Abstract
Objective
To determine whether preclinical Alzheimer disease (AD), as detected by the amyloid-imaging agent Pittsburgh Compound B (PiB) in cognitively normal older adults, is associated with risk of symptomatic AD.Design
A longitudinal cohort study of cognitively normal older adults assessed with positron emission tomography (PET) to determine the mean cortical binding potential for PiB and followed up with annual clinical and cognitive assessments for progression to very mild dementia of the Alzheimer type (DAT).Setting
The Alzheimer's Disease Research Center, Washington University, St Louis, Missouri.Participants
One hundred fifty-nine participants with a mean age of 71.5 years with a Clinical Dementia Rating (CDR) of 0 on a PET PiB scan at baseline.Main outcome measure
Progression from CDR 0 to CDR 0.5 status (very mild dementia).Results
Twenty-three participants progressed to CDR 0.5 at follow-up assessment (range, 1-5 assessments after PET PiB). Of these, 9 also were diagnosed with DAT. Higher mean cortical binding potential values for PiB (hazard ratio, 4.85; 95% confidence interval, 1.22-19.01; P = .02) and age (hazard ratio, 1.14; 95% confidence interval, 1.02-1.28; P = .03) predicted progression to CDR 0.5 DAT. The CDR 0.5 DAT group showed decline in 3 cognitive domains (episodic memory, semantic memory, and visuospatial performance) and had volume loss in the parahippocampal gyrus (includes entorhinal cortex) compared with individuals who remained at CDR 0.Conclusion
Preclinical AD as detected by PET PiB is not benign, as it is associated with progression to symptomatic AD.Free full text

PIB Imaging Predicts Progression from Cognitively Normal to Symptomatic Alzheimer’s Disease
Abstract
Objective
To determine whether preclinical Alzheimer’s disease (AD), as detected by the amyloid imaging agent Pittsburgh Compound B (PIB) in cognitively normal older adults, is associated with risk of symptomatic AD.
Design
A longitudinal cohort study of cognitively normal older adults assessed with positron emission tomography (PET) to determine the mean cortical binding potential for PIB and followed with annual clinical and cognitive assessments for progression to very mild dementia of the Alzheimer type (DAT).
Setting
Alzheimer’s Disease Research Center
Participants
One hundred and fifty-nine participants with mean age of 71.5 y in a longitudinal study of memory and aging had a PET PIB scan when cognitively normal with Clinical Dementia Rating (CDR) of 0.
Outcome Measure
Progression from CDR 0 status to CDR 0.5 (very mild dementia).
Results
Twenty-three participants progressed to CDR 0.5 at follow-up assessment (range: 1–5 assessments after PET PIB). Of these, 9 also were diagnosed with DAT. Higher MCBP values for PIB (hazard ratio 4.85, 95% CI, 1.22–19.01, p = .02) and age (hazard ratio 1.14, 95% CI 1.02–1.28, p = .03) predicted progression to CDR 0.5 DAT. The CDR 0.5 DAT group showed decline in three cognitive domains (episodic memory, semantic memory, and visuospatial performance) and had volume loss in the parahippocampal gyrus (includes entorhinal cortex) compared with individuals who remained CDR 0.
Conclusions
Preclinical AD, as detected by PET PIB, is not benign as it is associated with progression to symptomatic AD.
INTRODUCTION
The concept of preclinical Alzheimer’s disease (AD) holds that the Alzheimer pathological process operates for many years before producing clinically detectable impairment.1 A key corollary of this concept is that preclinical AD is not benign and will eventually produce sufficient synaptic and neuronal damage to cause cognitive decline and other symptoms of AD.2 Support for preclinical AD comes from postmortem observations showing that the neuropathology of AD, including cerebral deposits of amyloid-β (Aβ) and neurofibrillary changes associated with hyperphosphorylated tau, is present in an age-dependent manner in a substantial proportion of cognitively normal older adults.1,3–5 Autopsy studies, however, are by nature cross sectional in design and thus cannot predict whether these individuals would have developed symptomatic AD had they lived longer.
Cerebrospinal fluid (CSF) biomarkers of AD, antecedent to the symptomatic stages of the illness, may identify older adults who are destined to develop mild cognitive impairment (MCI) or dementia.6–8 Our group, for example, reported that the ratio of CSF tau to Aβ42 in cognitively normal individuals with a mean age of about 75 years predicted the development of symptoms of AD within 3 to 4 years.9 To our knowledge, however, there have been no studies to show that nondemented persons with Aβ cerebral deposits, as imaged by positron emission tomography (PET) using amyloid tracers, is associated with greater risk of symptomatic AD. We now provide preliminary data to indicate that preclinical AD, identified by the presence of fibrillar cerebral Aβ deposits detected in vivo with the [11C] benzothiazole amyloid tracer Pittsburgh Compound B (PIB),10 is associated with progression to symptomatic AD.
