-α (AI-2 deficient) were used as negative controls; CM of BB170 was used as positive control. The luminescence values expressed in specific light units (SLU = RLU (relative light units)/OD620 nm) (y axis) are plotted versus time (h) (x axis). Data shown are representative of three independent experiments. Similar data were obtained with CM of the RDN29 derivative strains.
Then, we analyzed the role of the luxS/AI-2 system in adaptation to an environment that resembles in vivo conditions by using RPMI medium (used for culturing eukaryotic cells) and 10% serum. We observed reduced luxS expression at the transcriptional level in wild-type cultures grown in RPMI medium compared to cultures grown in the nutrient rich THY medium (Fig. (Fig.4A).4A). Furthermore, AI-2 activity in CM from wild-type cultures grown in RPMI was reduced to a level similar to CM from luxS-deficient cultures (Fig. (Fig.4B).4B). During growth in RPMI, due to the presence of buffer salts and cultivation in the presence of CO2, no acidification occurs. Therefore, under this growth condition, regulation of luxS expression cannot be attributed to low pH. Since low concentration of iron in the RPMI medium could be responsible for the observed phenotype, we supplemented RPMI medium with iron and depleted THY medium from iron. In both cases, no change in luxS expression could be observed (data not shown). Our data thus indicate that under specific conditions encountered in the host (acidic conditions and in vivo-like environment), there is an advantage for GAS to lower the expression of luxS and the production of AI-2 like activity.
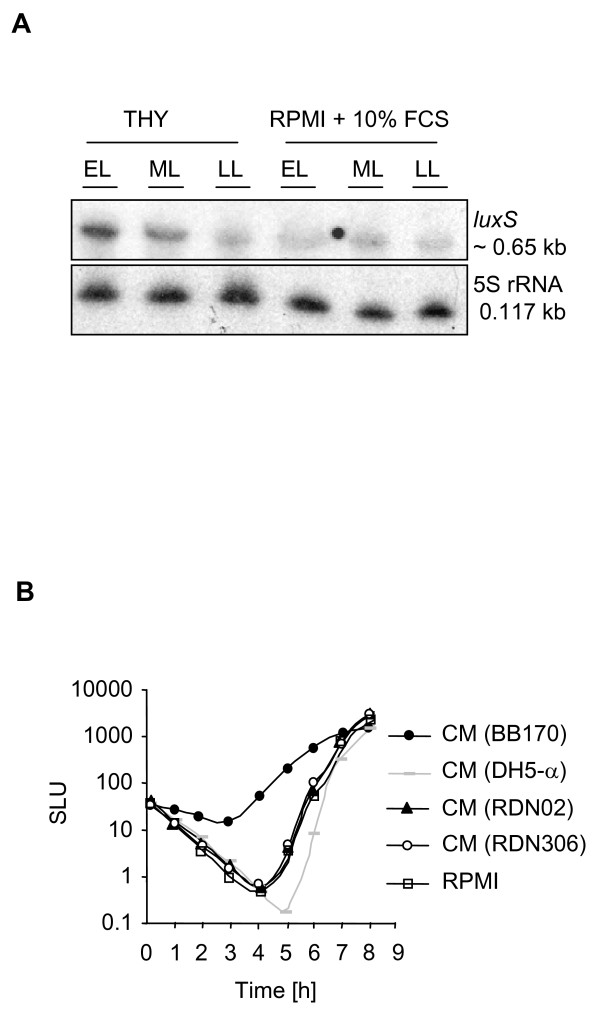
(A) Analysis of luxS expression in RPMI medium. Northern blot analysis of total RNA isolated from RDN02 (wild-type M19 strain) cultures at early-logarithmic (EL), mid-logarithmic (ML) and late-logarithmic (LL) phase of growth. Blots were hybridized with probes specific to luxS with 5S rRNA as loading control. The estimated sizes of transcripts are indicated. The blots shown are representative of three independent experiments. Identical luxS expression profiles were observed with RDN29 (wild-type M1 strain). (B) Induction of luminescence in V. harveyi reporter strain BB170 by CM of S. pyogenes. RDN02: M19 wild-type, RDN306: isogenic luxS-deficient mutant. RPMI medium and CM of DH5-α (AI-2 deficient) were used as negative controls; CM of BB170 was used as positive control. CM was prepared from cultures at late-logarithmic phase of growth in RPMI medium. The luminescence values expressed in specific light units (SLU = RLU (relative light units)/OD620 nm) (y axis) are plotted versus time (h) (x axis). Data shown are representative of three independent experiments. Similar data were obtained with CM of derivative strains of the RDN29 background.
Role of luxS in internalization and survival in human epithelial cells and macrophages. These results prompted us to determine whether a lack of luxS expression gives a beneficial advantage for GAS to survive in an intracellular environment. Interactions of wild-type and luxS-deficient strains of both serotypes M1 and M19 with monolayers of human pharyngeal epithelial cells were analyzed. No differences in the number of bacteria adhering to the epithelial cells (30 min, 1 h and 2 h after infection) were observed when comparing wild-type and mutant strains (data not shown). Using an antibiotic protection assay, the internalization and survival rates of the luxS mutant strains of both M1 and M19 serotypes were significantly higher (about 5-fold) at 2 h and 5 h after the addition of antibiotics compared to those of wild-type strains (Fig. (Fig.5A).5A). Analyzing the number of bacteria that survived intracellularly in macrophages led to the same results. The luxS deficient mutants showed significantly higher internalization and survival rates (about 2.5-fold) in macrophages at 0 h, 1 h and 2 h after the addition of antibiotics compared to the wild-type parents (Fig. (Fig.5B).5B). With both serotypes and in both epithelial and macrophage cells, complementing the mutant strains with the luxS allele in trans restored the intracellular survival rates to almost wild-type levels. Since the role of luxS in the AMC-related metabolic pathway seems limited, we analyzed the possibility of a signalling function of the luxS/AI-2 system in GAS survival in host cells. Antibiotic protection assays were performed with mixed cultures of wild-type and luxS-deficient cells (ratio 1:1) comparing with non-mixed wild-type and luxS-deficient cultures. The survival rates of the mixed culture in both eukaryotic cell types were almost identical to those of the wild-type strain (data not shown), thus suggesting a potential complementation effect of the luxS-deficient cells by the wild-type parent cells possibly by AI-2 production and signaling. In summary, the data indicate that lowering the expression of luxS provides GAS an advantage to survive the harsh conditions encountered in host cells.
