Abstract
Free full text
Calcium Flux in Neutrophils Synchronizes β2 Integrin Adhesive and Signaling Events that Guide Inflammatory Recruitment
Abstract
Intracellular calcium flux is an early step in the signaling cascade that bridges ligation of selectin and chemokine receptors to activation of adhesive and motile functions during recruitment on inflamed endothelium. Calcium flux was imaged in real time and provided a means of correlating signaling events in neutrophils rolling on E-selectin and stimulated by chemokine in a microfluidic chamber. Integrin dependent neutrophil arrest was triggered by E-selectin tethering and ligation of IL-8 seconds before a rapid rise in intracellular calcium, which was followed by the onset of pseudopod formation. Calcium flux on rolling neutrophils increased in a shear dependent manner, and served to link integrin adhesion and signaling of cytoskeletally driven cell polarization. Abolishing calcium influx through membrane expressed store operated calcium channels inhibited activation of high affinity β2 integrin and subsequent cell arrest. We conclude that calcium influx at the plasma membrane integrates chemotactic and adhesive signals, and functions to synchronize signaling of neutrophil arrest and migration in a shear stress dependent manner.
Introduction
Leukocytes accomplish a critical immune function by negotiating a complex series of signaling events in coordinating arrest and transmigration through vascular endothelium during recruitment to inflamed blood vessels. In the conventional model, neutrophils are captured by selectins and activated through chemokines presented on the apical surface of inflamed venular endothelium, which activate cell arrest via high affinity β2 integrins.42 Neutrophil activation enters a second stage with the polarization of the cell, forming a distinct pseudopod at the leading edge and uropod at the trailing edge in preparation for directed migration across the endothelium. Current evidence indicates that engagement of selectin and integrin bonds serve not only to decelerate neutrophils in the blood stream but also signal activation of cell arrest and migration.1,2,16 However the secondary messengers through which leukocytes integrate chemotactic signals with those following leukocyte engagement of integrin ligands and selectins are largely unknown.
It is widely accepted that an increase in cytoplasmic Ca2+ (calcium flux) is central to the transduction of the neutrophil response to chemokine. IL-8 activates phospholipase (PLC) by receptor mediated release of the Gβγ subunit from CXCR1 and CXCR2, the G-protein coupled receptors associated with this chemokine.40,51 PLC cleaves diacylglycerol (DAG) and inositol-1,4,5 triphosphate (IP3) from membrane lipids, resulting in calcium release from intracellular calcium stores and activation of protein kinase C (PKC).5 In conjunction with PKC, calcium mediates activation of neutrophil adhesion, superoxide production, and exocytosis of secretory granules containing additional integrins and digestive enzymes.5,13,45,48,49 In the context of recruitment, calcium dependent degranulation upregulates the surface expression of the integrin Mac-1 (αMβ2) and potentiates neutrophil adhesion to substrates bearing fibrinogen (a Mac-1 ligand) under static conditions. There is also evidence that calcium participates in the directionality of migration across surfaces. It has been shown that elevated cytoplasmic calcium is necessary for vesicular integrin cycling to the forward edge of a migrating neutrophil.13,23,29,30
Because of its central role in signaling downstream of chemokine ligation and adhesion function, intracellular calcium concentration has proven to be a sensitive indicator of the level of neutrophil activation during recruitment to inflammatory substrates under shear stress.21 However, studies of the signaling role of calcium have been conducted largely with neutrophils in suspension or sedimented on a fibronectin or glass substrate and fewer have recapitulated the physiological milieu.47 An applied force is required for effective activation and ligand binding by β2 integrins on a leukocyte, raising the possibility of a role for shear stress in the interactions between integrin and calcium flux.1 Indeed, binding of Mac-1 and LFA-1 to target ligands is essential for maximum calcium flux and activation of calcium dependent kinases.8,18,19,33,36,37 Because shear stress mediated force transduction and integrin ligation have striking affects on the activation and recruitment of neutrophils, the relationship between calcium and physiologically relevant mechanical loading of integrins is of great interest.
We report that IL-8 mediated calcium signaling is enhanced by neutrophil rolling under shear stress in a model of vascular inflammation. Influx of intracellular calcium was found to play an essential role for both neutrophil arrest and subsequent polarization on a selectin bearing substrate in shear flow. Correlation of the kinetics of recruitment and calcium flux yielded the remarkable finding that neutrophil deceleration mediated by high affinity β2 integrins and transition to a migratory state is synchronized by chemokine dependent calcium flux. Furthermore, neutrophils must be exposed to physiological shear stress in order to elicit the maximal calcium flux and ensuing directed polarization. Compared to rolling neutrophils, suspended neutrophils in the absence of shear stress or a selectin bearing substrate produced far less calcium signal and minimal upregulation of β2 integrin affinity. Thus, calcium functions in the integration of chemotactic and adhesive signaling pathways that serve to organize neutrophil arrest and orient neutrophil migration to vascular sites of inflammation.
Methods
Neutrophil Isolation
Human neutrophils were isolated from whole blood by density gradient centrifugation as previously described.43 Briefly, freshly donated human blood was layered atop a ficoll/percoll solution (Thermo Fisher Scientific, New Hampshire) and centrifuged for 30 min at 1800 rpm according to manufacturer instructions. The neutrophil band was removed and diluted in endotoxin tested HEPES buffered salt solution containing 0.1% human serum albumin. Although there has been some evidence that isolating neutrophils from peripheral blood in this manner causes activation,11,14 all experiments were conducted in parallel with un-stimulated controls. Any background activation of calcium flux caused by isolation thus simply raises the baseline calcium concentration which is exceeded or suppressed in experimental conditions.
