Abstract
Purpose
Methotrexate plasma concentration is related to its clinical effects. Our aim was to identify the genetic basis of interindividual variability in methotrexate pharmacokinetics in children with newly diagnosed acute lymphoblastic leukemia (ALL).Patients and methods
We performed a genome-wide analysis of 500,568 germline single-nucleotide polymorphisms (SNPs) to identify how inheritance affects methotrexate plasma disposition among 434 children with ALL who received 3,014 courses of methotrexate at 2 to 5 g/m(2). SNPs were validated in an independent cohort of 206 patients.Results
Adjusting for age, race, sex, and methotrexate regimen, the most significant associations were with SNPs in the organic anion transporter polypeptide, SLCO1B1. Two SNPs in SLCO1B1, rs11045879 (P = 1.7 x 10(-10)) and rs4149081 (P = 1.7 x 10(-9)), were in linkage disequilibrium (LD) with each other (r(2) = 1) and with a functional polymorphism in SLCO1B1, T521C (rs4149056; r(2) > 0.84). rs11045879 and rs4149081 were validated in an independent cohort of 206 patients (P = .018 and P = .017), as were other SLCO1B1 SNPs residing in different LD blocks. SNPs in SLCO1B1 were also associated with GI toxicity (odds ratio, 15.3 to 16.4; P = .03 to .004).Conclusion
A genome-wide interrogation identified inherited variations in a plausible, yet heretofore low-priority candidate gene, SLCO1B1, as important determinants of methotrexate's pharmacokinetics and clinical effects.Free full text

Germline Genetic Variation in an Organic Anion Transporter Polypeptide Associated With Methotrexate Pharmacokinetics and Clinical Effects
Abstract
Purpose
Methotrexate plasma concentration is related to its clinical effects. Our aim was to identify the genetic basis of interindividual variability in methotrexate pharmacokinetics in children with newly diagnosed acute lymphoblastic leukemia (ALL).
Patients and Methods
We performed a genome-wide analysis of 500,568 germline single-nucleotide polymorphisms (SNPs) to identify how inheritance affects methotrexate plasma disposition among 434 children with ALL who received 3,014 courses of methotrexate at 2 to 5 g/m2. SNPs were validated in an independent cohort of 206 patients.
Results
Adjusting for age, race, sex, and methotrexate regimen, the most significant associations were with SNPs in the organic anion transporter polypeptide, SLCO1B1. Two SNPs in SLCO1B1, rs11045879 (P = 1.7 × 10−10) and rs4149081 (P = 1.7 × 10−9), were in linkage disequilibrium (LD) with each other (r2 = 1) and with a functional polymorphism in SLCO1B1, T521C (rs4149056; r2 > 0.84). rs11045879 and rs4149081 were validated in an independent cohort of 206 patients (P = .018 and P = .017), as were other SLCO1B1 SNPs residing in different LD blocks. SNPs in SLCO1B1 were also associated with GI toxicity (odds ratio, 15.3 to 16.4; P = .03 to .004).
Conclusion
A genome-wide interrogation identified inherited variations in a plausible, yet heretofore low-priority candidate gene, SLCO1B1, as important determinants of methotrexate's pharmacokinetics and clinical effects.
INTRODUCTION
Whole-genome studies have identified genetic variations associated with risk of complex diseases.1–5 However, whole-genome pharmacogenetic studies are less common despite considerable speculation that such information will transform drug therapy.6–10 Methotrexate is used to treat malignancies, including acute lymphoblastic leukemia (ALL), as well as autoimmune disorders.11–15
The pharmacologic effects of methotrexate are well characterized, and its systemic exposure has been related to cure and toxicity in childhood ALL16–19 and other diseases.20,21,19,22,23 The genetic origins of interindividual pharmacokinetic and pharmacodynamic variability of methotrexate remain poorly understood, with conflicting results on candidate pharmacogenetic predictors for methotrexate.24,25 A genome-wide approach might identify new candidate genes whose polymorphisms forecast which patients might benefit from tailoring dosage on the basis of genomic variation. Herein, we performed a genome-wide association analysis of 434 patients with newly diagnosed ALL and surveyed 500,568 germline single-nucleotide polymorphisms (SNPs).