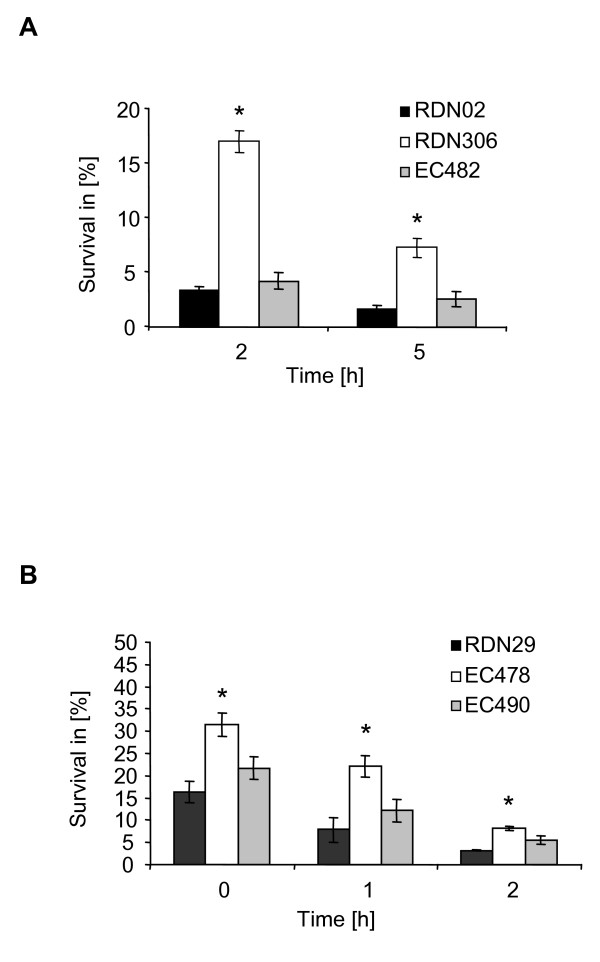
Internalization assays of S. pyogenes in epithelial cells and macrophages. RDN29 (wild-type strain), EC478 (isogenic luxS-deficient mutant) and EC490 (RDN29 (pEC85)); RDN02 (wild-type strain), RDN306 (isogenic luxS-deficient mutant) and EC482 (RDN29 (pEC85)). Bacterial cultures were used to infect (A) HEp-2 human pharyngeal epithelial cells and (B) RAW 264.7 macrophages in an antibiotic protection assay. For this, bacterial cultures were grown to mid-logarithmic (RAW 264.7) and late-logarithmic (HEp-2) phases, growth time points showing optimal internalization rates in RAW 264.7 and HEp-2, respectively. The percent intracellular invasion represents the average CFU/ml number of viable intracellular bacteria recovered at different times following addition of antibiotics versus the average CFU/ml number of inoculated bacteria × 100. The average values and standard deviations of three independent assays are shown. The asterisks indicate that the differences in survival rates between the luxS-deficient mutants and the wild-type strains were statistically significant (P < 0.01 for Hep-2 epithelial cells, P < 0.05 for RAW 264.7 macrophages). Similar data were obtained for both M1 and M19 serotypes.
Virulence gene expression in luxS mutants
We also studied whether luxS had regulatory effects on pathogenicity functions other than those already described (e.g. effect on cysteine protease, SLS and hyaluronic acid production) [26,27]. Expression of a number of genes encoding virulence factors and their regulators [16,21,28,31-36] was then studied by Northern blot analysis throughout the entire growth phase (Table (Table2).2). A consistent decrease in the fasX (encoding the effector molecule of the fasBCA regulatory system [21]) transcript and a consistent enhancement of the sibA (psp) (encoding a secreted immunoglobulin binding protein [32]) transcript were detected in the luxS-deficient RDN02 (M19) strain compared to the wild-type parent, however not in the M1 background (Fig. 6A, B). This finding does not only add fasX and sibA to the list of genes being influenced by luxS but underlines the fact that regulatory pathways linking the luxS/AI-2 pathway to virulence-associated gene expression can differ among different GAS strains or serotypes.
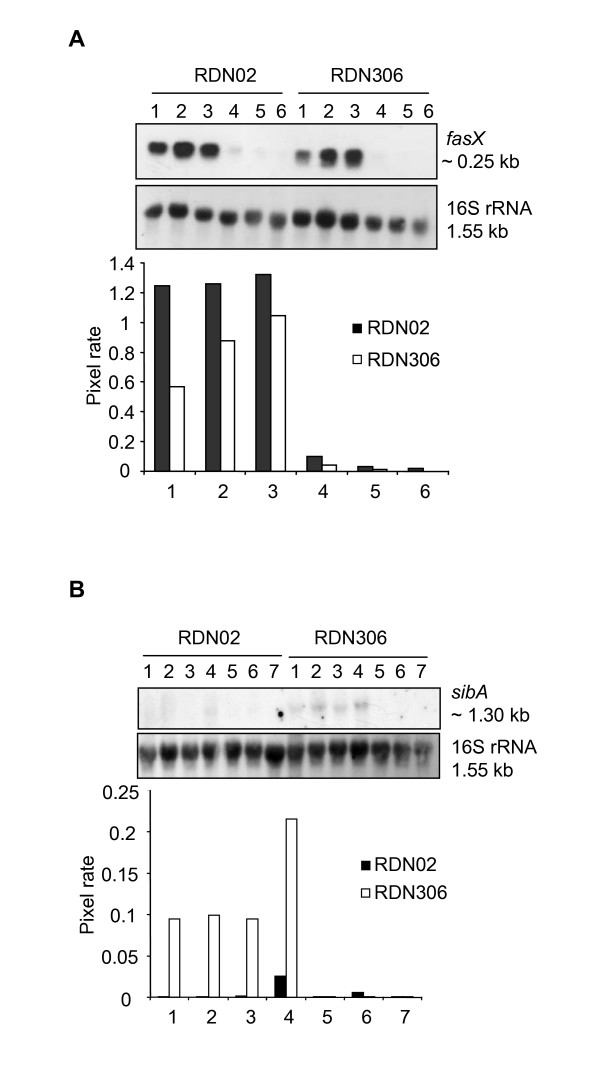
Transcriptional expression of virulence-associated genes in RDN02 (wild-type M19 strain) and RDN306 (isogenic luxS-deficient mutant). Northern blot analysis was performed with total RNA isolated from strains grown to (1) lag, (2) early-logarithmic, (3) mid-logarithmic, (4) mid-late-logarithmic, (5) late-logarithmic, (6) early-stationary and (7) stationary phase (O/N). Blots were hybridized with probes specific to fasX (A) and sibA (psp) (B) with a probe specific to 16S rRNA as loading control. The estimated sizes of transcripts are indicated. Pixel rates of signals (pixel counts obtained with probe of interest versus pixel counts obtained with the 16S rRNA probe) are shown for the different time points during growth. The blots shown are representative of three independent experiments.