Calcium Detection
Calcium concentration was detected within the cytoplasm of neutrophils by measuring emission from Fura-2 when excited by 380 and 340 nM light, and calibrating the measured ratio to calcium concentration standards (Invitrogen). A non-ratiometric calcium dye, Fluo-4 was used to detect intracellular calcium with higher time resolution. These reporters were loaded into neutrophils by incubating cells at a concentration of 1 × 106/mL with 1 μM of the acetoxymethyl-ester (AM) conjugated form of each dye for 30 min at 37 °C in calcium free HEPES buffered saline. Following loading, neutrophils were pelleted (centrifuged 30 s at 500 g), resuspended in HEPES buffered saline containing 1.5 mM CaCl2, and incubated a further 30 min at 37 °C.
Inhibitors
For certain conditions, calcium flux was suppressed by loading 1 × 106/mL neutrophils with 50 μM BAPTA-AM for 30 min at 37 °C in calcium free HEPES buffered saline. Although 50 μM BAPTA has been shown to prevent detectable increases in intracellular calcium,17 it is possible that calcium chelation allows a transient increase in local concentration proximal to the cell membrane and calcium stores due to the high rate of calcium transport.
As a supplementary method to calcium chelation, we sought to block calcium channels at the plasma membrane. The calcium channel inhibitor 2-aminoethoxydiphenyl borate has been shown to inhibit calcium flux multiple leukocyte types including monocytes, T-cells and neutrophils. There has been some controversy over the nature of the calcium channels inhibited by 2-APB, and the molecule was initially classified as an inhibitor of IP3 mediated release of calcium from intracellular stores. However, further evidence has strongly linked 2-APB to inhibition of store operated calcium channels at concentrations higher than 30 μM, and its use contributed to the discovery of the agent of store operated calcium flux, Orai1. Therefore, we also inhibited store operated calcium flux by incubating 1 × 106 neutrophils/mL in 100 μM 2-APB for 8 min at room temperature. All inhibitors were dissolved in dimethylsulfoxide (DMSO), and therefore all control experiments were conducted in the presence of 0.1% DMSO.
Microfluidic Flow Chamber
In order to replicate the fluid shear stress and geometry of a venule, we have designed vascular mimetic microfluidic chambers (VMMCs) as previously described.39 Briefly, we produced flow channel patterns on silicon wafers using photolithography, and created replicas by curing poly(dimethylsulfoxide) (PDMS) above the wafers. Flow and vacuum access holes were punched into the PDMS using symmetrically sharpened 16 gauge needles. The VMMC uses a network of vacuum channels to reversibly bond to a monolayer of substrate cells or adhesion molecules. The particular design used in this study consists of a single channel 600 μm in width and 100 μm in height, and requires a withdrawal flow rate of 12 μL/min to produce an average wall shear stress of 2 dynes/cm2 as estimated from Newtonian flow between infinite parallel plates. Sample buffer containing neutrophils or a chemical of interest is contained in an open 100 μL reservoir upstream from the flow channel, and less than 2 μL of dead volume exists between the reservoir and channel entrance. This allows the analyte fluid to be changed during the course of an experiment with an immediate affect on conditions within the flow channel.
Inflammatory Substrates
Inflamed endothelial cells express a mixture of selectins, integrin ligands, and chemokines that together recruit neutrophils and cause them to arrest. In order to independently measure the affects of selectin, integrin, and chemokine under physiological shear stress, we grew confluent monolayers of murine L-cells transfected to express human E-selectin (L–E) to serve as experimental substrates. L-cells were transfected and maintained as previously described.43
Phase Contrast Videomicroscopy
Neutrophils were isolated from human blood as previously described.15 Isolated neutrophils, diluted to a concentration of 1 × 106 mL−1 in HEPES buffered saline (110 mM NaCl, 10 mM KCl, 10 mM glucose, 1 mM MgCl2, and 30 mM HEPES (pH 7.35)) with 1.5 mM added CaCl2, were drawn into the microchannel at a shear stress of 2 dynes/cm2 by way of a syringe pump (Harvard Apparatus/Kent Scientific) and visualized using a 20× phase contrast objective within an enclosed 37 °C incubator atop an inverted microscope (Nikon TE200). Image sequences from these experiments were digitized by an analog to digital frame grabber (Scion) and recorded for analysis. Neutrophils captured by the relevant monolayer were identified by their brightness under phase contrast illumination. In certain experiments, inhibitors were loaded into the neutrophils as described above prior to input into the flow chamber.
Real Time Fluorescence Imaging and Velocimetry
In order to measure intracellular calcium concentration during the process of rolling, neutrophils pre-loaded with Fura-2-AM were perfused through the microchannel over L–E monolayers atop an inverted fluorescence microscope equipped with a mercury lamp and automated filter wheel. Using software to control filter position and camera acquisition (SimplePCI 5.3) we took a sequence of dual images of emission under 340 and 380 nM excitation. After subtracting background fluorescence, we used the ratiometric method to calculate intracellular calcium concentration as a function of time. Likewise, we used the monochrome calcium sensitive dye Fluo-4-AM in conjunction with 488 nM exitation and intensity measurements to observe calcium transients with greater time resolution. Rolling velocity was measured in neutrophils rolling on L–E monolayers by the distance traveled by the neutrophil centroid between video frames. An average instantaneous velocity was compiled from the velocity traces of individual neutrophils, rejecting frame-to-frame velocities higher than 4 μm/s from the average which corresponded to periods of neutrophil detachment and produced unusual spikes in acceleration/deceleration. Average instantaneous deceleration of neutrophil populations were calculated from average velocity by Eq. (1).

