Abstract
Free full text

This article has been retracted. Retraction in:
p73α Regulation by Chk1 in Response to DNA Damage
Abstract
The checkpoint kinase 1 (Chk1) is an essential component of the DNA damage checkpoint. Previous studies have demonstrated an indispensable role for the p53-related transcription factor p73α in DNA damage-induced apoptosis. Here, we provide evidence that p73α is a target of Chk1. We found that endogenous p73α is serine phosphorylated by endogenous Chk1 upon DNA damage, which is a mechanism required for the apoptotic-inducing function of p73α. Consistent with this, we discovered that endogenous p73α interacts with Chk1 and is phosphorylated by Chk1 at serine 47 in vitro and in vivo. In contrast, Chk2 does not phosphorylate p73α in vitro. Moreover, mutation of serine 47 abolishes both Chk1-dependent phosphorylation of p73α upon DNA damage in vivo and the ability of Chk1 to upregulate the transactivation capacity of p73α. Our data indicate a novel biochemical pathway through which the p73α proapoptotic function requires DNA damage-triggered p73α phosphorylation by Chk1.
In eukaryotes, damaged DNA can initiate a signaling pathway resulting in cell cycle arrest or, in the case of irreparable damage, in cell death. Because DNA damage can be caused by myriad types of assaults, multiple signals might be triggered. While some signaling pathways are poorly understood, there has been significant progress in elucidating pathways involving the ATM and ATR mediator protein kinases and their downstream effector kinases Chk1 (34) and Chk2 (27). The p53 tumor suppressor protein provides a well-studied example of a DNA damage-responsive checkpoint factor whose induction leads either to cell cycle arrest or to apoptosis (28). p53 is a member of the gene family comprising p73 and p63. In contrast to p53, the p73 gene encodes a group of full-length isoforms that vary in their N (ΔN-p73) and C (p73α to -δ) termini. Ectopically overexpressed p73α and p73β largely mimic p53 activities, including induction of apoptosis, cell cycle arrest, and transactivation of an overlapping set of target genes (17, 40). Moreover, endogenous p73 is differently upregulated by some cellular or viral oncogenes, T-cell receptor hyperactivation, and some forms of DNA damage (33, 37). Although multiple posttranslational modifications, for example, phosphorylation and acetylation, have been shown to occur and to determine p53 functions in response to DNA damage (19, 25, 29), the role of such modifications in the activation and regulation of its close relative p73 have just begun to be studied (18, 21). Endogenous p73 is both activated and stabilized in response to γ-irradiation and cisplatin by a c-Abl-dependent pathway, participating in an apoptotic response to DNA damage (1, 12, 36). Sumoylation of the C-terminal region (Lys-627) occurs specifically in p73α but not in p73β in vitro, altering its subcellular localization and partially increasing its degradation rate (26). Additionally, it has been documented that DNA damage-induced acetylation makes the apoptotic function of p73 possible by increasing its ability to activate the transcription of specific proapoptotic target genes (6). More recent work has reported that p73 is required for p53-dependent apoptosis in response to DNA damage (10).
Since it is unclear how the functional activity of p73 is regulated under normal conditions or upon genotoxic stress and since Chk1 is a conserved kinase that plays a critical role in the DNA checkpoint pathway (30), we decided to explore a potential role of p73 phosphorylation by Chk1 in its activation by DNA damage as well as in the regulation of its effector functions. In this study, we found that once Chk1 is activated and once p73α is accumulated after DNA damage, endogenous p73α is serine phosphorylated by a Chk1-dependent pathway, contributing to the p73α apoptotic response to DNA damage. Interaction between endogenous p73α and Chk1 was detected and was abolished in the presence of Chk1-specific short inhibitory RNA (siRNA). Finally, mutation of serine-47 (p73α-S47A) eliminates both Chk1-dependent phosphorylation of p73α upon DNA damage and the Chk1 capacity to upregulate p73α transactivation ability.
MATERIALS AND METHODS
Cell cultures and transfections.
H1299 and SAOS2 cells were maintained in RPMI and McCoy's 5a medium plus 10% fetal bovine serum, respectively, whereas 293 and HCT116-3 cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum. Sf9 insect cells were grown in TC100 medium (GIBCO BRL) at 30°C. H1299 and 293 cells were plated at 0.6 × 105/35-mm-diameter dish and 24 h later were transfected by the Fugene method (Roche, Inc.) with the plasmids (8 μg) indicated in the corresponding figure legends. Sf9 cells were plated in six-well plates and infected with 50 μl of virus. Cells were harvested 40 h after transfection or infection. For p73 activation, H1299 cells and HCT116-3 cells were treated with 5 or 50 μM cisplatin (Sigma), 0.22 μM daunorubicin (Dauno; Oncogene Research Products), or 300 nM UCN-01 (generously provided by the Drug Synthesis and Chemistry Branch, National Institutes of Health, Bethesda, Md.) for 24 h.
siRNA.
The siRNA duplexes were made by using protocols supplied by Dharmacon Research. The sequence of Chk1 siRNA oligonucleotides was 5′-AAGCGTGCCGTAGACTGTCCA-3′ (38). The control siRNA used was 5′-GCCATTCTATCCTCTAGAGGATG-3′, against luciferase. Cells were transfected with siRNA duplexes by use of Oligofectamine (Invitrogen) according to the manufacturer's instructions, and they were analyzed at 36 h posttransfection.
Plasmids.