Table 2
Oligonucleotides used in this study
| Oligo | aF/R | Sequence 5'-3' | bSpecificity |
| oliRN110 | F | CGCTGCGTAAAAGATACG | aphIII |
| oliRN111 | R | AATGGAGTGTCTTCTTCC | |
| oliRN130 | F | TTGAAATGACAAAAGAAGTT | luxS |
| oliRN129 | R | AAAGAGGCTATGATCCTTAT | |
| OLEC287OLEC288 | F | AGTTAAGTGACGATAGCCTAG | 5S rRNA |
| R | CTAAGCGACTACCTTATCTCA | ||
| oliRN242 | F | CGGTAACTAACCAGAAAGGG | 16S rRNA |
| oliRN243 | R | CGTTGTACCAACCATTGTAGC | |
| Virulence genes | |||
| OLEC137 | F | GACCTACTCAGGCAAATC | fbp54 |
| OLEC138 | R | CTTGTCTAAGGCAATCTC | |
| oliRN204oliRN203 | F | TGGTAAAAATGCCTATACCC | isp2 |
| R | GACCTCGTGAAAACTTAGCC | ||
| oliRN248 | F | AGGAATAAATTGGTCCTCTT | nga |
| oliRN249 | R | GGCAGTTTCAATAAACTCAT | |
| OLEC268 | F | AAGAGATCGAAGAAAGTCTT | scl |
| OLEC269 | R | TTTGGTTAGCTTCTTTGTCA | |
| oliRN202oliRN201 | F | AGTTAAGGCACAAGAACAGG | sibA (psp) |
| oliRN246 | R | TACTTGAGGCACTTTGAACC | |
| oliRN247 | F | ACTAGGAGCTACACAACCAG | sic |
| oliRN263 | R | TACCCTGTACCTAATGCTTC | |
| oliRN264 | F | ACATCTCAAGAATTACTAGC | ska |
| oliRN279 | R | TTGTTGTAGAGTAGTTTAGC | |
| oliRN50 | F | ATGGAAAATATTCTGATATCTTAG | slo |
| oliRN47 | R | CCCAAAGGATTTCATATTGAGC | |
| F | CATAATTACAGTCACTGATT | speC | |
| oliRN48 | R | ATCGAAATGACTAAAGTTCTTCAT | |
| oliRN55 | F | TTTTCAATGGTAGCTCTTG | speF (mf) |
| oliRN56 | R | TTCTTGAGCTCTTTGTTCG | |
| Stand-alone regulators | |||
| OLEC274 | F | ATAGCCTAGAAACAGAATTA | codY |
| OLEC275 | R | TTCTGAATAAGAAAGCGTAT | |
| oliRN170 | F | TAAGTAAGTTGTTTACAAGTCAAC AGTGGAG | mga |
| oliRN136 | R | AAAGGCGTAGATCAATTGG | |
| OLEC337 | F | ATGACGCCTAATAAAGAAGA | mtsR |
| OLEC338 | R | CTGTGACATAAAGTTGCTTA | |
| OLEC315 | F | TGGACATTCATTCACATCAG | perR (spf) |
| OLEC316 | R | ATCAGGTTGGTCTTTTGCTT | |
| oliRN73 | F | AAATACTTGGAATCATCAATCG | rofA |
| oliRN74 | R | TTTTCTTGAGCTAATGCAACCG | |
| OLEC278 | F | TAGACAACTTGAATGTCAAT | ropB |
| OLEC279 | R | AAGCTTTATCATACTCTTGT | |
| OLEC276 | F | ACATTTTGCAACGGTATATT | srv |
| OLEC277 | R | AATAGGGTCATTAAGTCATA | |
| Two-component systems | |||
| oliRN175 | F | CTGCTTGACTTAATGTTACC | covR (CovRS) |
| oliRN176 | R | TTTGACAATAATTCTTCACG | |
| OLEC182 | F | GAGCAATAACATTTTAGG | fasX (effector molecule, FasBCA) |
| OLEC183 | R | TTACAATCAGCTGATGTG | |
| OLEC270 | F | ATTCGAAAGACATCAGATGT | Irr (Ihk/Irr) |
| OLEC271 | R | CATATGTCATCCATCAATTG | |
| OLEC339 | F | GATATCATGATGCCGATTAA | sptR (SptRS) |
| OLEC340 | R | TAGCTAGTATCTTTATCACC | |
| OLEC280 | F | TACTTATTGTGGATGATGAA | vicR (VicRK) |
| OLEC281 | R | AATCATATCCCCAAACAATT | |
| oliRN23 | F | AATTGTGAATTCTATCATAATTGTGG (EcoRI) | Cassette aphIII for luxS- deficient mutants |
| oliRN24 | R | TAAATCAAGCTTCTAAAACAATTCATCCAG (HindIII) | |
| oliRN20 | F | ACTAGTGAATTCTCAAACATAACAATCC (EcoRI) | Fragment luxSdown for luxS- deficient mutants |
| oliRN19 | R | TTCTGAGGATCCCCACCATCCAGCC (BamHI) | |
| oliRN22 | F | AATCAAGCATGCTTACTTGGAAAAGAACCCAACC (SphI) | Fragment luxSup for luxS- deficient mutants |
| oliRN21 | R | AAGTGGAAGCTTGTGGAGGAGAAAAGGC (HindIII) | |
| oliRN267 | F | CATCGCTGCGCCTCTTGCTAC | Confirmation of the luxS- deficient mutants |
| oliRN268 | R | GGGTCATTCCAGAACCACAGA | |
| oliRN70 | F | GCTATTTTTTGACTTACTGGG | |
| oliRN69 | R | TCCGTATCTTTTACGCAGCGG | |
| oliRN205 | F | GTCAATGGATCCCCAGCTCTATTGCACC (BamHI) | PluxS-luxS-TT insert for luxS complementation plasmid pEC83 |
| oliRN280 | R | TATCTAGAGCTCTAGATTACTGAGAAAATC (SacI) | |
| oliRN250 | R | ATTCTTCAGAAATAAGACG | Primer extension |
a F, forward; R, reverse. Underlined sequences indicate restriction sites.
b fbp54 (fibronectin-binding protein), isp2 (immunogenic secreted protein homologue), nga (NAD-glycohydrolase), scl (streptococcal collagen-like protein), sibA (secreted immunoglobulin binding protein), sic (streptococcal inhibitor of complement), ska (streptokinase), slo (streptolysin O), speC (streptococcal pyrogenic exotoxin C), speF (mf) (mitogenic factor).
Discussion
Among the quorum sensing systems described to date, the luxS/AI-2 pathway has been shown to be involved in the regulation of virulence in a number of gram-negative and gram-positive bacteria [1,5,11,12]. In this study, we analyzed the expression of the streptococcal luxS/AI-2 system, its possible role in the AMC metabolic pathway and its function in adaptation to diverse host-induced stress conditions in two GAS clinical isolates of serotypes M1 and M19.
Expression analysis of luxS indicated that the luxS transcript is monocistronic (644 bases) in GAS serotypes M1 and M19. In a previous report, which was based on sequence analysis, the luxS transcript in GAS (M6 strain) was predicted to be polycistronic as a distal member of the fatty acid metabolism operon [26]. In our study, Northern blot analysis of luxS expression did not reveal any additional transcript of higher size. Furthermore, we showed that the monocistronic transcript is expressed in a growth phase-dependent manner, a finding already reported for bacterial species like Streptococcus bovis, whereas transcription of luxS in species like Salmonella typhimurium and Vibrio fischeri has been shown to be constitutive [37-39]. Accordingly, production of AI-2 like activity occurred in a temporal fashion in the serotypes analyzed, however, shifted out of phase compared to luxS expression. AI-2 like activity peaked at late-logarithmic phase when the luxS transcript had already declined being just above the detectable expression level. We also investigated the possibility of an auto-regulation mechanism of luxS expression in GAS. Induction experiments by Northern blot analysis failed to show a regulation of luxS expression by AI-2 dependent CM. These findings are in accordance with previous work in S. typhimurium, where AI-2 production and luxS transcription were not in phase either, also suggesting that luxS expression was not regulated by AI-2 [38].