PATIENTS AND METHODS
Patients and Treatment
The discovery cohort included 434 children (median age, 5.92 years; range, 1.02 to 18.85 years) with ALL enrolled and treated on St. Jude Children's Research Hospital Total XIIIB and Total XV protocols (Data Supplement Table 1).26–28 The validation cohort consisted of the next set of 206 patients (median age, 5.10 years; range 1.17 to 18.67 years) treated on St. Jude's Total XV protocol (Data Supplement Table 1) who were enrolled after the initial analysis began. Total XIIIB included consolidation therapy of two weekly doses of methotrexate (2 g/m2 over 2 hours, followed by leucovorin) and 6-mercaptopurine (75 mg/m2 per night for 2 weeks).26,27 Subsequent therapy included identical methotrexate and 6-mercaptopurine doses administered every 8 weeks up to 1 year. Total XV included four doses of methotrexate during consolidation, each given over 24 hours with dosage adjusted to achieve a steady-state plasma concentration of 33 μmol/L (low-risk arm) or 65 μmol/L (standard/high-risk arm).28 Serum creatinine was measured in the 24 hours before methotrexate treatment. Parents and patients gave informed consent and assent as appropriate, and the study was approved by the institutional review board. All grade 3 and 4 toxicities were prospectively scored using the National Cancer Institute (NCI) Cancer Therapy Evaluation Program (CTEP) (http://ctep.cancer.gov) toxicity criteria during the consolidation and continuation periods.26 The most common toxicities during consolidation, when only methotrexate and 6-mercaptopurine were given, were GI (mucositis) and infection. Detailed procedures for methotrexate administration (Data Supplement Table 2) and evaluations are provided in the Data Supplement.
Pharmacokinetic Data
Clearance was estimated on the basis of three to four methotrexate plasma concentrations per course up to 48 hours from the start of infusion. Clearance was estimated (CL = ke × V) using a two-compartmental linear pharmacokinetic model and a Bayesian approach, via ADAPT software (University of Southern California, Los Angeles, CA), as described.26,29,30 Population priors, mean (variance), were 9.0 L/m2 (22.1) for Vc, 0.70 hours−1 (0.05) for Ke, 0.08 hours−1 (0.002) for Kcp, and 0.11 hours−1 (0.000014) for Kpc as described.30 The average methotrexate clearance per patient across courses was calculated using all available postremission courses.
Genotyping and SNP Filtering Criteria
Germline DNA was extracted from blood after remission was achieved. DNA (500 ng) was applied to the 500K Array Set (Affymetrix, Santa Clara, CA). Chips were scanned and genotype calls were made using the Bayesian Robust Linear Multichip with Mahalanobis Distance (BRLMM) algorithm for 500,568 interrogated SNPs. Initial missing SNP genotypes were imputed using the fastPHASE software (Data Supplement; University of Washington, Seattle).31 SNPs were excluded for genotyping call rates less than 95% (n = 29,314), minor allele frequency less than 1% (n = 63,110), or if allele frequencies deviated from Hardy-Weinberg equilibrium (n = 10,944; P < 1 × 10−7). Some SNPs were excluded that met more than one criterion, leaving 398,699 SNPs in the analysis.
For a subset of patients in the validation cohort (n = 103), SNP genotypes were obtained using Affymetrix Genome-Wide Human SNP Array 6.0 (Affymetrix). The nonsynonymous SNP, SLCO1B1 T521C (rs4149056) was not represented on the Affymetrix arrays and was independently genotyped (DNAPrint Genomics, Sarasota, FL); call rates were more than 97% for this SNP.
Statistical Analysis
Analyses were conducted using R (http://www.r-project.org/) and SAS (SAS Institute, Cary, NC). Associations between average methotrexate clearance and patient characteristics (sex, age, race, and treatment regimen) were tested using analysis of variance (ANOVA). Nongenetic factors (eg, hydration and alkalinization) were standardized for each treatment regimen (Data Supplement). Associations between germline SNP genotypes and methotrexate clearance were evaluated using a general linear model, treating genotype as a numerical variable (0 = AA, 1 = AB, and 2 = BB) and including age, race, sex, and treatment regimen as covariates. Age was coded in years and treated as a continuous variable. Treatment regimen was coded as a categoric variable, and Total XIIIB was considered the baseline covariate in the multivariate analysis. Ancestry was determined using the actual genotypes, computing each patient's probability of having European, African, or Asian ancestry (Data Supplement). Ancestry was alternatively characterized using Eigenstrat software (Harvard Medical School, Boston, MA) on the basis of a principal component analysis of SNP genotypes.32 We found good concordance (94%) between genomically determined ancestry and self-declared race. SNPs whose P value for association with methotrexate clearance was less than 1 × 10−7 were considered reaching genome-wide significance. Because only SNPs annotated to the SLCO1B1 gene were tested for association with methotrexate clearance in the validation cohorts, a P value less than .05 was considered statistically significant. Multiple linear regression analysis was used to estimate percent variation explained by genotypes and other covariates.
A genome-wide linear mixed effect model33 was also conducted with each individual course of methotrexate clearance as a dependent variable, including course number (course 1 to 10 for patients on Total XIIIB and course 1 to 4 for patients on Total XV) and serum creatinine for each course as covariates. A linear mixed effect model was also used to estimate the intra- and interpatient variability in methotrexate clearance in the discovery cohort.