The cDNAs for different p73 domains were generated by PCR, with the full-length wild-type p73α cDNA as a template. The following oligonucleotide primers were used: 5′ end, 5′-GCTAGCTTCGACACCATGTCGCCGGCG-3′, and 3′ end, 5′-GCGGCCGCCTCGTTCAGGGCCTGCTGCTC-3′, for p73α-DB; 5′ end, 5′-GCTAGCTCCCAGCCATGTCCATGTCG-3′, and 3′ end, 5′-GCGGCCGCGGAGTCCACCAGTGGCTGCGG-3′, for p73α-OD; 5′ end, 5′-GCTAGCGCCCAGTCCACCGCCACCTCC-3′, and 3′ end, 5′-GCGGCCGCCTCGTTCAGGGCCTGCTGCTC-3′, for p73α-TAD-PxxP-DBD; 5′ end, 5′-GCTAGCGCCCAGTCCACCGCCACCTCC-3′, and 3′ end, 5′-GCGGCCGCGGAGTCCACCAGTGGCTGCGG-3′, for p73αΔ-TDI-II-SAM; 5′ end, 5′-GCTAGCTTCGACACCATGTCGCCGGCG-3′, and 3′ end, 5′-GCGGCCGCGGAGTCCACCAGTGGCTGCGG-3′, for p73α-DB-OD; 5′ end, 5′-GCTAGCGCCCAGTCCACCGCCACCTCC-3′, and 3′ end, 5′-GCGGCCGCGGTGGAGCTGGGTTGTGCGTA-3′, for p73α-TAD-PxxP; 5′ end, 5′-GCTAGCTATCGGCAGCAGCAGCAGCTC-3′, and 3′ end, 5′-GCGGCCGCTCAGTGGATCTCGGCCTCCGT-3′, for p73α-TDI-TDII-SAM; 5′ end, 5′-GCTAGCGCCCAGTCCACCGCCACCTCC-3′, and 3′ end, 5′-GCGGCCGCTGGCGGAGTGCAGTGGGACCC-3′, for p73αΔSAM. All truncated p73 versions were cloned into the NheI and NotI sites of the pCMV-HA vector. Phosphorylation-site mutants were made with the Quick Change mutagenesis kit (Stratagene), following the protocol suggested by the manufacturer. In each of these cases, alanine substitutions at potential serine phosphorylation sites were performed by use of pCMV-HAp73α-TAD-PxxP as a template, and for HAp73αS47A we used pCMV-HAp73α as a template. Both Chk1- and Chk1D130A-expressing plasmids were generated as previously described (32). The pCMV-Cdc25C fragment (195-256) was generated by PCR with a 5′ primer (CGCGCGGATCCCCATGGAGTTTTCCCTGAAA) and a 3′ primer (CCAGTGAATTCCCTCAGCCCTTCCTGAGCTTTCC), with wild-type cDNA as template. The product was digested with BamHI and EcoRI and was ligated into the pCMV-HA vector. All constructs were further verified by sequencing.
Immunoprecipitation analysis and immunoblotting.
Cellular extracts and immunoprecipitations were carried out essentially as described previously (11). Briefly, the soluble fraction was added to protein A-Sepharose beads cross-linked to hemagglutinin (HA) monoclonal antibody (MAb) 12CA5 (BabCo) or was incubated with anti-Chk1 (G4; Santa Cruz Biotech), anti-p73 (C17 and H79; Santa Cruz Biotech), or anti-Flag M2 (Sigma) antibody and rocked at 4°C for 6 h. The immunoprecipitates were then washed with wash buffer and resuspended in 2× sodium dodecyl sulfate (SDS) gel-loading buffer. Cellular extracts (40 μg) were resolved on SDS-4 to 20% polyacrylamide gel electrophoresis (PAGE) gels and transferred onto nitrocellulose membranes. The membranes were blocked with 5% nonfat milk, washed, and incubated with the following primary antibodies. For identifying p53 and wild-type or truncated HA-tagged p73 proteins, PAb1801 MAb (1:1,500) (NeoMarkers) and 12CA5 MAb (1:2,000) (BabCo), respectively, were used. His-Chk1-Flag and Flag-Chk2 proteins were detected by use of a Flag epitope MAb (1:1,000) (Sigma), while Cdc25C was detected by use of the 25C15 MAb (1:1,000) (NeoMarkers). Specific antibodies for Chk1 and phosphorylated Chk1 on serine 345 were purchased from Santa Cruz Biotech (G4; 1:500) and Cell Signaling (1:400), respectively. For specific p73 detection, anti-p73α antibodies (C17, 1:500; ER13, 1:500) (Santa Cruz Biotech and Cell Signaling, respectively) were used; for detection of phosphorylated p73 proteins, antiphosphoserine (1:200) (Sigma), antiphosphotyrosine (1:200) (4G10; Upstate Biotech), and antiphosphothreonine (1:200) (Zymed) antibodies were used. Anti-Ran antibody (1:3,000) (Santa Cruz Biotech) was used to normalize the amount of loaded proteins. Horseradish peroxidase-conjugated secondary antibodies were used to detect the bound primary antibodies. Immune complexes were visualized with an enhanced chemiluminescence detection system (Amersham).
Expression and purification of recombinant proteins.
Expression and purification of Chk1, Chk1(D130A), Chk2, Chk2(D347A), p53, and p73 proteins from baculovirus-infected insect cells were done as previously described (11, 16, 32). For expression of His-Cdc25C, pRSET plasmid with Cdc25C cDNA was introduced into Escherichia coli BL21(DE3)LysE. Purification of His-Cdc25C proteins was performed as described for p53. The bound protein was eluted with 0.25 mM imidazole and further dialyzed. For the wild-type and truncated p73 proteins, the products were eluted with HA peptide (10 μg/ml) after immunoprecipitation in an elution buffer (11).
Labeling and 2D peptide mapping analysis.
For in vitro kinase assays, Chk1 or Chk2 kinase reaction mixtures (20 μl) contained 300 ng of the kinase and 500 ng of substrate (HA-p53, HA-p73α, HA-p73β, or Cdc25C) in 1× kinase buffer (20 mM Tris-HCl [pH 7.5], 10 mM MgCl2, 2 mM MnCl2, and 1 mM dithiothreitol) in the presence of 25 μM ATP and 5 μCi of [γ-32P]ATP. Purification of HA-p73αS47A was performed as described above for mutated HA-tagged p73 proteins. The mixtures were incubated at 30°C for 20 min, and the reactions were stopped by the addition of an equal volume of 2× SDS gel-loading buffer. Proteins were resolved by SDS-PAGE, followed by either autoradiography or Western blot analysis. For in vivo 32P labeling, 293 cells transfected with plasmids carrying the genes for HA-p73α and HA-p73αS47A were incubated for 2 h in phosphate-free Dulbecco's modified Eagle's medium containing 2 mCi of 32P-labeled inorganic phosphate per ml. Ectopically expressed HA-p73α and HA-p73αS47A were purified by immunoprecipitation, eluted with an excess of HA peptide, and further digested with 2 μg of trypsin per ml. Two-dimensional (2D) phosphopeptide mapping was resolved by isoelectric focusing using immobilized pH gradient trips from the IPGRunner System (Invitrogen), following the manufacturer's instructions. The strips were then subjected to SDS-PAGE followed by autoradiography analysis.
Luciferase assays.
SAOS2 cells were seeded in 12-well plates and transfected with the plasmids indicated (200 ng) in the corresponding figure legends, along with p53-responsive reporter constructs (p21-luc or Bax-luc), as previously described (7a). For internal controls, 250 ng of the Renilla luciferase vector pRL-CMV (Promega) was cotransfected with the above constructs. Dual luciferase activity assays were performed 24 h later according to the manufacturer's instructions (Promega).
Cell cycle analyses.