Where d1 is deceleration, Δt is the time step V2 is velocity 1 s after the current deceleration, and V0 is velocity 1 s prior to the current deceleration. An increase in deceleration greater than 0.1 μM/s2 was found to be a reliable indicator of neutrophil deceleration in response to stimulus. The instantaneous fraction of shape-polarized neutrophils was determined by recording the number of neutrophils in each frame that had a length to width ratio greater than 1.5 as determined by pixel measurements of the fluorescent image performed in Image Pro 5.1. In certain experiments, inhibitors were loaded into the neutrophils as previously described prior to input into the flow chamber. Finally, neutrophils were stimulated with interleukin-8 (IL-8) at concentrations between 0.01 and 5 nM, or 4-Bromo A23187 (A2) at concentrations between 300 nM and 2 μM. Acute IL-8 exposure was conducted by exchanging the contents of the microfluidic reservoir with buffer containing a known concentration of IL-8, resulting in a rapid increase in IL-8 concentration to a set value.39
Detection of Active β2 Integrin Expression
Leukocyte β2 integrins exist in active (high affinity for ligand) or inactive (low affinity) conformations. In order to transition from rolling to arrest, a neutrophil must greatly increase the number of active β2 integrins on its surface. This occurs by two mechanisms: a shift to the active conformation for existing integrins on the surface (primarily αLβ2 or LFA-1), and an increase in the number of total integrins on the surface through degranulation (primarily αMβ2 or Mac-1). In order to examine a potential role for Ca2+ in priming arrest, we measured the expression of active β2 integrin and Mac-1 on neutrophils. The mouse anti-human IgG 327C (a generous contribution from ICOS corporation) has been reported to bind only the active conformation of β2 integrin, and thus can serve as a reporter for β2 integrin activation.27 Neutrophils at a concentration of 5 × 105 were loaded with any inhibitors as described earlier, then exposed to 5 min of stimulus in the form of 0.1–5 nM IL-8 or 100–2000 nM A2 in the presence of 10 μg/mL anti-β2 integrin (327C) conjugated to Alexa 488 or 1 mg/mL anti-Mac-1 (2LPm19c) conjugated to R-PE at 37 °C. Samples were then placed on ice and incubated for 30 min. Labeled neutrophils were pelleted, then resuspended in HEPES buffer containing 0.1% HSA at 4 °C. Samples were read on a FACScan cytometer and analyzed for mean fluorescence intensity (MFI) using CellQuest software. During each experiment, a non-stimulated sample was used as a control baseline for antibody labeling, and data were converted to mean fold-increase ((sample MFI/baseline MFI)—1) over this baseline.
Results
Rolling Neutrophils Exhibit Rapid Calcium Flux in Response to IL-8
In order to characterize the dynamics of calcium flux in neutrophils rolling on E-selectin, we developed a technique to image the dynamics of neutrophil adhesive interactions and shape change along with intracellular release of calcium during their initial response to chemokine. Freshly isolated human neutrophils loaded with the calcium sensitive dye Fluo-4 were perfused through a microfluidic flow chamber having as a substrate an L-cell monolayer transfected to express E-selectin (L–E). L-cells are a mouse fibroblastic cell line that have been shown to possess surface ligands to Mac-1 such as albumin and collagen, but do not express human ICAM-1 or LFA-1 binding capacity.39,43 Neutrophils rolling on this E-selectin bearing substrate were imaged in the process of responding to a bolus of IL-8 delivered by replacing the contents of the entry reservoir with a defined concentration of IL-8 dissolved in HEPES buffered salt solution. Although chemokine is linked to the glycocalyx of inflamed endothelium and presented to leukocytes at the apical surface in a manner that enhances arrest and polarization, we have recently reported that soluble IL-8 infused over rolling neutrophils causes deceleration of rolling neutrophils by stimulating receptors on the surface of the neutrophil distant from the point of substrate contact.4,7,31,39 Changes in average Fluo-4 emission in response to IL-8 were measured in rolling neutrophils over the course of 1 min (Fig. 1a). As depicted in the representative field of cells, an abrupt increase in intracellular calcium was recorded with maximum response within ~3 s (Fig. 1b). Perfusion of 0.1 nM, but not 0.01 nM IL-8, into the flow chamber produced a robust increase in intracellular calcium in rolling neutrophils that reached a peak after 10 s and began to gradually decrease towards baseline levels by 1 min.
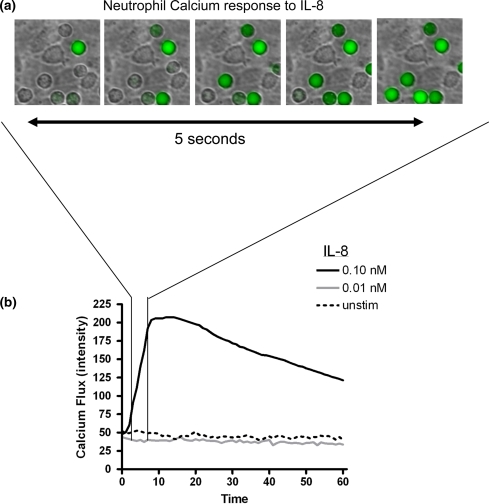
Dynamics of calcium flux in neutrophils rolling to arrest in presence of perfused chemokine. Neutrophils were loaded with Fluo-4 and perfused over monolayers expressing E-selectin at a shear stress of 2 dynes/cm2, then exposed to a dose range of IL-8 following 2 min of shear interaction. (a) Individual neutrophils that have rolled to arrest rapidly increase their intracellular calcium in response to IL-8 (0.1 nM) resulting in an increase in Fluo-4 emission. (b) On average, neutrophils exhibited a rapid increase in Fluo-4 emission indicative of calcium flux in response to IL-8 concentrations of 0.1 nM or higher, but did not significantly increase calcium in unstimulated or at low IL-8 of 0.01 nM. Plot is representative of 4 independent experiments with measurements from at least 60 neutrophils at each labeled concentration of IL-8
Neutrophil Rolling Amplifies Response to Chemokine and Calcium Flux
The combination of shear stress and E-selectin engagement has been shown to activate β2 integrins (CD18) in the absence of chemokine in rolling neutrophils.43 Therefore, we investigated the superposition of activation via E-selectin tethering and exposure to a dose range of IL-8 in eliciting calcium flux on rolling neutrophils. Neutrophils were loaded with Fura-2 and perfused over L–E monolayers and the kinetics of intracellular calcium release was recorded following perfusion of IL-8. In the absence of chemokine, neutrophils exhibited a significantly elevated intracellular calcium concentration when rolling under 2 dynes/cm2 shear stress compared to cell suspensions in the absence of shear (Fig. 2). For instance, stimulation with 0.1 nM IL-8 in suspension did not produce a significant calcium flux (no increase over 18 nM basal calcium), whereas an increase of ~135 nM was measured at this concentration in neutrophils rolling on L–E. Even when stimulated at 50-fold higher doses of IL-8 (i.e., 5 nM), neutrophils in suspension expressed approximately the same calcium flux as rolling neutrophils in the absence of IL-8. These data suggest that rolling on E-selectin superposes with signaling via chemokine to increase calcium flux by several orders over IL-8 stimulation in static suspension.