Logistic regression was used to analyze whether SLCO1B1 genotypes were associated with toxicity (GI toxicity and infection) as a dichotomous variable (yes or no) during consolidation. Patients with grade 3 or 4 toxicities (mucositis or infection) were considered as having toxicity. Weighted logistic regression26 was used to analyze the genotype-toxicity association in the 120-week continuation phase of therapy for Total XIIIB, accounting for recurrent episodes and censoring. The cumulative incidences of GI toxicity were compared among genotypes using Gray's test with incorporation of competing risks.34 To compute the odds ratio estimated for toxicity during the consolidation phase of therapy, we added a continuity adjustment of 0.5 to each cell count to deal with the zero event count for those with common homozygous genotypes.
For the gene level analysis, 24,836 genes were annotated to the interrogated SNPs, including all SNPs located within 5,000 base pairs (ie, cis-SNPs) of each transcript. We tested for significant associations between all the SNP genotypes (per gene) and methotrexate clearance using multiple linear regression, and we assessed the percent variation explained by the genotypes using r2 test statistic, the Akaike information criterion, and permutation analyses (Data Supplement).
RESULTS
In the discovery cohort, we estimated methotrexate clearance for 3,014 treatment courses given to 434 patients (Data Supplement Table 1). There was substantial interpatient variability in average (± standard deviation) methotrexate clearance (Fig 1), which differed among the 213 patients on the Total XIIIB (133 ± 25 mL/min/m2), the 114 patients on the Total XV low-risk (123 ± 28 mL/min/m2), and the 107 patients on the Total XV standard/high-risk (106 ± 22 mL/min/m2) treatment regimens (Data Supplement Fig 1; P < 2.2 × 10−16). The variability observed between treatment regimens is not surprising, given that dosage and the concomitant hydration and alkalinization (factors that can affect methotrexate clearance) differed (Data Supplement Table 2).22 Average methotrexate clearance differed by ancestral group (Data Supplement Fig 2; P = .005) with African American patients having the highest clearance. As previously noted,19,22 methotrexate clearance decreased with increasing age (Data Supplement Fig 3; P = 1.1 × 10-6). Thus, we adjusted all analyses for age, race, sex, and treatment regimen. Using a linear mixed effect model, the intrapatient variability in methotrexate clearance was 48%, while the interpatient variability was 52%.
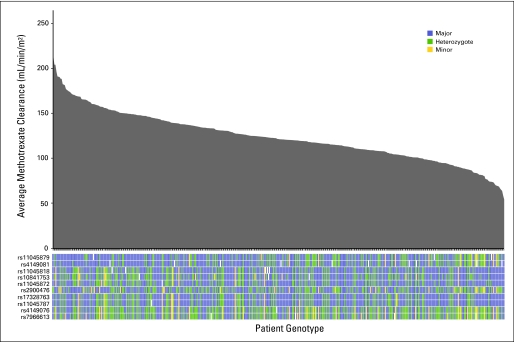
Average methotrexate clearance and linkage disequilibrium structure of the SLCO1B1 single-nucleotide polymorphisms (SNPs) in 434 children with acute lymphoblastic leukemia. The average methotrexate clearance for each of the 434 children in the discovery cohort is plotted from the highest to lowest (gray shaded area), and each patient's genotype at each of the SLCO1B1 SNP loci listed is indicated (colored bars below the x axis). Patients homozygous for the major allele are coded in blue, heterozygotes are coded in green, and those homozygous for the minor allele are coded in yellow.
We tested the association between 398,699 SNPs and average methotrexate clearance in 434 children in the discovery cohort. The strongest association signal was on chromosome 12 (Fig 2). The two top SNPs associated with methotrexate clearance were annotated to the transporter gene, SLCO1B1/OATP1B1, located on chromosome 12 (Table 1; Data Supplement Tables 3-5). These two SLCO1B1 SNPs remained significant (rs11045879, P = 1.7 × 10−10 and rs4149081, P = 1.7 × 10−9) even after correction for multiple testing. These two SLCO1B1 SNPs were in complete LD (r2 = 1) with each other (Fig 1) and were associated with methotrexate clearance across regimens (Fig 3).
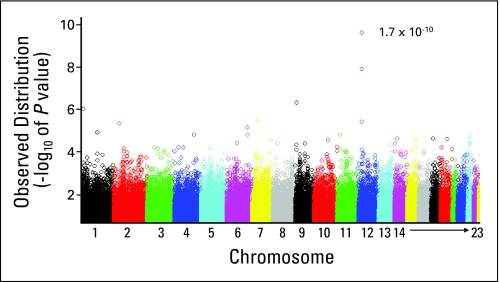
Genome-wide P values showing the association of single-nucleotide polymorphisms (SNPs) with methotrexate clearance in the discovery cohort of 434 children with acute lymphoblastic leukemia. Shown is the distribution of P values (as –log10 values) for the association of 398,699 SNP genotypes with methotrexate clearance in the discovery cohort. The P value of 1.7 × 10−10 is for the most significant association identified between a single SNP (located in SLCO1B1 on chromosome 12) and methotrexate clearance.