Despite intensive research on luxS and AI-2 during the last years, a clear separation of the possible metabolic function of AI-2 from its possible signaling activity could not be achieved. A recent comparative genomic and phylogenetic analysis of synthesis and signal transduction pathways showed that S. pyogenes contains the two-step enzymatic pathway catalyzed by the Pfs and LuxS enzymes to detoxify SAH leading to AI-2 production [13,40]. Interestingly, in S. pyogenes the AMC is incomplete as it lacks the homocysteine methyltransferases (MetE or MetH) that convert the SAH-derived metabolic product homocysteine to methionine. Nevertheless, S. pyogenes encodes the SAM synthetase ortholog (MetK) that permits conversion of methionine to SAM [40]. Therefore, either GAS cannot recycle homocysteine to methionine (as it has been described in Borrelia burgdorferi [41]) or recycling occurs through other types of methyltransferases. In the former case of a de novo methionine synthesis defect, bacterial growth would exclusively depend on sulfur provided from external sources. In our study, decreasing media concentrations of cysteine and methionine reduced to a similar extent bacterial growth and yields in both wild-type and luxS-deficient strains. These data indicate that GAS, as a poly-auxotrophic organism, is unable to recycle homocysteine to methionine. Moreover, we showed that inactivation of luxS does not lead to a metabolic burden that would influence growth or fitness. Although AI-2 can certainly be considered as a metabolite, the viability and lack of growth defects of the investigated luxS-deficient mutants in various media including minimal media depleted of sulfur sources argues against an essential role of luxS in AMC-related metabolism.
The previously described regulatory role of luxS in the production of virulence factors in GAS [26,27] prompted us to investigate the role of the luxS/AI-2 system in the adaptation to host-induced stress conditions. During an on-going infection, bacteria often have to face challenging conditions in particular niches of the host, including changes in pH, and therefore are forced to develop quickly an adaptive response, which requires fine-tuning of pathogenicity gene expression. Although we could not identify a possible involvement of the GAS luxS system in adaptation to oxidative and salt stress, we showed an increased survival of S. pyogenes under acidic conditions when the luxS/AI-2 system was down-regulated. This is in accordance with luxS expression and AI-2 production being significantly lowered when GAS cells were grown in low pH conditions. Interestingly, previous studies in S. mutans showed that acid sensitivity was enhanced in luxS deficient mutants [42,43] and that luxS expression was increased at low pH [44,45]. These reverse effects of luxS on tolerance to acidic conditions in S. pyogenes and S. mutans need to be considered in regard to their different living habitats and pathogenesis. Thus, our data reveal a link between the S. pyogenes luxS/AI-2 system and pathways involved in adaptation of the organism to stress [46,47]. In addition, we also observed a reduction of luxS expression and AI-2 like activity in GAS cells grown in serum enriched RPMI, a medium with a composition similar to that of human plasma. In a previous study, Marouni et al. reported that in an M6 serotype, the survival of a luxS-deficient mutant in epithelial cells at 4 h after infection was higher compared to the wild-type parent [27]. Here we analyzed further the role of luxS in interaction of S. pyogenes with host cells. No differences in adhesion rates to human pharyngeal epithelial cells were observed when comparing wild-type and luxS-deficient mutants in both M1 and M19 serotypes. However, the luxS-deficient mutants had a significant advantage to survive intracellularly in both epithelial cells (over a period of 7 h after infection) and macrophages (over a period of 3 h after infection). Taken together, we show that low level of luxS and AI-2 expression seems to provide a competitive advantage for GAS survival under specific conditions encountered during infection.
With luxS being linked to stress and adaptation to the host, we were interested in investigating additional effects of luxS on virulence factor expression in GAS. In an M6 serotype, luxS was shown to regulate streptolysin S (SLS) expression at the transcriptional level and SpeB cysteine protease activity [26]. In an M3 serotype, luxS was reported to regulate expression of SpeB and M protein at the transcriptional level and hyaluronic acid capsule at the post-transcriptional level [27]. In our report, we show that luxS had a positive effect on fasX expression and a negative effect on sibA (psp) expression at the transcriptional level. fasX is a small RNA molecule, effector of the fasBCA operon, which has regulatory functions on virulence factor expression. fasX was also suggested to be involved in local tissue destruction and general bacterial aggressiveness towards host cells [21,48]. sibA (psp) is a virulence gene encoding a secreted immunoglobulin binding protein [32]. Remarkably, the above described regulatory effects were only observed in the M19 strain, thus suggesting a strain or serotype dependent effect of luxS on the expression of these two targets. The strain- or serotype-dependent effect of luxS observed in this study emphasizes differences in regulatory pathways among different GAS isolates. Along these lines, previous reports demonstrated that mutations in GAS regulators might alter disease progression [49,50]. This is well illustrated with the two-component regulatory system CovRS where mutations in the sensor encoding gene covS have been shown to correlate with human disease severity [51,52]. Mutations in this gene can occur under selective pressure encountered in the host and can generate hypervirulent GAS variants with increased risk of systemic dissemination [51,52]. In the case of luxS, strain specificity manifestation of virulence-associated gene regulation by luxS has been reported previously in Neisseria meningitidis and Serratia marcescens [53,54]. Additionally, Marouni et al. attributed the dissimilar results of the effect of luxS on bacterial growth and the level of SpeB regulation in GAS to a possible strain-specific effect [27]. The finding that luxS can affect the expression of fasX RNA also provides an additional evidence for the notion that small RNAs have the ability to integrate cell density signals together with other environmental stimuli to affect gene expression [55].
Conclusion
Our data together with previous reports suggest a complex role of the luxS/AI-2 system in S. pyogenes. Here, we showed that expression of both luxS and AI-2 occurs in a temporal fashion but AI-2 like activity does not seem to have a regulatory effect on luxS expression. Analysis of the role of luxS in metabolism demonstrated a limited role of luxS in the AMC-related metabolism. However, studying the possible function of the luxS/AI-2 system in adaptation to stress revealed that a down-regulation of luxS expression provides an advantage for S. pyogenes to tolerate acidic conditions and grow in a host environment-mimicking medium. Accordingly, there was an increased ability of the luxS-deficient mutants to survive intracellularly in both epithelial cells and macrophages. Altogether, our data suggest an important function for the luxS/AI-2 system in survival and growth of GAS under conditions that are relevant during infections. Based on the data outlined in this article, it is tempting to speculate that in GAS, luxS is not exclusively a key part of a detoxification pathway but rather modulation of luxS expression levels would allow adjusting bacterial fitness in response to changing host conditions. Finally, our study revealed two novel virulence-associated targets of luxS in S. pyogenes: the regulator fasX RNA and the virulence gene sibA.