Dose dependence ofcalcium flux on stimulation with IL-8 in rolling or suspended neutrophils. Neutrophils were loaded with the ratiometric calcium indicator Fura-2 and perfused over a monolayer transfected with E-selectin. Calcium concentration was measured by ratiometric imaging in neutrophils sedimented onto the monolayer (Static) or rolling on the monolayer under shear stress (2 dynes/cm2) following exposure to a dose range of IL-8 from 0.001 to 5 nM. The average calcium concentration in all neutrophils in a field of view was measured over time, and the peak value was recorded. Data are the average of 4 or more independent experiments at each IL-8 concentration
Intracellular Calcium Directly Activates β2 Integrin
In order to elucidate the role of calcium flux in amplifying and directing the activation of CD18, we employed the β2 integrin activation reporter antibody 327C to measure how calcium signaling regulates the shift in integrin to a high affinity state.27 Treatment of neutrophils with the ionophore 4-bromo-A23187 (A2) resulted in a dose dependent increase in intracellular calcium (Fig. 3a) and subsequent upregulation of high affinity β2 integrin (Fig. 3b). We analyzed using FACS the surface expression of high affinity CD18 on a population of neutrophils stimulated with IL-8 under conditions that enhanced or suppressed calcium flux. Treatment with 1000 nM A2 was sufficient to elicit a peak in calcium flux comparable to that generated by 5 nM IL-8 (102 nM vs. 109 nM Ca2+). It is noteworthy that this concentration of ionophore was much less efficient than IL-8 since it only elicited a ~1-fold increase in CD18 activation compared to a ~10-fold increase with 5 nM IL-8 (Fig. 3b). In contrast, a calcium flux of 145 nM induced by addition of 2 μM A2, elicited an increase in CD18 expression comparable to that generated by IL-8. This result indicates that at maximal release, calcium is inherently capable of inducing nearly as much high affinity β2 integrin as stimulated by chemokine (Fig. 3c). The amplification of IL-8 mediated upregulation of high affinity CD18 was dependent on store operated calcium flux, as 327C expression was reduced to 5% of its maximal level by application of the inhibitor 2-aminoethoxydiphenyl borate (2-APB) at 100 μM. This concentration of inhibitor has previously been shown to abolish calcium flux in monocytes and T-cells.20,35 Likewise, chelation of intracellular calcium with BAPTA-AM substantially reduced expression of activated CD18 and eliminated calcium flux (Fig. 3c). Taken together, the data imply that calcium is both necessary and sufficient to upregulate high affinity β2 integrin.

Expression of high affinity β2 -integrins is regulated by intracellular calcium. (a) Peak calcium concentration in suspended neutrophils loaded with Fura-2 was measured by fluorescent microscopy following stimulation by 5 nM IL-8 or a range of calcium ionophore concentrations (A2). Bars represent the average calcium concentration measured in the neutrophils, and are representative of 3 independent experiments. (b) Flow cytometry was conducted on neutrophils that were labeled with the monoclonal antibody 327C-Alexa488, which binds specifically to the high affinity conformation of β2 integrins. Labeled neutrophils were stimulated with 5 nM IL-8 or a range of ionophore concentration. (c) As in (b), neutrophils were labeled with 327C-Alexa488 and analyzed by flow cytometry. Cells were stimulated with 5 nM or 0.1 nM IL-8, and in certain experiments incubated with the calcium channel inhibitor 2-APB at 100 μM. The upregulation of active CD18 induced by IL-8 or by each concentration of ionophore is displayed as the average fold-increase in 327C fluorescent signal compared to unstimulated controls from 3 independent experiments
Neutrophil Deceleration and Polarization Requires Calcium Flux
Since calcium flux is necessary for upregulation of high affinity β2 integrin in response to IL-8, we measured the dependence of arrest and shape polarization on calcium flux in rolling neutrophils. Infusing a bolus of IL-8 elicits rolling neutrophils to decelerate toward arrest and exhibit an increase in intracellular calcium concentration. Since 0.1 nM IL-8 was found to be a robust activator of calcium flux and arrest when neutrophils were rolling on a substrate, but not in suspension, this concentration was used to activate subtle changes in neutrophil recruitment. In order to provide a quantitative measure of the affect of calcium signaling on chemokine mediated recruitment, we measured the fraction of neutrophils that arrested 1 min following a 0.1 nM IL-8 bolus infusion (Fig. 4a). The transition to a migratory phenotype was quantified using a measure of cell shape polarization (i.e., an increase in the length to width ratio >1.5) (Fig. 4b). Treatment of neutrophils with BAPTA or 2-APB abolished any increase in neutrophil arrest or polarization on L–E monolayers following IL-8 stimulation. However, arrest and polarization were fully restored by administration of A2 (Figs. 4a and and4b)4b) or washout of 2-APB (data not shown) confirming that the defect in neutrophil recruitment is specific to calcium flux rather than a non-specific affect of 2-APB. Thus, blockade of plasma membrane calcium channels reduces the influx of calcium from the extracellular medium, which in effect blocks arrest and polarization of neutrophils.