Table 1.
SLCO1B1 SNPs and Methotrexate Clearance
| dbSNP ID | Location | Genomic Position | Alleles (A/B) | Discovery Cohort (n = 434) | Validation Cohort (n = 206) | Combined Cohort (n = 640) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MAF | Coefficient* | 95% CI | P | MAF | Coefficient* | 95% CI | P | MAF | Coefficient* | 95% CI | P | ||||
| rs11045879 | Intron | 21273886 | C/T | 0.17 | 13.1 | 9.1 to 17.1 | 1.7 × 10−10 | 0.17 | 6.05 | 1.0 to 10.9 | .018 | 0.16 | 10.8 | 7.6 to 14.0 | 8.2 × 10−11 |
| rs4149081 | Intron | 21269288 | A/G | 0.16 | 12.7 | 8.5 to 16.8 | 1.7 × 10−9 | 0.17 | 6.16 | 1.1 to 11.1 | .017 | 0.16 | 10.4 | 7.2 to 13.7 | 6.7 × 10−10 |
| rs11045818 | Synonymous | 21221028 | A/G | 0.14 | −9.2 | −13.8 to −4.5 | 2.2 × 10−4 | 0.13 | −11.7 | −17.1 to −6.3 | 3.7 × 10−5 | 0.14 | −9.3 | −12.9 to −5.7 | 6.3 × 10−7 |
| rs10841753 | Intron | 21212637 | C/T | 0.20 | −7.8 | −11.7 to −3.8 | 1.3 × 10−4 | 0.15 | −7.1 | −11.8 to −2.3 | .004 | 0.19 | −7.1 | −10.2 to −4.0 | 8.6 × 10−6 |
| rs11045872 | Intron | 21263611 | A/G | 0.16 | 8.1 | 3.7 to 12.5 | 5.0 × 10−4 | 0.15 | 7.1 | 2.0 to 12.2 | .007 | 0.16 | 7.3 | 3.9 to 10.7 | 3.4 × 10−5 |
| rs2900476 | Intron | 21227330 | C/T | 0.23 | 6.5 | 2.8 to 10.2 | 6.0 × 10−4 | 0.26 | 2.5 | −2.0 to 7.0 | .27 | 0.24 | 5.0 | 2.1 to 7.9 | .000796 |
| rs17328763 | 5′ upstream | 21173837 | C/T | 0.15 | −6.1 | −10.6 to −1.6 | .01 | 0.17 | −5.4 | −10.4 to −0.4 | .034 | 0.15 | −5.3 | −8.7 to −1.9 | .002676 |
| rs11045787 | Intron | 21191269 | G/T | 0.15 | −5.9 | −10.4 to −1.4 | .01 | 0.16 | −5.5 | −10.5 to −0.4 | .034 | 0.15 | −5.2 | −8.6 to −1.7 | .003227 |
| rs4149076 | Intron | 21262411 | C/T | 0.32 | 4.4 | 0.9 to 7.9 | .01 | 0.32 | −0.4 | −4.3 to 3.6 | .85 | 0.32 | 2.9 | 0.2 to 5.6 | .033823 |
| rs7966613 | intron | 21270899 | A/G | 0.32 | −3.6 | −7.0 to −0.1 | .03 | 0.33 | 0.8 | −3.1 to 4.8 | .67 | 0.32 | −2.3 | −5.0 to 0.4 | .093071 |
Abbreviations: SNP, single-nucleotide polymorphism; dbSNP, SNP database; ID, identification; MAF, minor allele frequency.

SLCO1B1 single-nucleotide polymorphism (SNP) rs11045879 is associated with methotrexate clearance and GI toxicity. (A) Association of SLCO1B1 SNP genotype (rs11045879) with methotrexate clearance in patients on Total XIIIB (TXIIIB), Total XV low-risk (TXVL), and Total XV standard/high-risk (TXVSH) treatment regimens. Numbers below each box plot indicate the sample size for each genotype. (B) Association of this SNP with GI toxicity. Logistic regression was used to analyze whether genotypes for SLCO1B1 SNPs were associated with toxicity as a dichotomous variable (yes or no) during the 2-week consolidation phase. The bar graphs display the percentage of patients (plotted on the y axis) per genotype (plotted on the x axis) who had grade 3 to 4 GI toxicity. Numbers above each bar represent the number of patients who had toxicity versus those who did not for the specified genotype. Similar relationships exist for SLCO1B1 SNP rs4149081 (data not shown).
We also used a linear mixed effects model to identify associations between SNP genotypes and methotrexate clearance; the results were similar to those assessing average clearance, with the same two top-ranked SNPs (P < 6.8 × 10−9) in SLCO1B1 (Data Supplement Table 4). Only 6.0% of the intracourse variability in clearance was accounted for by course number, even when accounting for the premethotrexate serum creatinine (Data Supplement Table 6).