Floating and adherent cells were collected, washed with cold phosphate-buffered saline, and then resuspended in 200 μl of binding buffer containing annexin V-fluorescein isothiocyanate (0.5 μg/ml) (Clontech Lab, Inc.) and 5 μg of propidium iodide (PI) (Sigma) per ml, in accordance with the manufacturer's instructions. After incubation at 20°C for 20 min, the stained cells were analyzed in a fluorescence-activated cell sorter (FACSCalibur; Becton Dickinson). The percentage of apoptotic cells was calculated by scoring for cells that were initially PI positive and were also positive for annexin V.
RESULTS
Chk1 phosphorylates p73α on serine residues in response to DNA damage.
We first studied whether Chk1 kinase has a role in inducing p73α posttranslational modifications upon DNA damage checkpoint activation. The phosphorylation status of both Chk1 and p73α proteins was studied in H1299 and HCT116-3 cells after cisplatin treatment and in the presence of Chk1-specific siRNAs in order to silence endogenous Chk1 expression (8, 39) (Fig. (Fig.1A).1A). Endogenous p73α and Chk1 proteins were purified by immunoprecipitation and analyzed by immunoblotting, using anti-p73α and antiphosphoserine (anti-P-Ser/IPp73) antibodies for specific p73α recognition and anti-Chk1 antibody for Chk1 recognition. As shown in Fig. Fig.1A,1A, Chk1-siRNA treatment resulted in the loss of endogenous Chk1 detection compared to the control siRNA-transfected cells. In addition, endogenous p73α serine phosphorylation was detectable only in those cells in which p73α protein levels were accumulated to the level detected after DNA damage and in which Chk1 expression was not reduced by Chk1-specific siRNAs (Fig. (Fig.1A,1A, lanes 4 and 12). Purified p73α proteins were also blotted with antiphosphothreonine and antiphosphotyrosine antibodies, but we were unable to detect p73 phosphorylation either in tyrosine or in threonine residues under the conditions described above (data not shown). It is noteworthy that commercially available p73α-specific antibodies allowed us to detect the induction of p73α after cisplatin action, but not endogenous p73α levels in the absence of treatment. Therefore, to rule out that Chk1 does not constitutively phosphorylate p73α and to prove that p73α phosphorylation by Chk1 is induced in response to DNA damage, H1299 and HCT116-3 cells were transfected with plasmids carrying genes for p73α. As shown in Fig. Fig.1A1A (lanes 5 to 8 and 13 to 16), ectopic p73α protein was serine phosphorylated only after cisplatin treatment and in the presence of control siRNAs (compare lanes 7 and 8 and lanes 15 and 16). For that reason, the effect of Chk1 on p73α after cisplatin treatment is not caused by the accumulation of p73α itself but rather by Chk1 regulation upon drug action. This was shown when cells accumulated p73α levels up to those induced by DNA damage and p73α was not phosphorylated except upon drug treatment. To demonstrate whether the DNA damage-induced serine phosphorylation of p73α is Chk1 dependent, H1299 cells were treated with cisplatin or daunorubicin for 24 h in the presence of UCN-01 (7-hydroxy staurosporine), an agent that inhibits Chk1 kinase activity and does not affect the activity of upstream kinases of Chk1 (Fig. (Fig.1B)1B) (9, 13, 39). Since it is known that Chk1 is activated by phosphorylation in response to DNA damage (22, 31, 38), analysis of cellular lysates showed that Chk1 was phosphorylated only in daunorubicin- and cisplatin-treated cells but not in untreated cells. In addition, endogenous p73α was consistently accumulated and only phosphorylated at serine residues in cells treated with cisplatin or daunorubicin in which the Chk1 activity was not inhibited by UCN-01 (lanes 2 and 3). Hence, these data show a potential Chk1-dependent p73α serine phosphorylation after DNA damage.
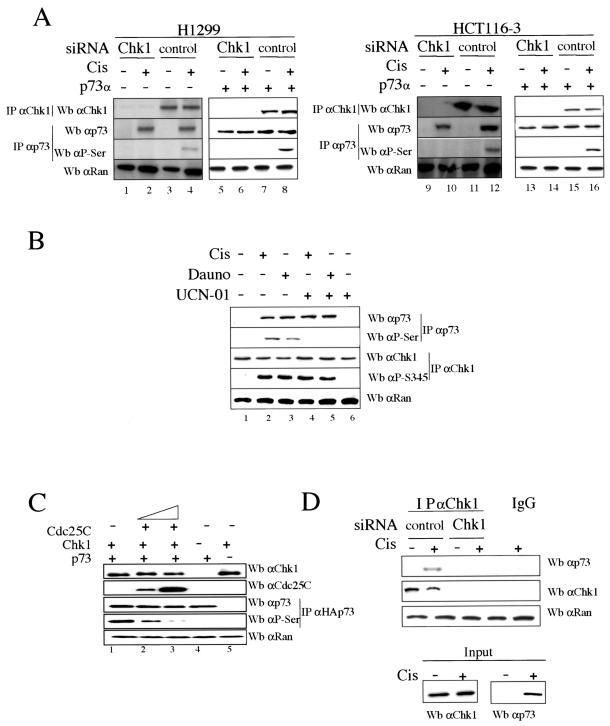
p73α serine phosphorylation by Chk1 upon DNA damage and interaction between p73α and Chk1. (A and B) p73α serine phosphorylation by Chk1. H1299 and HCT116-3 cells were transfected with plasmids expressing p73α along with siRNAs specific for either Chk1 or LucZ (control) for 36 h, in the combinations indicated. Cells were mock or cisplatin treated (Cis) and were harvested 24 h later (A). H1299 cells were untreated (−) or treated (+) with cisplatin, daunorubicin (Dauno), or UCN-01 for 24 h in the indicated combinations (B). Cellular extracts were prepared, and endogenous p73α and Chk1 proteins were revealed by immunoprecipitation (IP) and Western blot analysis (Wb) using anti-p73 (C-17) and antiphosphoserine (P-Ser/IPp73) antibodies for p73 and anti-Chk1 (G4) and antiphosphoserine 345 (P-Ser345) antibodies for Chk1. Anti-Ran immunoblotting was performed as a loading control. (C) Competition of p73 phosphorylation by Cdc25C. 293 cells were cotransfected for 24 h with plasmids expressing Cdc25C (195-256), Chk1, and p73α. Cellular extracts were prepared, and Chk1, Cdc25C, and Ran proteins were detected by immunoblotting using specific antibodies. HAp73 proteins were purified by immunoprecipitation using an HA epitope MAb, eluted with an excess of HA peptide, and identified by Western blotting using specific anti-p73α (ER-13) and antiphosphoserine (P-Ser/IPp73) antibodies. (D) Endogenous interaction between p73α and Chk1. H1299 cells were transfected with either Chk1 siRNA or Luc siRNA for 36 h. Cells were treated with cisplatin for 24 h, and cellular extracts were subjected to immunoprecipitation with an anti-Chk1 antibody (G4). The immune complexes were then subjected to Western blotting, and p73 and Chk1 proteins were detected by immunoblotting using both anti-p73 (C17) and anti-Chk1 (G4) antibodies. The presence of Chk1 immunoprecipitated into the resin was detected with an anti-Chk1 MAb, and 10% of the extract used for the immunoprecipitation was loaded and blotted with anti-p73 (ER13) antibody.