Dependence of IL-8 mediated arrest and polarization on intracellular calcium. Neutrophils were pre-treated with inhibitors or agonists to the calcium signaling pathway, and were observed adhering to L-cell monolayers expressing E-selectin by phase contrast microscopy. 0.1 nM IL-8 was introduced into the flow chamber triggering neutrophil arrest and shape change. (a) The fraction of total neutrophils that were arrested on the monolayer was measured 1 min after IL-8 influx under conditions which stimulated or inhibited calcium flux. (b) The fraction of neutrophils undergoing polarization (as defined in the methods) was likewise measured 3 min following IL-8 infusion. Both polarization and arrest are significantly suppressed by 2-ABP, and rescued when ionophore A2 is introduced along with 2-APB. Bars represent the average of at least 3 independent experiments, and error bars represent the SEM
Since neutrophil arrest, cell polarization, and calcium release are dynamic processes that occur during recruitment in shear flow, we correlated in time cell deceleration and shape change along with intracellular calcium flux in IL-8 activated neutrophils loaded with Fura-2-AM (Figs. 5a and and5b).5b). The displacement history along with the intracellular calcium concentration and the length to width ratio (i.e., polarization) of individual rolling neutrophils was recorded at 1 s intervals. Notably, when neutrophils were stimulated with IL-8, the onset of deceleration appeared to precede the onset of significant calcium flux by ~3 s (Fig. 5c). However, when store operated Ca2+ channels were inhibited with 2-APB or chelated with BAPTA, neutrophil deceleration was significantly impaired and this correlated with the elimination of the peak in calcium flux (Fig. 6). These data suggest that entry of extracellular calcium ions is vital for stabilizing arrest. The fact that infusion of the ionophore A2 with 2-APB partially restored the slope of calcium influx and arrest supports this conclusion. In contrast to the correlation between calcium flux and arrest, the onset of neutrophil polarization occurred after intracellular calcium plateaued at its maximum level. Directly liberating intracellular calcium stores by addition of 2 μM A2 was sufficient to elicit arrest, but not polarization in the absence of chemokine in a smaller population of neutrophils (Fig. 6). On the other hand, when neutrophils were co-stimulated with IL-8 and A2, the onset of polarization but not arrest was hastened by 10 s. These data demonstrate that low levels of intracellular calcium influx through plasma membrane channels support integrin activation leading to cell arrest, which is necessary to activate a much larger flux of intracellular calcium. Furthermore, these results suggest that the rise in intracellular calcium serves to synchronize the initiation of neutrophil polarization.
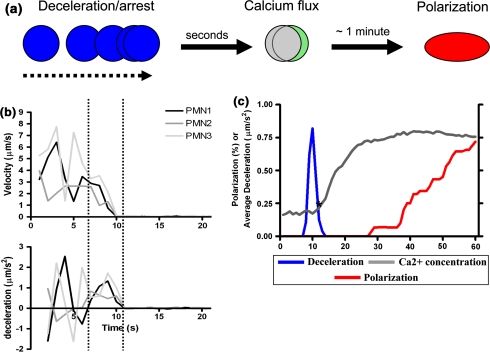
Neutrophils rolling on L–E cells decelerate to arrest before fluxing calcium and undergoing shape polarization. Neutrophils were pre-loaded with Fura-2 calcium indicator and their trajectories and shape change were simultaneously recorded at 1 s intervals in order to measure intracellular calcium, neutrophil deceleration, and polarization as a function of time. (a) The diagram depicts the stages of neutrophil response to infusion of IL-8. Neutrophils first decelerate from a steady state rolling velocity to a stable arrest in response to IL-8 stimulation, then exhibit a change in the ratiometric emission by Fura-2 indicating calcium flux. An increasing fraction of neutrophils become polarized (non-spherical) with time in preparation for migration as determined by the neutrophil aspect ratio. (b) A representative trace of instantaneous velocity and calculated deceleration of three individual neutrophils. Deviations in deceleration due to discontinuous rolling largely cancel each other outside of the two dotted lines, leaving a single spike in deceleration (downslope in velocity) marking arrest in response to IL-8. (c) Traces of intracellular calcium and deceleration in this panel are the average of at least 30 traces of individual neutrophils as in (b) from 3 separate experiments. The primary spike in average deceleration, intracellular calcium, and the fraction of neutrophils polarized are plotted for the minute following IL-8 infusion. Single asterisk indicates where calcium concentration significantly increases above background—3 s following significant deceleration

Kinetics of calcium flux, cell arrest and shape change in response to 2-APB and BAPTA. Traces of intracellular calcium and deceleration in this panel are the average of at least 30 traces of individual neutrophils as in (Fig. 5c) from 3 separate experiments. (a) BAPTA abolishes intracellular calcium altogether, resulting in defective neutrophil arrest and migration. Traces of average deceleration, calcium concentration, and polarization as affected by administration of 0.1 nM IL-8 plus (b) the calcium channel inhibitor 2-APB at 100 μM, (c) the ionophore A2 at 2 μM, (d) a mixture of 2-APB and A2, and (e) A2 without IL-8 are shown as indicated. Asterisks indicate that neutrophils pretreated with the ionophore A2 take significantly less time to polarize than wildtype neutrophils (p < 0.05)