Methods
Bacterial strains and growth conditions
Bacterial strains and plasmids used in this study are listed in Table Table1.1. S. pyogenes was routinely cultured in Todd Hewitt Broth (THB, Bacto, Becton Dickinson) supplemented with 0.2% yeast extract (Oxoid) (THY) without agitation or on trypticase soy agar supplemented with 3% sheep blood (TSA, BBL, Becton Dickinson). C-medium (peptide rich and carbohydrate poor), THY or RPMI medium supplemented with 10% foetal bovine serum (Gibco), metal ion-restricted medium, chemically defined medium (CDM) and sulfur-restricted CDM (CDM-S) were used in specific experiments [56,57]. For the eukaryotic-like environment, RPMI 1640 (PAA) was supplemented with 10% foetal bovine serum (Gibco) or 10% foetal bovine serum and 50 μM FeCl3. For metal-ion restricted medium, THY was treated overnight with 30 g of chelating resin Chelex 100 (Sigma)/liter, supplemented with 1 mM MgCl2 or 1 mM MgCl2and 1 mM FeCl3. Sulfur-restricted CDM (CDM-S) consisted of CDM with sulfur containing salts replaced with their chloride equivalents [58]. CDM contained L-methionine (670 μM), L-cysteine (4,125 μM) and L-cystine (208 μM). In sulfur-limiting conditions, L-cystine was eliminated and only L-cysteine (10 μM or 350 μM) and L-methionine (1 μM or 100 μM) were provided as sulfur source. All S. pyogenes cultures were incubated at 37°C in an atmosphere supplemented with 5% CO2. Escherichia coli was grown aerobically at 37°C in Luria-Bertani (LB) medium either in liquid with shaking or on agar plates. V. harveyi was cultured aerobically at 28°C in an Autoinducer Bioassay (AB) medium [9]. Transformation of E. coli and S. pyogenes was performed as previously described [59,60]. Whenever required, suitable antibiotics were added to the medium to the following final concentrations: erythromycin 300 μg/ml for E. coli and 3 μg/ml for S. pyogenes; kanamycin 25 μg/ml for E. coli and 300 μg/ml for S. pyogenes. Bacterial cell growth was monitored periodically by measuring the optical density of culture aliquots at 620 nm using a microplate reader (SLT Spectra Reader).
DNA manipulations
DNA manipulations including DNA preparation, amplification, digestion, ligation, purification, agarose gel electrophoresis and Southern blot analysis were performed according to standard techniques [60]. Synthetic oligonucleotides used as primers in this study (Table (Table2)2) were supplied by VBC-Biotech Services GmbH. Sequencing reactions were performed at VBC-Biotech Services GmbH.
Construction of the luxS-deficient mutants
Replacement of the luxS coding sequence with a kanamycin resistance cassette, aphIII [61], was performed selecting for a double cross-over event. For this purpose, a 1209 bp fragment upstream of luxS (luxS-up) and a 1107 bp fragment downstream of luxS (luxS-down) were amplified using wild-type genomic DNA as template and primers (containing flanking restriction sites) oliRN22/oliRN21 and oliRN20/oliRN19, respectively. The aphIII cassette was amplified using plasmid pAT21 as template and primers oliRN23/oliRN24. After digestion with the respective restriction enzymes, the three fragments were ligated and cloned into pUC19 (suicide vector for S. pyogenes). The resulting plasmid pEC131 was purified, linearized with the restriction enzyme ScaI (which cuts within the ampicillin resistance cassette) and used to transform electro-competent wild-type S. pyogenes. Kanamycin resistant clones were selected and the correct replacement event was checked by PCR analysis using combinations of primers oliRN267 and oliRN268 derived from flanking regions upstream and downstream of the luxS-up and luxS-down fragments and primers oliRN69 and oliRN70 specific to the aphIII cassette. Southern blot analysis was done to further confirm that the recombination events had not affected the DNA regions located upstream and downstream of luxS. The S. pyogenes mutants were grown in liquid medium without antibiotic unless otherwise specified.
Construction of plasmids for complementation studies
Plasmid pEC82 contains repDEG-pAMβ1 (the origin of replication of pAMβ1), ermAM (an erythromycin resistance gene with its own promoter and transcriptional terminator), ColE1 (a pUC19-based ColE1 origin of replication for E. coli) and an expanded MCS (multiple cloning site). To create the pEC83 luxS-complementation vector, a 1152 bp large DNA fragment (PluxS-luxS-TT) containing the luxS coding sequence, its putative promoter region and putative transcriptional terminator, was amplified from wild-type genomic DNA using primers (containing flanking restriction sites) oliRN205/oliRN280 and cloned into pEC82. Plasmids pEC82 and pEC83 were introduced in competent E. coli and S. pyogenes strains selecting for erythromycin resistant clones.
RNA analysis
Total RNA was prepared from culture samples harvested at different time points during growth and further processed for normalization and Northern blot analysis as previously described [62] with minor modifications. Specific α-32P-dATP labeled DNA probes corresponding to internal fragments of genes were created by amplification of wild-type genomic DNA using primers described in Table Table2.2. Primer extension analysis of the luxS transcript and DNA sequencing reactions were carried out according to standard protocols [60].
Phenotypical studies
Assays for bioluminescence, acid killing, hydrogen peroxide killing, salt stress and biofilm formation were performed as described previously [9,28,29,43,63] with some minor modifications. For the acid tolerance assay, mid-logarithmic phase bacterial cultures were harvested by centrifugation and washed once with 0.1 M glycine buffer (pH 7.0). Culture aliquots were removed for CFU determination and the remaining cultures were subjected to killing by incubating the cells in 0.1 M glycine buffer (pH 4) for 6 h. The number of viable bacteria was determined by plating appropriate dilutions in triplicate on TSA media. For each individual experiment, the ability of strains to survive the acid challenge was reported as survival in percent, defined by the ratio of average CFU/ml (triplicate measurements) recovered after challenge versus the average number of CFU/ml (triplicate measurements) present immediately before challenge × 100.
Preparation of conditioned medium for induction assays
Conditioned medium (CM) was prepared from bacterial cultures grown in THY buffered with 0.1 M HEPES (Sigma) (pH 7.5) to early-, mid- and late-logarithmic phase. Culture supernatants were separated from bacterial pellets by centrifugation at 9,500 × g for 30 min at 4°C, filter sterilized (0.45 μm) and stored at -20°C. For induction assays, early-logarithmic wild-type cultures were induced for 60 and 90 min with THY adjusted to pH 7.5, 6.0 and 5.0 or with CM of wild-type and luxS-deficient cultures from early-, mid- and late-logarithmic growth phase and then subjected to Northern blot analysis.
Bacterial adhesion, internalization and survival assays in host cells. HEp-2 cells and RAW 264.7 mouse macrophages were maintained in RPMI medium (HEp-2 cells) or Dulbecco's modified Eagle's medium (D-MEM, Gibco) (RAW 264.7 cells) supplemented with 10% foetal bovine serum (Gibco), penicillin (100 μg/ml) and streptomycin (100 μg/ml), and grown at 37°C in an atmosphere containing 5% CO2. Prior the infection assay, the cells were cultured overnight to semi-confluency (1.5 × 105 cells per well for HEp-2 cells and 2 × 105 cells per well for RAW264.7 cells) in 24-well tissue culture plates containing medium without antibiotic. GAS strains were grown to the same OD620 nm corresponding to mid-logarithmic (RAW 264.7) or late-logarithmic (HEp-2) phase, washed with PBS, suspended in fresh cell culture medium and added to the semi-confluent host cell monolayers at a multiplicity of infection (MOI) of 5:1 (HEp-2) or 100:1 (RAW 264.7) in triplicate. Times of incubation were 30 min, 1 h and 2 h (adhesion, HEp-2 cells), 2 h (internalization, HEp-2 cells) and 30 min (RAW 264.7 cells). For adhesion assays (HEp-2 cells), infected monolayers were washed extensively with PBS before lysing. For internalization assays (RAW 264.7 cells), extracellularly adhered bacteria were killed by incubation with fresh medium containing either 100 μg/ml gentamicin and 5 μg/ml penicillin (HEp-2 cells) or 60 μg/ml penicillin (RAW 264.7 cells). At indicated time points, the infected monolayers were washed extensively with PBS to remove the antibiotics. Cells were then lysed with chilled sterile distilled water. The number of viable intracellular bacteria released from the lysed cells was determined by plating appropriate dilutions of the lysates on TSA-blood plates in triplicates followed by 24 h incubation at 37°C. The number of bacteria that survived intracellularly was calculated using the following equation: average number of bacteria recovered (cfu/ml) per well (triplicate) at designated time point/inoculated number of bacteria (cfu/ml)) × 100. Average values were plotted.