Eight additional SLCO1B1 SNPs (Table 1) were associated with clearance (P < .05); these SNPs were not in LD (based on r2) with the top two SLCO1B1 SNPs (Fig 1) and were encompassed by multiple haplotype blocks (Data Supplement Figs 4A and and4B).4B). In a gene-level analysis, SLCO1B1 had a stronger association with methotrexate clearance (P < 1 × 10−6), with a higher r2 and a lower Akaike information criterion, than any other gene (Data Supplement Tables 7 and 11).

Incidence of toxicity based on SLCO1B1 rs11045879 genotype. Cumulative incidence of the first episode of GI toxicity significantly differed (P = .024) by SLCO1B1 rs11045879 genotype during the continuation phase of treatment during the Total XIIIB treatment regimen. Cumulative incidences of GI toxicity were compared among genotypes using Gray's test with incorporation of competing risks.
We genotyped a validation cohort of 206 additional patients (Table 1). Seven of the 10 interrogated SLCO1B1 SNPs remained associated (P < .05) with methotrexate clearance (Table 1). Nine of the 10 SLCO1B1 SNPs were associated with clearance (P < .05) when the two cohorts (discovery and validation) were combined (Table 1), and the strongest signal remained in SLCO1B1 (Data Supplement Fig 5).
The percentage of methotrexate clearance variability that could be explained by SLCO1B1 genotypes compared with the variability explained by factors such as race, sex, age, treatment regimen, or serum creatinine was estimated in a multivariate analysis for both the discovery and validation cohorts (Table 2). In a stepwise linear regression analysis, five SNPs were retained in the model (Table 2), such that SLCO1B1 genetic variation accounted for 9.3% of the interpatient variability in the discovery cohort and 11.3% in the validation cohort. This superseded the proportion of variability contributed by the significant factors of race, serum creatinine, and sex; only treatment regimen accounted for a higher proportion (17.9%) of the variation in clearance (Table 2). Average serum creatinine concentrations explained 3.2% and 1.9% of the variability in methotrexate clearance in the discovery and validation cohorts, respectively; in a linear mixed effects model (Data Supplement Table 6), 2.4% of interpatient and 0.4% of intrapatient variability was accounted for by serum creatinine. Treatment regimen and SLCO1B1 SNP genotypes accounted for 18.6% and 11.0% of variability in clearance, respectively. Results were similar for the first cycle only of methotrexate (Data Supplement Table 8).
Table 2.
Multivariate Analysis of Factors Associated With Average Methotrexate Clearance in the Discovery and Validation Cohorts
| Variables | Discovery Cohort (n = 434) | Validation Cohort With Serum Creatinine Data*(n = 125) | Validation Cohort† (n = 206) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient‡ | 95% CI | P§ | r2 (%) | Coefficient‡ | 95% CI | P§ | r2 (%) | Coefficient‡ | 95% CI | P§ | r2 (%) | |
| Ancestry¶ | .0007 | 2.1 | .32 | 1.6 | .021 | 4.1 | ||||||
    African African | Ref‡ | Ref‡ | Ref‡ | |||||||||
    European European | −13.0 | −19.8 to −6.3 | −7.9 | −18.5 to 2.8 | −7.3 | −16.7 to 2.0 | ||||||
    Asian Asian | −6.5 | −25.0 to 12.0 | 2.4 | −31.8 to 36.6 | 14.6 | −4.3 to 33.6 | ||||||
| Sex | .003 | 0.01 | .02 | 3.3 | .046 | 1.1 | ||||||
    Female Female | Ref‡ | Ref‡ | Ref‡ | |||||||||
    Male Male | 6.6 | 2.2 to 10.9 | 7.7 | 1.1 to 14.3 | 5.6 | 0.2 to 11.0 | ||||||
| Serum creatinine, mg/dL‖ | −60.2 | −86.8 to −33.7 | 8.6 × 10−6 | 3.2 | −33.6 | −71.8 to 4.6 | .09 | 1.9 | NA | NA | NA | |
| Age, years | 0.39 | −0.49 to 1.3 | .39 | 0.1 | 0.98 | −0.21 to 2.2 | .10 | 1.7 | −0.5 | −1.1 to 0.1 | .093 | 1.7 |
| Treatment regimen | 1.1 × 10−25 | 17.9 | .00013 | 9.3 | .001 | 2.8 | ||||||
    Total XV low-risk Total XV low-risk | Ref‡ | Ref‡ | Ref‡ | |||||||||
    Total XV standard/high-risk Total XV standard/high-risk | −15.9 | −22.1 to −9.7 | −15.0 | −22.8 to −7.3 | −7.2 | −13.1 to −1.4 | ||||||
    Total XIIIB Total XIIIB | 14.3 | 8.7 to 19.8 | NA | NA | ||||||||
| SLCO1B1 genotype# | 2.3 × 10−10 | 9.3 | .002 | 11.3 | 4.7 × 10−4 | 9.3 | ||||||
    rs4149081 (A/G) rs4149081 (A/G) | 14.0 | 8.5 to 19.5 | 5.2 | −3.3 to 13.8 | 6.6 | 0 to 13.2 | ||||||
    rs10841753 (C/T) rs10841753 (C/T) | −7.2 | −13.6 to −0.8 | 0.07 | −12.1 to 12.3 | 1.3 | −6.8 to 9.4 | ||||||
    rs11045818 (A/G) rs11045818 (A/G) | −8.3 | −19.4 to −2.9 | −18.6 | −34.7 to −2.4 | −17.8 | −29.7 to −5.8 | ||||||
    rs2900476 (C/T) rs2900476 (C/T) | 3.4 | −1.6 to 8.4 | 3.4 | −4.2 to 11.0 | 2.9 | −3.1 to 8.9 | ||||||
    rs17328763 (C/T) rs17328763 (C/T) | −8.4 | −18.5 to 1.6 | −6.0 | −17.4 to 5.3 | −6.2 | −14.7 to 2.1 | ||||||
Abbreviation: NA, Not applicable.