Shear Stress Catalyzes Potentitation of Calcium Release and Neutrophil Polarization
Expression of high affinity CD18 is largely dependent on calcium flux under static conditions when measured by flow cytometry in cell suspension (see Fig. 3). Furthermore, arrest under shear stress requires calcium flux presumably initiated by plasma membrane calcium channels (i.e., APB inhibition results of Figs. 4 and and6).6). We hypothesized that integrin ligation during neutrophil deceleration and arrest under shear flow is necessary for catalyzing intracellular calcium flux, a necessary step for subsequent directed migration. We simultaneously quantified polarization and calcium flux in arrested neutrophils activated by IL-8 under normal (2 dynes/cm2) and low (0.2 dynes/cm2) shear stress (Fig. 7a). In contrast to a robust response to IL-8 stimulation under 2 dynes/cm2, neutrophils under 0.2 dynes/cm2 shear stress exhibited minimal calcium flux over 2 min (Fig. 7b). Indeed, Fluo-4 emission in neutrophils stimulated under low shear was not significantly different from that detected when store operated calcium channels were inhibited with 2-APB (Fig. 7b). This abrogation of calcium flux also corresponded with a significant decrease in neutrophil polarization (Figs. 7a and and7b).7b). Furthermore, when shear stress was subsequently increased from 0.2 up to 2 dynes/cm2, neutrophils rapidly detached from the monolayer, while those continuously exposed to 2 dynes/cm2 remained adherent (Fig. 7c). This shear dependent increase in adhesion strength was rescued in neutrophils treated under low shear when exposed to calcium ionophore (data not shown). Taken together, these results suggest that shear stress upregulates chemokine dependent arrest and polarization of neutrophils in part by catalyzing a rapid increase in cytoplasmic calcium, which in turn upregulates the number of high affinity β2 integrins that themselves participate in outside-in signaling.
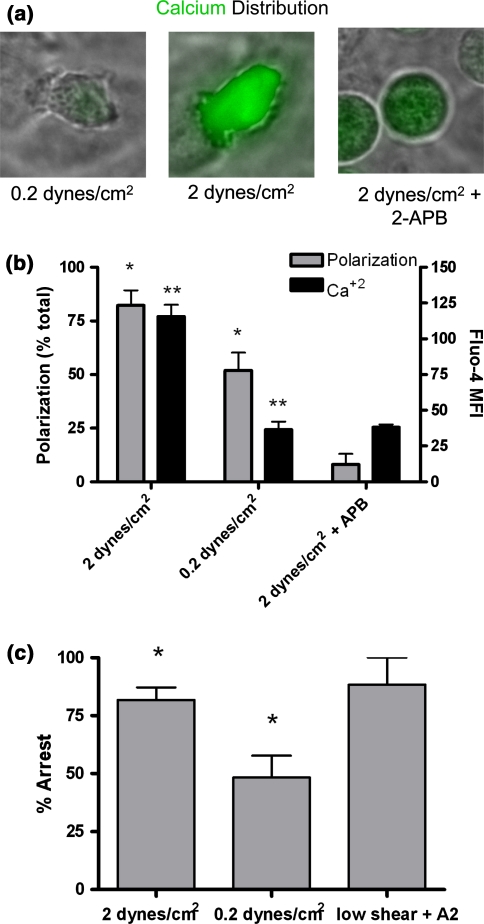
Shear stress activates calcium flux and cell polarization. Neutrophils were loaded with Fluo-4, perfused over an L–E monolayer, then stimulated with 0.1 nM IL-8 for 2 min at 0.2 dynes/cm2 (low) or 2 dynes/cm2 (normal) shear stress, and observed at high resolution with a 63× plan APO oil objective (Nikon). In some experiments, shear stress was increased to 2 dynes/cm2 after 2 min of incubation with 0.1 nM IL-8 at 0.2 dynes/cm2. (a) Changes in neutrophil shape and calcium flux following addition of IL-8 were monitored for 2 min by videomicrosopy in the presence and absence of 2-APB. (b) Intracellular calcium was quantified by the average fluorescent intensity of Fluo-4 emission (out of 255) at the centroid of the neutrophil and polarization was quantified by the fraction of visible cells that exhibited a radius ratio greater than 1.5. Both polarization and calcium flux were significantly lower in neutrophils under low shear than under normal shear conditions as indicated by * and ** respectively. Bars are representative of 3 or more independent experiments. (c) Neutrophils were pre-conditioned with 0.2 or 2 dynes/cm2 shear stress as indicated for 2 min, then observed 1 min at a shear stress of 2 dynes/cm2 (elicited by a step change in flow rate). Fraction of total neutrophils remaining adherent to an L–E monolayer in a microscope field were recorded. Bars are representative of <5 observations from 3 independent experiments and * indicates a significant difference (p < 0.05 by t-test)
Discussion
A fundamental question in the field of immunology remains how leukocytes sense and efficiently navigate the repulsive forces of flowing blood to efficiently arrest within microvascular sites of inflammatory injury. In the current study we applied video microscopy with in-situ fluorescence and phase imaging of neutrophils in a custom microfluidic channel to observe the process of signal transduction via selectins, chemokines, and integrins under hydrodynamic stress.
Integrin Activity is Regulated from the Inside-Out by Calcium Release
Calcium flux was necessary and sufficient to upregulate high affinity β2 integrins, which functioned to decelerate and arrest rolling neutrophils. Integrin activation was abrogated by chelation of intracellular calcium and reversibly inhibited by blocking store operated calcium channels. Induction of calcium flux with ionophore was comparable to IL-8 stimulation in producing a robust increase in high affinity CD18. These data led to the conclusion that integrin conversion to high affinity is dependent on calcium flux. These results in neutrophils are in line with a recent study by Hyduk et al. that SDF-1α dependent activation of VLA-4 and arrest of monocytes to VCAM-1 are dependent on functional calcium flux and the activity of PLC.20 Thus, calcium flux is a central mediator of chemokine dependent changes in integrin conformation that cause leukocyte arrest on inflamed endothelium. Moreover, these data are the first to reveal the central role of shear stress in the generation of calcium flux triggered by sub-nanomolar concentrations of chemokine, thereby implicating a mechanism by which shear forces enhances signaling through bound selectins and integrins during neutrophil rolling and arrest.