Chk1-mediated p73 activation was also verified by coexpression of Chk1 and p73α in the presence or absence of a fragment of Cdc25C without phosphatase activity (195-256), which is a Chk1 substrate previously used to monitor Chk1 activity (13, 31) (Fig. (Fig.1C).1C). When Cdc25C was co-overexpressed, p73 phosphoserine levels were greatly reduced in a Cdc25C dose-dependent manner, while the level of total p73α protein was not affected (lanes 2 and 3). Finally, to gain mechanistic insight into the link between Chk1 and p73, we investigated whether an endogenous association between Chk1 and p73α can be detected after DNA damage and in the presence or absence of Chk1 siRNA. As shown in Fig. Fig.1D,1D, endogenous p73α accumulated upon DNA damage was able to interact with Chk1 when control siRNA was transfected, but not in the absence of Chk1 protein. Our data suggest that once Chk1 is phosphorylated at Ser-345 and once endogenous p73α accumulates in response to DNA damage, p73α is serine phosphorylated by Chk1, likely due to a physical association between p73α and Chk1.
Chk1, but not Chk2, directly phosphorylates p73α in vitro.
Previous reports have described that Chk1 and Chk2 kinases share substrate specificity to some degree, e.g., Cdc25C phosphorylation at Ser-216 (2, 3, 24). They also play partially redundant roles in the Saccharomyces cerevisiae DNA damage checkpoint and the Schizosaccharomyces pombe replication checkpoint (14, 35). To extend our initial findings, we next examined whether both Chk1 and Chk2 kinases phosphorylate p73 protein in vitro. His-Chk1 and Flag-Chk2 were expressed and purified from baculovirus-infected insect cells, and both kinases were compared for the ability to phosphorylate baculovirus-purified HA-p73α, HA-p73β, and HA-p53 in vitro (Fig. (Fig.2A2A and B, left panels). Analysis of the products detected by autoradiography showed that both p73α and p73β proteins are substrates for Chk1 in vitro. Interestingly, p53 protein was phosphorylated by Chk2 kinase (32), but Chk2 did not phosphorylate either p73α or p73β. As controls, a kinase-dead mutant of hChk1 (Chk1D130A) (31, 32) and a kinase-defective mutant of Flag-Chk2 (Chk2D347A) (31) were also unable to phosphorylate any of these substrates (Fig. (Fig.2A2A and B, right panels). To rule out that the epitopes from both the Chk1 and Chk2 tags were not contributing to p73 phosphorylation and to avoid the comigration effect of both Chk2-P and p73α bands, similar experiments were performed with purified glutathione S-transferase-Chk1 and His-Chk2. These experiments confirmed that both p73α and p73β are phosphorylated by Chk1 and not by Chk2 in vitro (data not shown). The activity of both kinases was confirmed by use of His-Cdc25C purified from bacteria. As shown in Fig. Fig.2C,2C, efficient Cdc25C phosphorylation by Chk1 and Chk2 kinases was detected. We also investigated whether an association between Chk1 and p73α or p73β can be detected in insect cells (Fig. (Fig.2D).2D). Sf9 cells were coinfected with baculoviruses expressing His-Chk1-Flag or Flag-Chk2 along with HA-p73α or HA-p73β. Flag-tagged proteins were immunoprecipitated with anti-Flag antibody. The presence of p73α or p73β in the immunoprecipitates was detected by Western blotting using an anti-HA-tag antibody. We observed that the p73α and p73β isoforms were able to interact directly with Chk1, but no interaction between Chk2 and p73 isoforms was detected. These data, together with the results described above, suggest that p73α phosphorylation by Chk1 in vitro, but not by Chk2, is probably due to a physical association between p73α or p73β and Chk1. Taken together, this provides evidence for differential substrate specificity between human Chk1 and Chk2, as Chk1, but not Chk2, efficiently phosphorylates p73α and p73β in vitro.

p73 phosphorylation by Chk1 in vitro. (A and B) Phosphorylation of p73α and p73β by Chk1, but not by Chk2, in vitro. Expression and purification of these proteins from baculovirus-infected insect cells and further in vitro kinase assays were performed as described in Materials and Methods. Chk1, Chk1D130A, Chk2, and Chk1D347A were incubated alone or with purified HA-tagged p73 and p53 proteins. The products were visualized by autoradiography (upper panels) and by Western blotting (Wb) using an anti-HA-tag antibody (lower panels). (C) Chk1 and Chk2 phosphorylate Cdc25C in vitro. His-Chk1-Flag or Flag-Chk2 was incubated with Cdc25C proteins prepared from bacteria as described in Materials and Methods. The phosphorylation status of Cdc25C was analyzed as described above, and His-Chk1-Flag and Flag-Chk2 were visualized by Western blotting using an anti-Flag-tag antibody. (D) Interaction between p73α or p73β and Chk1, but not Chk2. Sf9 insect cells were coinfected with baculoviruses expressing wild-type Chk1 or Chk2 along with a p73 isoform (α or β). Cellular extracts were subjected to immunoprecipitation (IP) with an anti-Flag-tag antibody, and the immune complexes were then subjected to Western blotting. HA-tagged p73 proteins were detected with HA epitope MAb. The presence of immunoprecipitated Flag-tagged Chk1 or Chk2 in the resin was detected with an anti-Flag MAb. In the bottom panel, 10% of the extract (~20 μg) used for the immunoprecipitation was loaded.
Serine at position 47 is the major phosphorylation residue of p73α for Chk1.