GI toxicity (grade 3 or 4 mucositis) and infection were the most common toxicities observed during the methotrexate-intensive consolidation and continuation phases of Total XIIIB therapy.26 SNPs in SLCO1B1, rs11045879 T allele (OR, 16.4; 95% CI, 8.7 to 26.7; P = .004) and the G allele at rs4149081 (OR, 15.3; 95% CI, 7.9 to 24.6; P = .03; data not shown) were each associated with GI toxicity during consolidation (Fig 3). The same two SNPs were also associated with GI toxicity during the continuation phase (Fig 4). During the consolidation phase of Total XV, GI toxicity was less frequent (5% v 18% in Total XIIIB), and we found no association with SLCO1B1 SNP genotypes. Because all patients on Total XV had their methotrexate doses adjusted to achieve a common steady-state plasma concentration, relationships among toxicity and methotrexate exposure and SLCO1B1 SNPs may have been attenuated on this trial. We found no associations between SLCO1B1 SNP genotype and infectious toxicity in either trial (data not shown).
We also genotyped the nonsynonymous SLCO1B1 T521C (rs4149056) polymorphism in a subset of 489 patients; this subset included 387 patients from the discovery cohort and 102 patients from the validation cohort (Data Supplement Table 1). SLCO1B1 T521C was in high LD with the top SLCO1B1 SNPs rs11045879 (r2 = 0.86, discovery cohort; r2 = 0.89, validation cohort) and rs4149081 (r2 = 0.86, discovery cohort; r2 = 0.89, validation cohort). SLCO1B1 T521C was associated with methotrexate clearance in the discovery and the combined (discovery plus validation) patient cohorts (Data Supplement Table 9; P = 1.9 × 10−7 and P = 1.2 × 10−7, respectively). In addition, a marginal association (P = .07) was observed between this SNP genotype and the occurrence of GI toxicity during consolidation (Data Supplement Fig 6). In multivariate analysis, the T521C SNP was significantly associated with methotrexate clearance; however, when genotypes at T521C and at rs11045879 were allowed to compete, only the rs11045879 SNP remained in the model (Data Supplement Table 10).
DISCUSSION
Using a genome-wide interrogation of germline variation, we uncovered genetic variation that affects the disposition and effects of methotrexate in children with ALL. The strongest genetic variation associated with methotrexate pharmacokinetics and dynamics resided in a reasonable candidate gene, SLCO1B1, although this transporter was not previously studied as a candidate gene in clinical pharmacogenetic studies of methotrexate.35–38
The SLCO1B1 locus maintained significance after adjusting for covariates such as age, race, serum creatinine, sex, and treatment regimen and was verified in an independent validation set. The top-ranked SNPs (Data Supplement Tables 3-5), rs11045879 and rs4149081, were located in the gene (SLCO1B1) encoding an organic anion transporter (OATP1B1). Eight additional SNPs in SLCO1B1 had P values less than .05 for their association with methotrexate clearance (Table 1). The fact that multiple SNPs in this locus, some of which were not in LD, were independently associated with methotrexate clearance provides further evidence of the functional importance of SLCO1B1.