METHODS
Participants
Community-living individuals volunteered to participate in longitudinal studies of memory and aging at Washington University’s Alzheimer’s Disease Research Center (ADRC) and its affiliated programs. The recruitment protocol and assessment methods for these studies have been described.11 All participants were assessed annually with identical instruments and procedures with two exceptions: 1) the ADRC psychometric battery was modified for younger individuals (age 45–74 years) enrolled in the affiliated Adult Children Study;12 and 2) these younger participants were assessed every three years until they reached age 65 years, when they were evaluated annually.
Inclusion criteria for this study were: 1) age 50 years or older; 2) cognitively normal (including the absence of MCI) at the index clinical assessment, which was within 2 years prior or 1 month after their PET PIB scan, completed between April 2004 and November 2008; and 3) at least one assessment subsequent to the index assessment. Exclusion criteria included the presence at baseline of a clinically meaningful disorder (e.g., disabling stroke) that could interfere with longitudinal follow-up or contraindicate the assessment protocol (e.g., cardiac pacemaker precluding magnetic resonance imaging [MRI]).
All procedures were approved by the University’s Human Research Protection Office. Written informed consent was obtained from each participant and their collateral source (informant).
Clinical Assessment
At baseline and at each follow-up, experienced clinicians conducted semistructured interviews with the informant and separately with the participant to determine whether there had been decline in the participant’s cognitive abilities that were sufficient to interfere with the participant’s accustomed activities.13,14 The assessment protocol included demographic information, health history, an aphasia battery, medication history, a depression inventory, and the MiniMental State Examination (MMSE).15 After a neurological examination of the participant, the clinician synthesized all information to determine whether dementia was present or absent based on the principle of intraindividual cognitive decline relative to previously attained function.13,14 The clinician’s judgment was operationalized with the Clinical Dementia Rating (CDR),16 where CDR 0 corresponds to no dementia (i.e., cognitively normal) and CDR 0.5, 1, 2, and 3 represent very mild, mild, moderate, and severe dementia. An etiologic diagnosis of dementia (i.e., CDR ≥ 0.5) is made by the clinician in accordance with standard criteria.17
The CDR determination and dementia diagnosis were made without reference to the participant’s performance on the psychometric battery. The independence of the clinical and psychometric assessments allows the cognitive test results to be assessed longitudinally without the confounding that occurs when psychometric performance is used both to classify participants and to evaluate outcomes. The clinical assessment alone permits the detection of very mild cognitive decline at the CDR 0.5 stage, even when cognitive test deficits are too minimal to meet criteria for MCI (e.g., “preMCI”).14 Participants meeting the clinical phenotype for AD (e.g., gradual onset and progression of cognitive dysfunction; interference with conduct of accustomed activities) were diagnosed with dementia of the Alzheimer type (DAT).18 The accuracy of the diagnosis of DAT as confirmed by postmortem examination is 93% for our study overall11 and 92% for our CDR 0.5 participants who might be classified elsewhere as MCI or preMCI.14 Participants at the CDR 0.5 stage who do not fulfill the DAT phenotype were diagnosed with a nonAD disorder as appropriate or as “uncertain” dementia if no etiological condition was readily apparent; for this study, both categories together are considered to be “nonDAT”.
Psychometric Assessment
Within a few weeks of the clinical assessment, a 1.5 hour neuropsychological test battery was administered by psychometricians who were unaware of the results of any prior psychometric assessments. Individual measures in the test battery include Logical Memory and Associate Learning from the Wechsler Memory Scale (WMS)19 and the Free and Selective Reminding Test (sum of 3 free recall trials)20 to evaluate episodic memory, Information from the Wechsler Adult Intelligence Scale (WAIS)21 and the Boston Naming Test22 to evaluate semantic memory, Mental Control and Digit Span (forward and backward) from the WMS19 and word fluency for the letters S and P23 to evaluate working memory, and Block Design and Digit Symbol from the WAIS21 and the Trailmaking Test A24 to measure visuospatial ability and speeded psychomotor performance. Scores are converted to z scores using means and standard deviations from the initial assessment of a reference group of 310 participants who were CDR 0 at enrollment, had at least two follow-up assessments, and remained CDR 0 for as long as they were followed.25 These z scores were then averaged to form composites representing the 4 cognitive domains.