To define more specifically the p73α region(s) phosphorylated by Chk1 kinase, proteins containing different p73 functional domains were initially tested for the ability to be phosphorylated in vitro by recombinant Chk1 in the presence of [γ-32P]ATP (Fig. (Fig.3A).3A). HA-tagged p73 fusion proteins were purified by immunoprecipitation with anti-HA-tag antibody and then eluted with excess HA peptide. The experiment showed that all p73 proteins carrying the amino-terminal region were substrates of Chk1 in vitro (Fig. (Fig.3A,3A, lanes 2, 3, and 6). Furthermore, phosphorylation of truncated p73 proteins containing the DNA binding domain, the oligomerization domain, or the carboxy-terminal region could not be detected. These results indicate that the amino-terminal region is the most plausible candidate for Chk1 phosphorylation. To confirm and expand on this outcome, we wondered whether Chk1 could phosphorylate in vivo the same set of truncated p73 proteins. We cotransfected Chk1 along with different truncated versions of p73 in 293 cells (Fig. (Fig.3B).3B). Analysis of HAp73-purified proteins by elution with HA peptide and further Western blotting with an antiphosphoserine antibody (anti-P-Ser) demonstrated that those p73 forms that were phosphorylated in vitro were also serine phosphorylated in vivo (Fig. (Fig.3B,3B, lanes 2, 3, and 4). Thus, our results strongly indicate that Chk1 kinase phosphorylates p73 on serine residues localized within its transactivation and/or proline-rich domains, both in vitro and in vivo (Fig. (Fig.3C3C).
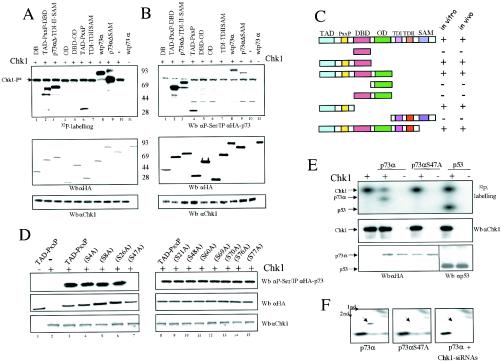
p73α is phosphorylated on serine 47 by Chk1 kinase. (A) Chk1 phosphorylates p73α transactivation and proline-rich domains in vitro. Flag-Chk1 prepared from baculovirus-infected insect cells was incubated with either HA-tagged wild-type p73 or truncated p73 proteins purified from transfected cells. The purification of p73α proteins and kinase activity assays were carried out as described in Materials and Methods. The phosphorylated proteins were visualized by autoradiography (upper panel). The protein levels of p73α and Chk1 were determined by Western blotting (Wb) with anti-HA-tag or anti-Chk1 antibody, respectively. (B) Chk1 phosphorylates similar p73α truncated versions in vivo. 293 cells were cotransfected with the Chk1 expression vector and different HAp73 mutants (described in panel C). Clarified cellular extracts were prepared, and Chk1 levels were analyzed by Western blotting using an antibody specific for Chk1. The HA-tagged p73s were then immunoprecipitated with an HA epitope MAb and further eluted with an excess of HA peptide. HA-tagged p73 proteins were detected with anti-HA-tag and antiphosphoserine antibodies. (C) Schematic representation of truncated p73 proteins. TAD, transactivation domain; DBD, DNA-binding domain; OD, oligomerization domain; TDI and TDII, transactivation domains I and II (between residues 382 and 491), including part of exon XI and exon XII, respectively (23); SAM, sterile α motif domain. Truncated proteins that were phosphorylated (+) or unphosphorylated (−) in vitro and in vivo are summarized. (D) p73 is phosphorylated on serine 47 in vivo. 293 cells were cotransfected with plasmids expressing Chk1 along with each p73αTAD-PxxP mutant carrying an alanine substitution in the serine residue indicated. Immunoprecipitation, elution, and detection of point-mutated HAp73 proteins were performed as described above. (E) p73αS47A is not phosphorylated by Chk1 in vitro. The purification of p73 proteins was done as described above, whereas HA-tagged p53 protein was obtained from baculovirus-infected insect cells. Glutathione S-transferase-Chk1 prepared from baculovirus-infected insect cells was incubated with p73α, p73αS47A, or p53. The kinase activity assay was performed as described for panel A. The phosphorylated proteins were visualized by autoradiography. The protein levels of p73, p53, and Chk1 were determined by Western blotting with anti-HA-tag, anti-p53, or anti-Chk1 antibody, respectively. (F) p73αS47A is not phosphorylated by Chk1 in vivo. Radiolabeled HAp73α-, HAp73αS47A-, and Chk1 siRNA-transfected cells were treated with cisplatin and were subjected to 2D phosphopeptide mapping in vivo. The purification of p73 proteins was done as described above, and 2D gel electrophoresis was resolved by use of pH 3 to 10 strips and SDS-PAGE. Samples were analyzed by autoradiography.
A precise target context for Chk1 phosphorylation has not been described, although a putative Chk1 consensus site for Cdc25 was previously defined (15). Examination of the p73α amino acid sequence for the Cdc25 canonical site did not show any putative serine residues as possible candidates. Thus, we focused on those serine residues that were conserved among p53 family members, carrying out site-directed mutagenesis on the majority of the serine residues present at the p73 amino-terminal region. To assess the ability of each mutant protein to act as a substrate for Chk1 in vivo, we used a similar approach to the one described above, but this time we cotransfected each point-mutated HAp73α-TAD-PxxP construct along with Chk1 (Fig. (Fig.3D).3D). Further analysis of these purified mutant forms showed that the mutation of serine 47 to alanine abolished serine phosphorylation of this region, as detected by Western blotting with antiphosphoserine antibodies. In contrast, phosphorylation of any other point mutant was not significantly altered. To prove that serine 47 is a p73α phosphorylation site for Chk1, purified p73αS47A mutant was also tested as a substrate for Chk1 by use of an in vitro kinase assay, as described above. As shown in Fig. Fig.3E,3E, wild-type p73α, but not p73αS47A, was phosphorylated by Chk1 in vitro. Finally, to confirm that serine 47 is a p73α phosphorylation site for Chk1, HAp73α-, HAp73αS47A-, and Chk1 siRNA-transfected cells were treated with cisplatin and were subjected to 2D phosphopeptide mapping (Fig. (Fig.3F).3F). HA-p73α and HA-p73αS47A proteins were purified by immunoprecipitation and digested with trypsin. Subsequently, peptides were resolved by 2D gel electrophoresis, and phosphopeptides were visualized by autoradiography. As shown in Fig. Fig.3F,3F, ,2D2D tryptic phosphopeptide mapping in the presence of ectopic p73α showed one central radiolabeled spot (denoted with an arrow in Fig. Fig.3F)3F) that was missing from p73αS47A-transfected cells and after expression of Chk1 siRNAs. Coomassie blue staining of the copurified p73 proteins showed that HA-p73α coimmunoprecipitated with Chk1 protein (Fig. (Fig.1D1D and and2D),2D), besides other contaminants (data not shown). This could explain the additional phosphorylation events that disappeared in part when Chk1 expression was reduced by Chk1 siRNAs (Fig. (Fig.3F;3F; compare left panel with right panel). Altogether, these observations indicate that p73α is phosphorylated, at least in part, on serine 47 by Chk1 kinase both in vivo and in vitro.