SLCO1B1 is localized at the sinusoidal membrane of hepatocytes, and its transcript has been detected in enterocytes.39–41 SLCO1B1 mediates uptake of substrates from sinusoidal blood, resulting in their net excretion from blood (likely via biliary excretion). SLCO1B1 has been shown to transport methotrexate in vitro,42,43 as well as other compounds such as bilirubin, bile acids, 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors,44,45 benzylpenicillin, rifampicin, angiotensin-converting enzyme inhibitors, and the active metabolite of irinotecan, SN-38.46,47
Previous studies have described functional consequences of nonsynonymous polymorphisms in SLCO1B1 in vitro43,48 and in vivo (eg, statins).49 The SLCO1B1 T521C SNP was in high LD (r2 = 0.84) with the SNPs interrogated on the genome-wide arrays we used, and our independent genotyping confirmed an association of lower methotrexate clearance (higher systemic exposure) with the C allele. This is consistent with prior studies that demonstrate greater plasma exposure to multiple SLCO1B1 substrates in those carrying the 521C allele than those homozygous for the 521T allele.49–51 Nonetheless, most of the SLCO1B1 SNPs that were associated with clearance (Table 1) were noncoding SNPs, not in LD with T521C, and in a multivariate model, SNPs other than T521C maintained an association with methotrexate clearance (Data Supplement Table 10). The fact that these SNPs resided throughout the gene suggest multiple mechanisms by which variations affect the function of SLCO1B1, perhaps by effects on transcription and post-transcriptional processing (eg, pre-mRNA splicing and mRNA translation). Moreover, noncoding SNPs may be in linkage with rare, nontyped coding SNPs. Thus it appears that the function of SLCO1B1 may be affected by several different polymorphisms.
SNPs in SLCO1B1 were associated not only with methotrexate clearance but also with GI toxicity (Fig 3) in Total XIIIB. Only 5% of patients in Total XV had toxicity, and this may in part be attributed to targeting of methotrexate to achieve a specific plasma concentration. The alleles that were associated with lower plasma exposure were always associated with increased GI toxicity. This finding is consistent with lower blood concentrations but higher GI tract or enterocyte concentrations of methotrexate being associated with GI toxicity. The fact that SLCO1B1 polymorphisms were not associated with the other common toxicity,26 infection, is consistent with GI concentrations being a determinant of methotrexate GI toxicity52,53 and being of relatively minor importance for infectious toxicity. Because 6-mercaptopurine was given during consolidation, toxicity (particularly infection) may have been partly due to 6-mercaptopurine. However, GI toxicity is more closely linked to methotrexate53 than to thiopurines.54,55
SLCO1B1 genotypes were associated with methotrexate clearance across three different treatment regimens (Fig 3) and were significant after adjusting for regimen differences and for sex, race, renal function, and age (Table 2 and Data Supplement Table 6). Moreover, clearance was measured on multiple occasions, and thus the average value of clearance is a composite that already accounts for intrapatient variability that may have been due to nongenetic causes. Accounting for each course independently in a mixed effects model, and accounting for variation in serum creatinine,56 polymorphisms in SLCO1B1 remained significant predictors of interpatient variability in clearance. The fact that serum creatinine explained a small fraction (2.4%) of the interpatient variability in methotrexate clearance may be because serum creatinine was monitored before administration of the drug, and renal dysfunction was a contraindication to high-dose methotrexate. The proportion (9.3%) of interpatient variability in drug clearance attributable to germline variation in a single gene is on par with or greater than the variability accounted for by a single gene for other complex phenotypes.57
Identifying patients at risk of low methotrexate clearance could be useful for monitoring and supportive care during high-dose methotrexate, particularly in settings in which rapid turnaround of plasma levels is not available. Moreover, identifying that SLCO1B1 accounts for a substantial degree of interpatient variability in clearance highlights that drug interactions are likely to occur if a potent SLCO1B1 substrate is given with methotrexate.
Methotrexate is metabolized to a 7-OH methotrexate metabolite.58 This metabolite could interact with SLCO1B1. Other genetic variations may influence methotrexate clearance, such as in SLCO1A2 (Data Supplement Tables 3-5).59 Moreover, there may be important insertion/deletion or germline copy number variations that were not adequately interrogated on the array we used.
Using a whole-genome approach, we identified a plausible candidate gene, SLCO1B1, that is associated with methotrexate pharmacokinetics and pharmacodynamics. This illustrates the proof of principle that genome-wide tools can lead to the discovery of important pharmacogenetic links between inherited genomic variation and drug response in humans.
Acknowledgment
We thank our protocol coinvestigators, clinical and research staff, particularly Pamela McGill, Natalie Lowery, Sean Freeman, Nancy Kornegay, and Jennifer Pauley, PharmD, as well as patients and families for their participation. We thank the staff from the St Jude Hartwell Center for Bioinformatics and Biotechnology.