Spatial-Temporal Aspects of Calcium Flux
By quantifying the concentration of intracellular calcium it was possible to precisely measure the temporal synchronization of chemokine mediated calcium flux and cell deceleration in rolling neutrophils. Surprisingly, we found that neutrophil deceleration and arrest preceded detection of intracellular calcium stores release by seconds. Nonetheless, the elimination of IL-8 mediated arrest and high affinity β2 integrin by 2-APB or BAPTA indicate that calcium is critical for arrest. We hypothesize that local influx of calcium via channels within the immediate region (i.e., sub-micron) of high affinity ligated integrins are activated to open and facilitate additional diffusion of calciuim into the cytoplasm.32,34 This phenomena of diffusion limited calcium response has previously been observed in neurons where calcium dependent neurotransmitter release occurs before detection of cytoplasmic calcium through indicators.38 Extrapolation to neutrophils would suggest that calcium diffusion from the extracellular fluid and intracellular stores is the rate limiting step in triggering cytoplasmic flux of calcium from endoplasmic reticulum and calciosomal stores.
The agent 2-ABP has been shown to target the recently discovered store operated calcium channel, Orai1, acting as an activator at concentrations below 10 μM, and a potent inhibitor at concentration over 30 μM.10,35,46 Thus, the fact that 100 μM 2-APB is an effective inhibitor of arrest and high affinity β2 integrin despite its incomplete inhibition of intracellular calcium (compared to BAPTA) supports the notion that integrin activation is regulated by the rate of calcium entry via store operated channels at the plasma membrane, rather than a global increase in cytoplasmic calcium. Calcium concentrations detected within nanometers of the plasma membrane are up to 3 orders of magnitude higher than the average concentration recorded in the neutrophil following chemotactic stimulus.6 These calcium rich microdomains within the neutrophil cortex may provide a focal region that is further regulated by calcium sensitive kinases that function to amplify the response.6,25,26,44 Indeed, patch clamp studies demonstrate, that neutrophil exocytosis is triggered at calcium concentrations only detected within calcium microdomains, indicating that granules, and possibly other signaling complexes, cluster around calcium channels.29,30 A rise in cytoplasmic calcium concentration may function at a secondary level to direct F-actin mediated neutrophil migration following cell deceleration and arrest. This conclusion is supported by the observation that shape polarization and migration followed within less than 1 min the peak in absolute calcium concentration (170 nM for 0.1 nM IL-8).
Calcium Flux is Catalyzed by Adhesion Molecule Ligation Under Shear Stress
Our data indicate that shear stress mediated buildup of tension on E-selectin and CD18 bonds are important regulators of calcium flux and serve to catalyze subsequent polarization of neutrophils. In the absence of shear stress or adhesion to the cell monolayer, neutrophils failed to produce significant calcium flux in response to 0.1 nM IL-8, while they produced 10-fold greater levels of intracellular calcium when rolling on E-selectin. Likewise, neutrophils exposed to 0.1 nM IL-8 under low shear stress failed to produce a calcium signal any greater than in neutrophils inhibited with 2-APB. Finally, rolling on E-selectin produced a small but significant increase in intracellular calcium in the absence of chemokine. These results indicate that rolling under physiological shear stress provides an important co-signal to activate calcium flux. One possible mechanism is that engagement of integrins or E-selectin ligands under tension directly stimulates stretch activated calcium channels, which are sensitive to 2-APB inhibition. Alternately, neutrophil–substrate interaction at sufficient shear stress (i.e., 2 dynes/cm2 but not 0.2 dynes/cm2) may increase force directed contact with the substrate and likewise amplification of selectin, integrin, and chemokine receptor ligation on rolling neutrophils.7,24 This hypothesis is supported by a recent study by McMeekin et al. who showed enhancement in the duration of PAF mediated calcium flux in the presence of soluble E-selectin even in the absence of shear stress.28 In this case, rolling under shear stress simply serves to increase the rate of E-selectin engagement by the neutrophil and in turn enhance calcium flux. In either case, the defect in calcium flux under low shear may explain the marked reduction in neutrophil polarization found in stimulated neutrophils in that forces were insufficient to trigger opening of calcium channels, or that integration of signals on the L–E monolayer was impaired. Indeed, it has been frequently observed that leukocyte transmigration across inflamed endothelium is highly dependent on physiological shear stress.3 Thus, membrane tension may play a key role in catalyzing calcium dependent signal during leukocyte attachment to inflamed endothelium.