Chk1 phosphorylates p73α Ser47 upon DNA damage and upregulates p73α transactivation ability.
To investigate the ability of p73αS47A to be phosphorylated by Chk1 in response to DNA damage, we carried out similar approaches to those described for Fig. Fig.1B,1B, but this time we overexpressed either p73α or p73αS47A for 24 h and then treated the cells with cisplatin, daunorubicin, or UCN-01 for an additional 24 h (Fig. (Fig.4A).4A). To detect both p73 and Chk1 phosphorylation, HA-p73 and Chk1 proteins were immunoprecipitated with an HA epitope MAb and an anti-Chk1 (G4) antibody, respectively. HA-p73 proteins were eluted with an excess of HA peptide and identified by Western blotting using specific anti-p73α and antiphosphoserine (anti-P-Ser) antibodies. Consistent with our previous results, serine phosphorylation of p73α was only detected for cells expressing p73α in the absence of the Chk1 inhibitor UCN-01, and only after treatment with daunorubicin or cisplatin (Fig. (Fig.4A,4A, lanes 3 to 6). However, we did not observe serine phosphorylation of p73αS47A under any circumstance. Hence, the absence of serine at position 47 abolishes detectable Chk1-dependent p73α serine phosphorylation upon DNA damage. These data support the existence of a signaling pathway that involves both p73 and Chk1 as important mediators in the cellular response to DNA damage.

Chk1 phosphorylates p73α serine 47 upon DNA damage and enhances p73 transactivation activity. (A) In response to DNA damage, p73α serine 47 is a substrate for Chk1 kinase. H1299 cells were transfected with plasmids expressing either p73α or p73αS47A for 24 h and were left untreated (−) or treated (+) with the indicated chemotherapeutic agents for an additional 24 h, as described in Materials and Methods and the legend for Fig. Fig.1B.1B. Chk1, HAp73α, and HAp73αS47A proteins were immunoprecipitated (IP) with an anti-Chk1 (G4) antibody and HA-epitope MAb, respectively. HAp73 proteins were eluted with an excess of HA peptide and identified by Western blotting (Wb) using specific anti-p73α (ER-13) and antiphosphoserine (P-Ser/IPp73) antibodies. The protein levels of Chk1 were determined by Western blot analysis with anti-Chk1 or anti-phospho-Chk1-S345 (P-S345). Anti-Ran immunoblotting was performed as a loading control. (B) Chk1 kinase enhances p73α-mediated transactivation. SAOS2 cells were transiently transfected with plasmids expressing p73α, p73α(R292H), p73β, p53, p53 R175H, Chk1, and Chk1 (D130A), along with a luciferase reporter plasmid (p21-Luc), in the combinations indicated. In all cases, total DNA was normalized with equivalent amounts of the control pCMV vector. Cellular extracts were prepared for luciferase assays (B and C). Luciferase activity was quantified and normalized to the internal Renilla reporter assay. Histograms represent the means of three experiments performed in triplicate in relative luminescence units (RLU); bars indicate standard deviations of the means.
In addition, we wanted to know whether modification by Chk1 affects p73 function as a transcriptional regulator. SAOS2 cells were transfected with a luciferase reporter construct (7) controlled by a p53 response element from the p21 promoter (p21-Luc), together with a plasmid that expressed p73α, p73α (R292H), p73β, p53, p53R175H, Chk1, or Chk1(D130A) (Fig. (Fig.4B).4B). The magnitude of both p53 and p73α transactivation activities was consistent with previously published data (11, 28). When p73α was coexpressed with Chk1, a marked increase in p73 transcription ability was detected. Note that significant changes were not found with a kinase-inactive Chk1 (Chk1D130A) (Fig. (Fig.4B,4B, compare columns 2, 10, and 11). As controls, Chk1, p73α (R292H), a mutant defective for DNA binding and transcriptional activation, and p53R175H, a tumor-derived p53 mutant, had no effect on the reporter gene activity. This ability of Chk1 to increase p73 transactivation activity was also tested by use of another luciferase reporter construct under the control of the Bax-2 promoter, with similar results (data not shown). Since these results suggest that Chk1 increases p73-dependent transcription, we tested whether increasing Chk1 protein levels affects p73 transactivation activity by using the luciferase reporter construct (p21-Luc). As shown in Fig. Fig.4C,4C, the ability of Chk1 to stimulate p73 transcriptional activation was augmented in a Chk1 dose-dependent manner. Interestingly, this effect was not observed when Chk1 was coexpressed with full-length p73αS47A (Fig. (Fig.4C,4C, compare columns 3 and 7). Taken together, these findings strongly demonstrate that Chk1 improves p73 transactivation function by serine 47 phosphorylation. We conclude that there might be a correlation between the capacity of Chk1 to phosphorylate p73α at serine 47 and its ability to upregulate p73α transactivation ability.
p73α regulation by Chk1 upon DNA damage allows enhancement of p73α apoptotic function.
Although a role for p73 in DNA damage-induced apoptosis was discovered in cell-based experiments, recent genetic studies with knockout mice suggest that not only p53 is necessary to activate apoptosis in response to DNA damage, but also p73 (and p63) is required for DNA damage to cause apoptosis (10). To assess the functional consequences of p73 phosphorylation by Chk1, we explored whether Chk1 regulation affected the p73α apoptotic function by using low apoptotic doses of cisplatin (1, 12, 33, 37). H1299 cells were mock transfected or transfected with plasmids expressing p73α, HA-p73αS47A, or HA-Chk1D130A and were then treated with cisplatin (5 μM) at various times. We avoided high apoptotic doses of cisplatin (50 μM) because the percentage of apoptotic cells rose to 48% after 48 h (data not shown), masking a potential induction mediated by p73α. After transfection with the backbone expression plasmid and cisplatin action, cellular extracts were analyzed by immunoprecipitation and immunoblotting with anti-p73α and antiphosphoserine (anti-P-Ser/IPp73) antibodies for specific p73α recognition and anti-Chk1 and antiphosphoserine 345 antibodies for Chk1 detection. The induction of endogenous p73α after DNA damage allowed us to detect its serine phosphorylation (Fig. (Fig.5A,5A, lanes 2 to 4) as well as a weak apoptotic response (8% after 48 h) (Fig. (Fig.5B).5B). In addition, we detected that exogenously expressed HA-p73α was serine phosphorylated only after cisplatin treatment (Fig. (Fig.5A,5A, compare lanes 5 and 6). Consistent with previous results shown in Fig. Fig.1A,1A, we again confirmed that p73α regulation by Chk1 is indeed DNA damage dependent. Flow cytometric analysis of cells stained with annexin V revealed that ectopic p73α works in combination with cisplatin action to considerably increase apoptotic levels (from 3 to 20%) (Fig. (Fig.5B),5B), consistent with previously published results (12, 17). In HAp73αS47A-transfected cells, HAp73 proteins were purified by the HA epitope MAbs and eluted with HA peptide. We observed that the lack of Chk1 regulation abolished p73 serine phosphorylation (Fig. (Fig.5A,5A, lanes 10 to 12) and that the apoptotic level decreased to the control level, likely by the effect of endogenous p73α. Blocking Chk1-dependent activity by Chk1D130A overexpression caused a minor apoptotic response, likely as a consequence of endogenous p73α that accumulated but was not serine phosphorylated (Fig. (Fig.5A,5A, lanes 14 to 16). These data suggest that the synergistic effect of p73 and cisplatin is likely due to p73 phosphorylation by Chk1, because we did not detect this result in transfected cells with either p73αS47A or Chk1D130A. Indeed, we again used Chk1 siRNAs to block Chk1 expression and function in the presence of ectopic p73α (Fig. (Fig.5C5C and D). In those cells in which Chk1 expression was reduced by Chk1-specific siRNAs (Fig. (Fig.5D),5D), we reverted the ability of ectopic p73α to potentiate cisplatin-induced apoptosis in H1299 and HCT116-3 cells (Fig. (Fig.5C).5C). Taken together, these data suggest the existence of a signaling pathway by which the proapoptotic function of p73α requires DNA damage-triggered p73 phosphorylation by Chk1.