Authors' contributions
MS, RPJ, ZAP, CH and DZ carried out the experimental work. MS, RPJ and CH participated in the design of the study. MS helped to draft and critically revised the manuscript. MS designed the figures. EC conceived the study and wrote the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We thank Dr. Bonnie Bassler (Princeton University, USA) for her generous gift of V. harveyi strains. We thank Nina Gratz and Pavel Kovarik for their help with the macrophage work. We are grateful to Dr. Eszter Nagy (Intercell AG, Vienna, Austria) for helpful discussions and critical review of the manuscript. We thank Dr. Jason Rosch (St. Jude Children's Research Hospital, Memphis, USA) for helpful discussions. This work was supported by grant No. P17238-B09 from the Austrian Science Fund (Fonds zur Förderung der wissenschaftlichen Forschung, FWF; to EC), grant No. H-1020-2004 from the University Jubilee Foundation of the City of Vienna (Hochschuljubilaümsstiftung der Stadt Wien, HJST; to EC) and grant No. 10802 from the Austrian Nationalbank (die Österreichische Nationalbank, ÖNB; to EC). RPJ and ZAP were recipients of scholarships from the Austrian Exchange Service (Der Österreichische Austauschdienst, ÖAD) and the Higher Education Commission of Pakistan (HEC), respectively.
References
- Henke JM, Bassler BL. Bacterial social engagements. Trends Cell Biol. 2004;14:648–656. [Abstract] [Google Scholar]
- Lazazzera BA. Quorum sensing and starvation: signals for entry into stationary phase. Curr Opin Microbiol. 2000;3:177–182. [Abstract] [Google Scholar]
- Lyon GJ, Novick RP. Peptide signaling in Staphylococcus aureus and other Gram-positive bacteria. Peptides. 2004;25:1389–1403. [Abstract] [Google Scholar]
- Parsek MR, Greenberg EP. Sociomicrobiology: the connections between quorum sensing and biofilms. Trends Microbiol. 2005;13:27–33. [Abstract] [Google Scholar]
- Walters M, Sperandio V. Quorum sensing in Escherichia coli and Salmonella. Int J Med Microbiol. 2006;296:125–131. [Abstract] [Google Scholar]
- Williams P, Winzer K, Chan WC, Camara M. Look who's talking: communication and quorum sensing in the bacterial world. Philos Trans R Soc Lond B Biol Sci. 2007;362:1119–1134. [Europe PMC free article] [Abstract] [Google Scholar]
- Podbielski A, Kreikemeyer B. Cell density-dependent regulation: basic principles and effects on the virulence of Gram-positive cocci. Int J Infect Dis. 2004;8:81–95. [Abstract] [Google Scholar]
- Suntharalingam P, Cvitkovitch DG. Quorum sensing in streptococcal biofilm formation. Trends Microbiol. 2005;13:3–6. [Abstract] [Google Scholar]
- Bassler BL, Greenberg EP, Stevens AM. Cross-species induction of luminescence in the quorum-sensing bacterium Vibrio harveyi. J Bacteriol. 1997;179:4043–4045. [Europe PMC free article] [Abstract] [Google Scholar]
- Surette MG, Miller MB, Bassler BL. Quorum sensing in Escherichia coli, Salmonella typhimurium, and Vibrio harveyi: a new family of genes responsible for autoinducer production. Proc Natl Acad Sci USA. 1999;96:1639–1644. [Europe PMC free article] [Abstract] [Google Scholar]
- De Keersmaecker SC, Sonck K, Vanderleyden J. Let LuxS speak up in AI-2 signaling. Trends Microbiol. 2006;14:114–119. [Abstract] [Google Scholar]
- Vendeville A, Winzer K, Heurlier K, Tang CM, Hardie KR. Making 'sense' of metabolism: autoinducer-2, LuxS and pathogenic bacteria. Nat Rev Microbiol. 2005;3:383–396. [Abstract] [Google Scholar]
- Winzer K, Hardie KR, Williams P. LuxS and autoinducer-2: their contribution to quorum sensing and metabolism in bacteria. Adv Appl Microbiol. 2003;53:291–396. [Abstract] [Google Scholar]
- Bisno AL, Brito MO, Collins CM. Molecular basis of group A streptococcal virulence. Lancet Infect Dis. 2003;3:191–200. [Abstract] [Google Scholar]
- Cunningham MW. Pathogenesis of group A streptococcal infections. Clin Microbiol Rev. 2000;13:470–511. [Europe PMC free article] [Abstract] [Google Scholar]
- Kreikemeyer B, McIver KS, Podbielski A. Virulence factor regulation and regulatory networks in Streptococcus pyogenes and their impact on pathogen-host interactions. Trends Microbiol. 2003;11:224–232. [Abstract] [Google Scholar]
- Salim KY, de Azavedo JC, Bast DJ, Cvitkovitch DG. Role for sagA and siaA in quorum sensing and iron regulation in Streptococcus pyogenes. Infect Immun. 2007;75:5011–5017. [Europe PMC free article] [Abstract] [Google Scholar]
- Beyer-Sehlmeyer G, Kreikemeyer B, Horster A, Podbielski A. Analysis of the growth phase-associated transcriptome of Streptococcus pyogenes. Int J Med Microbiol. 2005;295:161–177. [Abstract] [Google Scholar]
- Chaussee MS, Phillips ER, Ferretti JJ. Temporal production of streptococcal erythrogenic toxin B (streptococcal cysteine proteinase) in response to nutrient depletion. Infect Immun. 1997;65:1956–1959. [Europe PMC free article] [Abstract] [Google Scholar]
- Smoot LM, Smoot JC, Graham MR, Somerville GA, Sturdevant DE, Migliaccio CA, Sylva GL, Musser JM. Global differential gene expression in response to growth temperature alteration in group A Streptococcus. Proc Natl Acad Sci USA. 2001;98:10416–10421. [Europe PMC free article] [Abstract] [Google Scholar]
- Kreikemeyer B, Boyle MD, Buttaro BA, Heinemann M, Podbielski A. Group A streptococcal growth phase-associated virulence factor regulation by a novel operon (Fas) with homologies to two-component-type regulators requires a small RNA molecule. Mol Microbiol. 2001;39:392–406. [Abstract] [Google Scholar]
- Mangold M, Siller M, Roppenser B, Vlaminckx BJ, Penfound TA, Klein R, Novak R, Novick RP, Charpentier E. Synthesis of group A streptococcal virulence factors is controlled by a regulatory RNA molecule. Mol Microbiol. 2004;53:1515–1527. [Abstract] [Google Scholar]
- Neely MN, Lyon WR, Runft DL, Caparon M. Role of RopB in growth phase expression of the SpeB cysteine protease of Streptococcus pyogenes. J Bacteriol. 2003;185:5166–5174. [Europe PMC free article] [Abstract] [Google Scholar]
- McIver KS, Scott JR. Role of mga in growth phase regulation of virulence genes of the group A streptococcus. J Bacteriol. 1997;179:5178–5187. [Europe PMC free article] [Abstract] [Google Scholar]