Signaling Pathways that Link Membrane Calcium Receptors to Shear Stress and Neutrophil Function
Recent evidence that chemokines and integrins are activated in close spatial proximity suggests that calcium flux may participate in directing outside-in signaling as integrins bind ICAMs under shear flow.1,41 The notion that integrins and calcium channels are located within nanometers of each other within the plane of the plasma membrane could explain how shear stress, calcium flux, and chemokine mediated signaling correlate with time and force in facilitating neutrophil activation and integrin mediated deceleration in less than a second.41
Our observations suggest a model in which chemokine rapidly activates intracellular calcium channels through PLCβ and IP3, exposing a membrane associated signaling complex to high concentrations of calcium and producing transient activation of β2 integrin as depicted in Fig. 8. Shear stress applies tensile force to high affinity integrin bonds which may further stabilize the high affinity β2 integrin conformation and facilitate diffusion to form multivalent high avidity clusters.12,22 High affinity β2 integrin produces an “outside-in” signal through focal adhesion kinase and Src family kinases, which are known to interact with PLCγ, potentiating further calcium store depletion, and influx through store operated membrane channels.50 We hypothesize that plasma membrane calcium channels adjacent to stressed integrins are opened resulting in a calcium microdomain that converts nearby β2 integrin to a high affinity state and stabilizes arrest (Fig. 8b). Following arrest, diffusion of calcium into the cytoplasm may activate calcium sensitive secondary kinases such as p38 MAPk and RhoA which direct F-actin formation and stress mediated orientation of cytoskeletal forces that direct migration.9,15,22,34,43 Thus, synchronization of critical events along the pathway from rolling to arrest to polarization is achieved by calcium flux initiated by chemokine signaling, which leads to integrin binding that in turn drives the localized of release of calcium and arrest followed by a global increase in calcium and migration (Fig. 8c).

Synchronization of selectin and chemokine signaling of neutrophil arrest via calcium flux. (a) β2 integrins in a resting neutrophil are expressed in a low affinity conformation clustered around a calcium channel and in close proximity to a chemokine receptor (CXCR 1). Upon recognition of IL-8, a G-protein coupled signal (represented here with PLCβ) is transmitted from the receptor to a nearby low affinity β2 integrin, via store release calcium triggering a shift to high affinity. (b) Under shear stress, high affinity β2 integrin is stabilized by binding to ICAM-1 and is put under tension, resulting in an outside-in signal which results in association of Src family kinases with the cytoplasmic domain of the integrin and PLCγ. (c) Depletion of a peripheral calcium store rapidly activates a local calcium channel, triggering calcium flux. A local hemispherical domain of high calcium concentration activates RhoA, causing surrounding β2 integrins to shift to high affinity, which is stabilized by ICAM-1 ligation, causing neutrophil deceleration and arrest
This model places the decisive event in leukocyte recruitment within the region of endothelial contact we have denoted the inflammatory synapse.42 Within this region, high affinity integrins and stress activated calcium channels may function together as effective force transducers that directionally guide cytoskeletal contractile forces. Ongoing studies in our laboratory have focused on investigating the dynamics in spatial association between high affinity β2 integrins, peripheral calcium stores, and plasma membrane calcium channels that may underlie the organizational role of calcium.
Acknowledgments
We thank Dr. Noo Lee Jeon at UC Irvine for assistance in design and fabrication of the microfluidics flow channels used in this study. Generous support from NIH R01 AI47294 to SIS. Grant Numbers and Sources of Support: NIH R01 AI47294 to SIS.
Abbreviations
| A2 | 4-Bromo-A23187 |
| 2-APB | 2-Aminoethoxydiphenyl borate |
| VMMC | Vascular mimetic microfluidic chamber |
| L–E | L-cells transfected with E-selectin |
References
Articles from Annals of Biomedical Engineering are provided here courtesy of Springer
Full text links
Read article at publisher's site: https://doi.org/10.1007/s10439-008-9453-8
Read article for free, from open access legal sources, via Unpaywall:
https://link.springer.com/content/pdf/10.1007%2Fs10439-008-9453-8.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/134345982
Article citations
Candida albicans induces neutrophil extracellular traps and leucotoxic hypercitrullination via candidalysin.
EMBO Rep, 24(11):e57571, 05 Oct 2023
Cited by: 4 articles | PMID: 37795769 | PMCID: PMC10626426
Local and Systemic Effects of Porphyromonas gingivalis Infection.
Microorganisms, 11(2):470, 13 Feb 2023
Cited by: 10 articles | PMID: 36838435 | PMCID: PMC9963840
Review Free full text in Europe PMC
Spatiotemporal characteristics of P-selectin-induced β2 integrin activation of human neutrophils under flow.
Front Immunol, 13:1023865, 10 Nov 2022
Cited by: 8 articles | PMID: 36439190 | PMCID: PMC9692129
Nexinhib20 Inhibits Neutrophil Adhesion and β2 Integrin Activation by Antagonizing Rac-1-Guanosine 5'-Triphosphate Interaction.
J Immunol, 209(8):1574-1585, 07 Sep 2022
Cited by: 8 articles | PMID: 36165184 | PMCID: PMC9529951
β2-Integrin Adhesive Bond Tension under Shear Stress Modulates Cytosolic Calcium Flux and Neutrophil Inflammatory Response.
Cells, 11(18):2822, 09 Sep 2022
Cited by: 1 article | PMID: 36139397 | PMCID: PMC9497066
Go to all (58) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Neutrophil Inflammatory Response Is Downregulated by Uptake of Superparamagnetic Iron Oxide Nanoparticle Therapeutics.
Front Immunol, 11:571489, 09 Dec 2020
Cited by: 8 articles | PMID: 33362760 | PMCID: PMC7757401
Selectin catch-bonds mechanotransduce integrin activation and neutrophil arrest on inflamed endothelium under shear flow.
Blood, 130(19):2101-2110, 15 Aug 2017
Cited by: 53 articles | PMID: 28811304 | PMCID: PMC5680610
Shear-dependent capping of L-selectin and P-selectin glycoprotein ligand 1 by E-selectin signals activation of high-avidity beta2-integrin on neutrophils.
J Immunol, 172(12):7780-7790, 01 Jun 2004
Cited by: 78 articles | PMID: 15187162
E-selectin ligands as mechanosensitive receptors on neutrophils in health and disease.
Ann Biomed Eng, 40(4):849-859, 24 Jan 2012
Cited by: 43 articles | PMID: 22271244 | PMCID: PMC3680630
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NIAID NIH HHS (2)
Grant ID: R01 AI047294
Grant ID: R01 AI47294