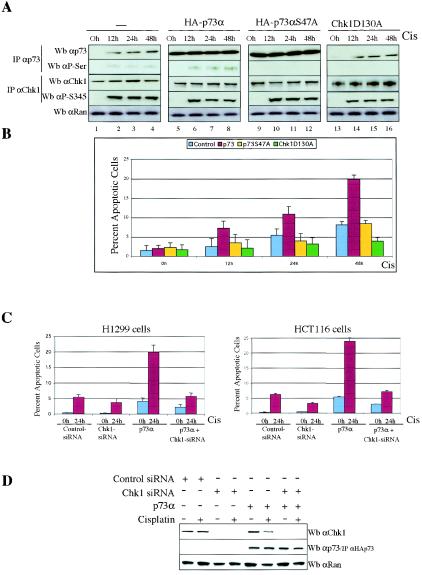
Chk1 regulation enhances the apoptosis function of p73. (A and B) p73 serine phosphorylation is reduced in the presence of HA-p73αS47A or HA-Chk1D130A and p73-induced apoptosis. H1299 cells were either mock transfected or transfected with DNA encoding p73α, HA-p73αS47A, or HA-Chk1D130A for 24 h and were left untreated (0 h) or treated with cisplatin at the indicated times. Cells were harvested, and duplicate aliquots were purified by immunoprecipitation (IP) and then analyzed by Western blotting (Wb) with the antibodies indicated (A) or stained with annexin V. (B) Percentages of apoptotic cells were quantified by flow cytometry. Data are means of three independent experiments; bars indicate standard deviations. (C and D) Reduction in endogenous Chk1 protein results in attenuation of p73-induced apoptosis. H1299 and HCT116-3 cells were transfected with control siRNAs, Chk1 siRNAs, or p73α, in the combinations indicated. Cells were mock or cisplatin treated (Cis) and were harvested 24 h later. Cells were harvested and apoptosis was quantified (C). Cellular extracts were prepared, and p73 and Chk1 proteins were revealed by Western blot analysis using anti-p73 (C-17) antibody for p73 and anti-Chk1 (G4) antibody for Chk1. Anti-Ran immunoblotting was performed as a loading control (D).
DISCUSSION
Findings from the present study allow us to propose a model for p73α regulation by Chk1 kinase upon DNA damage. Support for this model is based on the following observations. Because p73α accumulates and Chk1 is activated upon DNA damage, Chk1 is able to serine phosphorylate endogenous p73α in several different cell lines. Phosphorylation at serine residues of both endogenous and ectopic p73α by Chk1 was demonstrated in H1299 and HCT116-3 cells in the presence of cisplatin, daunorubicin, UCN-01, a Chk1 kinase-dead mutant, or Chk1-specific siRNA expression (Fig. (Fig.1A1A and B). Phosphorylation of p73α was also shown to occur because of Chk1 overexpression in vivo by competition studies with a Cdc25C fragment without phosphatase activity (Fig. (Fig.1C).1C). Parallel studies indicated that both p73α and p73β proteins were phosphorylated by human Chk1 in vitro, and interestingly, both substrates were not phosphorylated by Chk2 (Fig. (Fig.2A2A and B). Additionally, serine 47 was identified as a site of Chk1 phosphorylation in vivo and in vitro (Fig. (Fig.3).3). In addition, we have confirmed that p73α is serine phosphorylated in a Chk1-dependent manner after cisplatin treatment, but when cells overexpressed either Chk1D130A (Fig. (Fig.5A)5A) or Chk1-specific siRNA (Fig. (Fig.1A),1A), we were unable to detect p73α serine phosphorylation. The expression of a single point mutant of p73α lacking the phosphorylation site (p73αS47A) impaired this phosphorylation with and without treatment (Fig. (Fig.4A4A and and5A).5A). However, since Chk1 is a serine/threonine kinase, we favor the idea that human p73α is phosphorylated on Ser 47 in response to DNA damage or replication blocks. The activation of Chk1 following these treatments is not surprising, as Chk1 appears to be relatively specific in response to cisplatin or daunorubicin (38). These findings, in conjunction with those that demonstrated a physical interaction between p73α and Chk1 (Fig. (Fig.1D1D and and2C),2C), argue that Chk1 may directly phosphorylate p73α in vivo and in vitro on serine 47 upon DNA damage. While it is tempting to speculate a new role for Chk1 as a p73α upstream regulator, more extensive analyses are needed to make any definitive statements. We cannot rule out the fact that under different experimental conditions, additional p73α phosphorylation sites by Chk1 could be identified.
Although some conservation at the amino acid level of Chk1, Chk2, and the upstream proteins among species suggests that the basic cell cycle machinery is probably preserved, the checkpoints in which each effector kinase participates appear not to be conserved (22, 30). We checked whether the phosphorylation seen in cells was a result of a direct phosphorylation of p73α by Chk1 and did not involve other intermediate kinases. Using in vitro kinase assays, we showed that p73α and p73β are phosphorylated by Chk1, but not by Chk2 (Fig. (Fig.2A2A and B). We also verified the kinase activity of Chk1 and Chk2 by Cdc25 phosphorylation (Fig. (Fig.2C).2C). This enforced the idea that both Chk1 and Chk2 share substrate specificities, e.g., for Cdc25C, in response to DNA damage, but they might also target different proteins, such as p73α and p53, when the signals that activate the checkpoint are different.