- Eran Y, Getter Y, Baruch M, Belotserkovsky I, Padalon G, Mishalian I, Podbielski A, Kreikemeyer B, Hanski E. Transcriptional regulation of the sil locus by the SilCR signalling peptide and its implications on group A streptococcus virulence. Mol Microbiol. 2007;63:1209–1222. [Abstract] [Google Scholar]
- Lyon WR, Madden JC, Levin JC, Stein JL, Caparon MG. Mutation of luxS affects growth and virulence factor expression in Streptococcus pyogenes. Mol Microbiol. 2001;42:145–157. [Abstract] [Google Scholar]
- Marouni MJ, Sela S. The luxS gene of Streptococcus pyogenes regulates expression of genes that affect internalization by epithelial cells. Infect Immun. 2003;71:5633–5639. [Europe PMC free article] [Abstract] [Google Scholar]
- Brenot A, King KY, Caparon MG. The PerR regulon in peroxide resistance and virulence of Streptococcus pyogenes. Mol Microbiol. 2005;55:221–234. [Abstract] [Google Scholar]
- Dalton TL, Scott JR. CovS inactivates CovR and is required for growth under conditions of general stress in Streptococcus pyogenes. J Bacteriol. 2004;186:3928–3937. [Europe PMC free article] [Abstract] [Google Scholar]
- Loughman JA, Caparon M. Regulation of SpeB in Streptococcus pyogenes by pH and NaCl: a model for in vivo gene expression. J Bacteriol. 2006;188:399–408. [Europe PMC free article] [Abstract] [Google Scholar]
- Bates CS, Toukoki C, Neely MN, Eichenbaum Z. Characterization of MtsR, a new metal regulator in group A streptococcus, involved in iron acquisition and virulence. Infect Immun. 2005;73:5743–5753. [Europe PMC free article] [Abstract] [Google Scholar]
- Fagan PK, Reinscheid D, Gottschalk B, Chhatwal GS. Identification and characterization of a novel secreted immunoglobulin binding protein from group A streptococcus. Infect Immun. 2001;69:4851–4857. [Europe PMC free article] [Abstract] [Google Scholar]
- Liu M, Hanks TS, Zhang J, McClure MJ, Siemsen DW, Elser JL, Quinn MT, Lei B. Defects in ex vivo and in vivo growth and sensitivity to osmotic stress of group A Streptococcus caused by interruption of response regulator gene vicR. Microbiology. 2006;152:967–978. [Europe PMC free article] [Abstract] [Google Scholar]
- Malke H, Steiner K, McShan WM, Ferretti JJ. Linking the nutritional status of Streptococcus pyogenes to alteration of transcriptional gene expression: the action of CodY and RelA. Int J Med Microbiol. 2006;296:259–275. [Abstract] [Google Scholar]
- Reid SD, Montgomery AG, Musser JM. Identification of srv, a PrfA-like regulator of group A streptococcus that influences virulence. Infect Immun. 2004;72:1799–1803. [Europe PMC free article] [Abstract] [Google Scholar]
- Shelburne SA, Sumby P, Sitkiewicz I, Granville C, DeLeo FR, Musser JM. Central role of a bacterial two-component gene regulatory system of previously unknown function in pathogen persistence in human saliva. Proc Natl Acad Sci USA. 2005;102:16037–16042. [Europe PMC free article] [Abstract] [Google Scholar]
- Asanuma N, Yoshii T, Hino T. Molecular characterization and transcription of the luxS gene that encodes LuxS autoinducer 2 synthase in Streptococcus bovis. Curr Microbiol. 2004;49:366–371. [Abstract] [Google Scholar]
- Beeston AL, Surette MG. pfs-dependent regulation of autoinducer 2 production in Salmonella enterica serovar Typhimurium. J Bacteriol. 2002;184:3450–3456. [Europe PMC free article] [Abstract] [Google Scholar]
- Lupp C, Ruby EG. Vibrio fischeri LuxS and AinS: comparative study of two signal synthases. J Bacteriol. 2004;186:3873–3881. [Europe PMC free article] [Abstract] [Google Scholar]
- Sun J, Daniel R, Wagner-Dobler I, Zeng AP. Is autoinducer-2 a universal signal for interspecies communication: a comparative genomic and phylogenetic analysis of the synthesis and signal transduction pathways. BMC Evol Biol. 2004;4:36. [Europe PMC free article] [Abstract] [Google Scholar]
- von Lackum K, Babb K, Riley SP, Wattier RL, Bykowski T, Stevenson B. Functionality of Borrelia burgdorferi LuxS: the Lyme disease spirochete produces and responds to the pheromone autoinducer-2 and lacks a complete activated-methyl cycle. Int J Med Microbiol. 2006;40:92–102. [Abstract] [Google Scholar]
- Sztajer H, Lemme A, Vilchez R, Schulz S, Geffers R, Yip CY, Levesque CM, Cvitkovitch DG, Wagner-Dobler I. Autoinducer-2-regulated genes in Streptococcus mutans UA159 and global metabolic effect of the luxS mutation. J Bacteriol. 2008;190:401–415. [Europe PMC free article] [Abstract] [Google Scholar]
- Wen ZT, Burne RA. LuxS-mediated signaling in Streptococcus mutans is involved in regulation of acid and oxidative stress tolerance and biofilm formation. J Bacteriol. 2004;186:2682–2691. [Europe PMC free article] [Abstract] [Google Scholar]
- Hasona A, Zuobi-Hasona K, Crowley PJ, Abranches J, Ruelf MA, Bleiweis AS, Brady LJ. Membrane composition changes and physiological adaptation by Streptococcus mutans signal recognition particle pathway mutants. J Bacteriol. 2007;189:1219–1230. [Europe PMC free article] [Abstract] [Google Scholar]
- Lemos JA, Brown TA, Jr, Burne RA. Effects of RelA on key virulence properties of planktonic and biofilm populations of Streptococcus mutans. Infect Immun. 2004;72:1431–1440. [Europe PMC free article] [Abstract] [Google Scholar]
- Azcarate-Peril MA, McAuliffe O, Altermann E, Lick S, Russell WM, Klaenhammer TR. Microarray analysis of a two-component regulatory system involved in acid resistance and proteolytic activity in Lactobacillus acidophilus. Appl Environ Microbiol. 2005;71:5794–5804. [Europe PMC free article] [Abstract] [Google Scholar]
- DeLisa MP, Bentley WE. Bacterial autoinduction: looking outside the cell for new metabolic engineering targets. Microb Cell Fact. 2002;1:1–9. [Europe PMC free article] [Abstract] [Google Scholar]
- Klenk M, Koczan D, Guthke R, Nakata M, Thiesen HJ, Podbielski A, Kreikemeyer B. Global epithelial cell transcriptional responses reveal Streptococcus pyogenes Fas regulator activity association with bacterial aggressiveness. Cell Microbiol. 2005;7:1237–1250. [Abstract] [Google Scholar]