Footnotes
Supported by National Cancer Institute Grants No. CA 51001, CA 078224, and CA 21765; National Institutes of Health/National Institute of General Medical Sciences Pharmacogenetics Research Network and Database Grants No. U01 GM61393, U01 HL65899, and U01GM61374; a Center of Excellence grant from the State of Tennessee; and by the American Lebanese Syrian Associated Charities.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Mary V. Relling
Financial support: Mary V. Relling, William E. Evans
Administrative support: Ching-Hon Pui, William E. Evans, Mary V. Relling
Collection and assembly of data: Lisa R. Treviño, Noriko Shimasaki
Data analysis and interpretation: Lisa R. Treviño, Noriko Shimasaki, Wenjian Yang, John C. Panetta, Cheng Cheng, Deqing Pei, Diana Chan, Alex Sparreboom, Kathleen M. Giacomini, William E. Evans, Mary V. Relling
Manuscript writing: Lisa R. Treviño, Mary V. Relling
Final approval of manuscript: Lisa R. Treviño, Noriko Shimasaki, Wenjian Yang, John C. Panetta, Cheng Cheng, Deqing Pei, Diana Chan, Alex Sparreboom, Kathleen M. Giacomini, Ching-Hon Pui, William E. Evans, Mary V. Relling
REFERENCES
Articles from Journal of Clinical Oncology are provided here courtesy of American Society of Clinical Oncology
Full text links
Read article at publisher's site: https://doi.org/10.1200/jco.2008.20.4156
Read article for free, from open access legal sources, via Unpaywall:
https://ascopubs.org/doi/pdfdirect/10.1200/JCO.2008.20.4156?role=tab
Subscription required at intl.jco.org
http://intl.jco.org/cgi/content/full/27/35/5972
Free to read at intl.jco.org
http://intl.jco.org/cgi/content/abstract/27/35/5972
Subscription required at intl.jco.org
http://intl.jco.org/cgi/reprint/27/35/5972.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Association of microRNA Polymorphisms with Toxicities Induced by Methotrexate in Children with Acute Lymphoblastic Leukemia.
Hematol Rep, 15(4):634-650, 20 Nov 2023
Cited by: 1 article | PMID: 37987321 | PMCID: PMC10660515
Review Free full text in Europe PMC
The influence of methotrexate-related transporter and metabolizing enzyme gene polymorphisms on peri-engraftment syndrome and graft-versus-host disease after haplo-hematopoietic stem cell transplantation in pediatric patients with malignant hematological diseases.
Front Immunol, 14:1229266, 05 Sep 2023
Cited by: 0 articles | PMID: 37731501 | PMCID: PMC10507719
A genome-wide association study identifies 41 loci associated with eicosanoid levels.
Commun Biol, 6(1):792, 31 Jul 2023
Cited by: 5 articles | PMID: 37524825 | PMCID: PMC10390489
Case report: Hepatotoxicity and nephrotoxicity induced by methotrexate in a paediatric patient, what is the role of precision medicine in 2023?
Front Pharmacol, 14:1130548, 02 May 2023
Cited by: 2 articles | PMID: 37201023 | PMCID: PMC10185764
Germline genetic variants and pediatric rhabdomyosarcoma outcomes: a report from the Children's Oncology Group.
J Natl Cancer Inst, 115(6):733-741, 01 Jun 2023
Cited by: 2 articles | PMID: 36951526 | PMCID: PMC10248851
Go to all (204) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
SNPs (Showing 11 of 11)
- (11 citations) dbSNP - rs11045879
- (9 citations) dbSNP - rs4149081
- (3 citations) dbSNP - rs4149056
- (2 citations) dbSNP - rs11045818
- (2 citations) dbSNP - rs2900476
- (2 citations) dbSNP - rs17328763
- (2 citations) dbSNP - rs10841753
- (1 citation) dbSNP - rs11045787
- (1 citation) dbSNP - rs4149076
- (1 citation) dbSNP - rs11045872
- (1 citation) dbSNP - rs7966613
Show less
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Germline genetic variations in methotrexate candidate genes are associated with pharmacokinetics, toxicity, and outcome in childhood acute lymphoblastic leukemia.
Blood, 121(26):5145-5153, 07 May 2013
Cited by: 94 articles | PMID: 23652803
Rare versus common variants in pharmacogenetics: SLCO1B1 variation and methotrexate disposition.
Genome Res, 22(1):1-8, 06 Dec 2011
Cited by: 165 articles | PMID: 22147369 | PMCID: PMC3246196
Polymorphisms of the SLCO1B1 gene predict methotrexate-related toxicity in childhood acute lymphoblastic leukemia.
Pediatr Blood Cancer, 57(4):612-619, 08 Mar 2011
Cited by: 81 articles | PMID: 21387541
Methotrexate Disposition in Pediatric Patients with Acute Lymphoblastic Leukemia: What Have We Learnt From the Genetic Variants of Drug Transporters.
Curr Pharm Des, 25(6):627-634, 01 Jan 2019
Cited by: 10 articles | PMID: 30931851
Review
Funding
Funders who supported this work.
NCI NIH HHS (6)
Grant ID: R01 CA078224
Grant ID: R01 CA051001
Grant ID: CA 078224
Grant ID: CA 51001
Grant ID: CA 21765
Grant ID: P30 CA021765
NHLBI NIH HHS (2)
Grant ID: U01 HL65899
Grant ID: U01 HL065899
NIGMS NIH HHS (4)
Grant ID: U01GM61374
Grant ID: U01 GM061393
Grant ID: U01 GM061374
Grant ID: U01 GM61393