Although sequence substrate specificity of Chk1 has not been reported, a comparison of the sequences surrounding the known Chk1 phosphorylation sites for Cdc25 isoforms, in humans, Xenopus laevis, and Schizosaccharomyces pombe, reveals certain conserved basic and hydrophobic residues near the N-terminal targeted serine. However, there are no obvious Cdc25 consensus motifs on p73α. This suggests that Chk1 kinase may be extremely flexible in its sequence requirements and substrate specificity. We believe that our powerful and standard experimental strategies do ascertain that serine 47 is a potent candidate for phosphorylation by Chk1 in vivo and in vitro (Fig. (Fig.3),3), as is functionally demonstrated in additional assays. However, more work is needed to elucidate the sequence and structural requirements of Chk1 among its known substrates.
DNA damage-induced events, such as phosphorylation, acetylation, or even dephosphorylation, might be involved in the regulation of p73α transactivation capacity and its turnover. Consistent with this postulate, we observed that there is a strong association between the ability of Chk1 to phosphorylate p73α and its capacity to increase p73α transcriptional activity, as demonstrated by coexpressing the p73αS47A construct along with Chk1 (Fig. (Fig.4B4B and C). Also relevant is the fact that expression of a kinase-inactive form of Chk1 did not stimulate such p73α function. Additional studies are required to determine the mechanisms by which Chk1 regulates p73 function. Thus, previous observations support the hypothesis that a direct interaction between Chk1 and p73 might be sufficient to activate p73 in vivo, although other proteins could be involved.
It has been reported that cisplatin activates endogenous p73 by a posttranscriptional mechanism (14). Protein stability of endogenous and exogenous p73α has been shown to be regulated in part through proteasome-dependent degradation, since p73α is accumulated after treatment with proteasome inhibitors (20). Furthermore, ionizing γ-irradiation activates p73 through c-Abl-mediated tyrosine phosphorylation without protein stabilization (1, 34). In accordance with this notion, we have demonstrated that endogenous p73α is accumulated and phosphorylated by Chk1 upon DNA damage (Fig. (Fig.11 and and5).5). This indicates that cisplatin treatment is capable of both inducing the accumulation of p73α and converting a latent inactive p73α form to an active one by Chk1 regulation. As a consequence, we showed that Chk1-dependent activation of p73α by DNA damage could contribute to the induction of apoptosis. Additional support to connect apoptosis and p73α phosphorylation by Chk1 is shown by p73α overexpression, which increases an apoptotic response as a result of cisplatin-induced DNA damage (Fig. (Fig.1A1A and and5),5), as previously demonstrated (5). H1299 and HCT116 cells were transfected with wild-type p73α, the nonphosphorylatable mutant p73αS47A expression vector, or Chk1-specific siRNAs and were further exposed to low apoptotic doses of cisplatin. Apoptosis was measured by the appearance of phosphatidylserine molecules on the external surface of the plasma membrane (binding to annexin V). The percentage of apoptotic cells revealed that exogenously expressed wild-type p73α cooperates with cisplatin action to significantly increase levels of apoptosis only in the presence of Chk1 (from 6 to 23%; Fig. Fig.5C).5C). Therefore, both accumulation of endogenous p73α over a certain threshold and serine phosphorylation of p73 seem to be required for the induction of apoptosis by p73α. Since the mechanisms that regulate the levels and activity of p73 have not been fully elucidated, and because of its ability to function as a transcription factor, our model predicts that p73 transcriptional activity might be tightly associated with its proapoptotic function.
Due to the fact that p73, unlike p53, is not frequently mutated in human cancer (4), we propose that p73 itself or agents that can activate the proapoptotic p73 pathway can be used as potential therapeutic agents against p53-defective tumors.
Acknowledgments
We are indebted to Eva Hernando for her remarkable help in this work. We thank Pier-Paolo Pandolfi, Isabel Martinez-Lacaci, and Miguel Alaminos-Mingorance for helpful discussions and critical comments on the manuscript. The technical assistance of Ella Freulich is acknowledged.
S.G. was supported by Human Frontier Science Program long-term fellowship LT0299/2000. This work was supported by an NCI PO1CA87497 grant award.
REFERENCES
Articles from Molecular and Cellular Biology are provided here courtesy of Taylor & Francis
Full text links
Read article at publisher's site: https://doi.org/10.1128/mcb.23.22.8161-8171.2003
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc262369?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
The p53 family member p73 in the regulation of cell stress response.
Biol Direct, 16(1):23, 08 Nov 2021
Cited by: 39 articles | PMID: 34749806 | PMCID: PMC8577020
Review Free full text in Europe PMC
Epigenetic modification in the expression of p73 p73 - epigenetic target for anticancer therapy.
Oncol Rev, 13(2):421, 24 Jul 2019
Cited by: 1 article | PMID: 31410249 | PMCID: PMC6661529
p53 and its isoforms in DNA double-stranded break repair.
J Zhejiang Univ Sci B, 20(6):457-466, 01 Jun 2019
Cited by: 12 articles | PMID: 31090271 | PMCID: PMC6568222
Review Free full text in Europe PMC
Evaluation of CHK1 activation in vulvar squamous cell carcinoma and its potential as a therapeutic target in vitro.
Cancer Med, 7(8):3955-3964, 02 Jul 2018
Cited by: 3 articles | PMID: 29963769 | PMCID: PMC6089182
p73 coordinates with Δ133p53 to promote DNA double-strand break repair.
Cell Death Differ, 25(6):1063-1079, 06 Mar 2018
Cited by: 28 articles | PMID: 29511339 | PMCID: PMC5988805
Go to all (52) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
p73 induction after DNA damage is regulated by checkpoint kinases Chk1 and Chk2.
Genes Dev, 18(24):3041-3054, 01 Dec 2004
Cited by: 160 articles | PMID: 15601819 | PMCID: PMC535915
Identification of protein kinase A catalytic subunit beta as a novel binding partner of p73 and regulation of p73 function.
J Biol Chem, 280(17):16665-16675, 21 Feb 2005
Cited by: 18 articles | PMID: 15723830
TAp73 induction by nitric oxide: regulation by checkpoint kinase 1 (CHK1) and protection against apoptosis.
J Biol Chem, 286(10):7873-7884, 06 Jan 2011
Cited by: 9 articles | PMID: 21212274 | PMCID: PMC3048674
Funding
Funders who supported this work.
NCI NIH HHS (2)
Grant ID: P01 CA087497
Grant ID: P01 CA 87497