- Kreikemeyer B, Nakata M, Koller T, Hildisch H, Kourakos V, Standar K, Kawabata S, Glocker MO, Podbielski A. The Streptococcus pyogenes serotype M49 Nra-Ralp3 transcriptional regulatory network and its control of virulence factor expression from the novel eno ralp3 epf sagA pathogenicity region. Infect Immun. 2007;75:5698–5710. [Europe PMC free article] [Abstract] [Google Scholar]
- Luo F, Lizano S, Bessen DE. Heterogeneity in the Polarity of Nra Regulatory Effects on Streptococcal Pilus Gene Transcription and Virulence. Infect Immun. 2008;76:2490–2497. [Europe PMC free article] [Abstract] [Google Scholar]
- Sumby P, Whitney AR, Graviss EA, DeLeo FR, Musser JM. Genome-wide analysis of group A streptococci reveals a mutation that modulates global phenotype and disease specificity. PLoS Pathog. 2006;2:e5. [Europe PMC free article] [Abstract] [Google Scholar]
- Walker MJ, Hollands A, Sanderson-Smith ML, Cole JN, Kirk JK, Henningham A, McArthur JD, Dinkla K, Aziz RK, Kansal RG, et al. DNase Sda1 provides selection pressure for a switch to invasive group A streptococcal infection. Nat Med. 2007;13:981–985. [Abstract] [Google Scholar]
- Coulthurst SJ, Kurz CL, Salmond GP. luxS mutants of Serratia defective in autoinducer-2-dependent 'quorum sensing' show strain-dependent impacts on virulence and production of carbapenem and prodigiosin. Microbiology. 2004;150:1901–1910. [Abstract] [Google Scholar]
- Dove JE, Yasukawa K, Tinsley CR, Nassif X. Production of the signalling molecule, autoinducer-2, by Neisseria meningitidis : lack of evidence for a concerted transcriptional response. Microbiology. 2003;149:1859–1869. [Abstract] [Google Scholar]
- Bejerano-Sagie M, Xavier KB. The role of small RNAs in quorum sensing. Curr Opin Microbiol. 2007;10:189–198. [Abstract] [Google Scholar]
- Lyon WR, Gibson CM, Caparon MG. A role for trigger factor and an rgg-like regulator in the transcription, secretion and processing of the cysteine proteinase of Streptococcus pyogenes. EMBO J. 1998;17:6263–6275. [Europe PMC free article] [Abstract] [Google Scholar]
- Rijn I van de, Kessler RE. Growth characteristics of group A streptococci in a new chemically defined medium. Infect Immun. 1980;27:444–448. [Europe PMC free article] [Abstract] [Google Scholar]
- Doherty N, Holden MT, Qazi SN, Williams P, Winzer K. Functional analysis of luxS in Staphylococcus aureus reveals a role in metabolism but not quorum sensing. J Bacteriol. 2006;188:2885–2897. [Europe PMC free article] [Abstract] [Google Scholar]
- Caparon MG, Scott JR. Genetic manipulation of pathogenic streptococci. Methods Enzymol. 1991;204:556–586. [Abstract] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: a Laboratory Manual. 2. Cold Spring Harbor, NY edn: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Trieu-Cuot P, Courvalin P. Nucleotide sequence of the Streptococcus faecalis plasmid gene encoding the 3'5"-aminoglycoside phosphotransferase type III. Gene. 1983;23:331–341. [Abstract] [Google Scholar]
- Herbert S, Barry P, Novick RP. Subinhibitory clindamycin differentially inhibits transcription of exoprotein genes in Staphylococcus aureus. Infect Immun. 2001;69:2996–3003. [Europe PMC free article] [Abstract] [Google Scholar]
- Lembke C, Podbielski A, Hidalgo-Grass C, Jonas L, Hanski E, Kreikemeyer B. Characterization of biofilm formation by clinically relevant serotypes of group A streptococci. Appl Environ Microbiol. 2006;72:2864–2875. [Europe PMC free article] [Abstract] [Google Scholar]
Articles from BMC Microbiology are provided here courtesy of BMC
Full text links
Read article at publisher's site: https://doi.org/10.1186/1471-2180-8-188
Read article for free, from open access legal sources, via Unpaywall:
https://bmcmicrobiol.biomedcentral.com/counter/pdf/10.1186/1471-2180-8-188
Open access at BioMedCentral
http://www.biomedcentral.com/1471-2180/8/188/abstract
Open access at BioMedCentral
http://www.biomedcentral.com/content/pdf/1471-2180-8-188.pdf
Open access at BioMedCentral
http://www.biomedcentral.com/1471-2180/8/188
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1186/1471-2180-8-188
Article citations
luxS contributes to intramacrophage survival of Streptococcus agalactiae by positively affecting the expression of fruRKI operon.
Vet Res, 54(1):83, 27 Sep 2023
Cited by: 0 articles | PMID: 37759250 | PMCID: PMC10536698
How does Quorum Sensing of Intestinal Bacteria Affect Our Health and Mental Status?
Microorganisms, 10(10):1969, 05 Oct 2022
Cited by: 7 articles | PMID: 36296244 | PMCID: PMC9611604
Review Free full text in Europe PMC
Production of a Class IIb Bacteriocin with Broad-spectrum Antimicrobial Activity in Lactiplantibacillus plantarum RUB1.
Probiotics Antimicrob Proteins, 13(6):1820-1832, 23 Aug 2021
Cited by: 10 articles | PMID: 34423377
Deciphering Streptococcal Biofilms.
Microorganisms, 8(11):E1835, 21 Nov 2020
Cited by: 16 articles | PMID: 33233415 | PMCID: PMC7700319
Review Free full text in Europe PMC
Challenges and Limitations of Anti-quorum Sensing Therapies.
Front Microbiol, 10:2473, 31 Oct 2019
Cited by: 34 articles | PMID: 31736912 | PMCID: PMC6834643
Go to all (26) article citations
Other citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The LuxS/AI-2 Quorum-Sensing System of Streptococcus pneumoniae Is Required to Cause Disease, and to Regulate Virulence- and Metabolism-Related Genes in a Rat Model of Middle Ear Infection.
Front Cell Infect Microbiol, 8:138, 04 May 2018
Cited by: 28 articles | PMID: 29780750 | PMCID: PMC5945837
Role of luxS in immune evasion and pathogenicity of piscine Streptococcus agalactiae is not dependent on autoinducer-2.
Fish Shellfish Immunol, 99:274-283, 11 Feb 2020
Cited by: 5 articles | PMID: 32058098
Regulation of autoinducer 2 production and luxS expression in a pathogenic Edwardsiella tarda strain.
Microbiology (Reading), 154(pt 7):2060-2069, 01 Jul 2008
Cited by: 82 articles | PMID: 18599834
The LuxS/AI-2 system of Streptococcus suis.
Appl Microbiol Biotechnol, 102(17):7231-7238, 25 Jun 2018
Cited by: 30 articles | PMID: 29938319
Review
Funding
Funders who supported this work.
Austrian Science Fund FWF (1)
Regulation of virulence by a regulatory RNA in GAS
Prof. Dr. Emmanuelle CHARPENTIER, Helmholtz Centre for Infection Research
Grant ID: P 17238

 1,2
1,2