Imaging Assessment
For MRI, one to four MPRAGE T1-weighted images were acquired (1mm × 1mm × 1.25mm) in one scanning session on a Sonata 1.5T (N = 3), Vision 1.5T (N = 48), or Trio 3T (N = 67) scanner. Image processing was conducted as described26–28 and includes motion correction, averaging across scans, atlas transformation, and inhomogeneity correction. Regional volume estimates were obtained with the Freesurfer image analysis suite, where each voxel in an image is assigned a neuroanatomical label based on probabilistic information from a manually labeled training set that included cognitively normal older adults and those with symptomatic AD.27,29 This technique generates volumes that correspond well to manually generated volumes. Regions of interest (ROI) included the prefrontal cortex (combined superior, middle, and inferior frontal gyri), lateral parietal (combined inferior and superior parietal and supramarginal regions), temporal neocortical (combined superior, middle, and inferior temporal gyri), anterior cingulate, posterior cingulate, precuneous, hippocampal, and parahippocampal (including entorhinal cortex) regions. Intracranial volume was used to adjust ROI for head size variation based on a covariance approach. Freesurfer-derived quantitative estimates have been shown to be reliable in spite of biases across different scanners.30 Volume data were not included for 40 participants due to processing errors (n = 39) and protracted delay between PET PIB and MRI scans (n = 1).
The PET PIB imaging also has been described in detail.31 PET imaging was conducted with a Siemens 961 HR ECAT scanner (CTI, Knoxville, KY) or a Siemens 962 HR+ ECAT scanner. Participants kept their eyes closed during scanning and a mask was used to minimize head motion. After radiochemical synthesis of [11C] PIB,32 approximately 12mCi was administered intravenously with simultaneous initiation of a 60-minute dynamic PET scan. PIB image analysis was achieved by registering each participant’s PET PIB image set to the MPRAGE MRI that was registered to a standard atlas33 target designed to minimize bias due to atrophy.26 The ROIs for PET (detailed information on the boundaries for the ROIs is available)31 were individually drawn on the MRI and applied to unblurred images of the PET dynamic data, yielding high-resolution regional time-activity curves. These curves were analyzed for PIB specific binding by the Logan graphical analysis, using the cerebellum as a reference tissue input function.34 The Logan analysis yields a tracer distribution volume ratio (DV ratio) which then was converted to an estimate of the binding potential (BP) for each ROI: BP = DV ratio-1.31 The BP expresses regional PIB binding values in a manner directly proportional to the number of binding sites. The BP values from the prefrontal cortex, gyrus rectus, lateral temporal cortex, and precuneous ROIs were averaged in each participant to calculate the mean cortical BP (MCBP); these ROIs have high PIB uptake in participants with symptomatic AD.31
Genotyping
Using DNA extracted from peripheral blood samples in each participant, genotyping for apolipoprotein E (APOE) was performed using standard procedures as previously described.35
STATISTICAL ANALYSIS
A Cox proportional hazard model was used to examine associations between levels of fibrillar Aβ cerebral deposits as estimated by the MCBP and time to first diagnosis of DAT. The models were adjusted for age at PET PIB scan, education, gender, and whether the participant was an APOE ε4 carrier. Data from participants who did not receive a DAT diagnosis over the follow-up period (including those who received a diagnosis for nonDAT dementia) were censored at the date of their most recent clinical assessment. A similar analysis was conducted testing time to any diagnosis of dementia (i.e., a CDR>0) rather than time to a diagnosis of DAT specifically. In this analysis, data from participants who did not develop dementia were censored at the date of their most recent clinical assessment. Mixed linear models (PROC MIXED; SAS Institute, Inc; Cary, NC) controlling for age, gender, education, and APOE ε4 carrier status were used to determine whether there were differences in the slope of the cognitive domain composite scores for participants who (1) remained cognitively normal, (2) developed nonDAT dementia, and (3) developed DAT over the follow-up period.
A series of ANCOVAs with regional volume (e.g., hippocampus) as a continuous dependent variable and longitudinal cognitive status (i.e., remained cognitively normal, developed nonDAT dementia, or developed DAT) as a categorical independent variable, and age at PET PIB scan, education, gender and APOE ε4 status as covariates were conducted to examine whether there were group differences in structural brain integrity at the time of the PET PIB scan.
RESULTS
One hundred and fifty-nine participants met the inclusion and exclusion criteria. Data from many of the 159 participants have appeared in other reports from our ADRC.28,36,37 In particular, one of the participants is the subject of a case report38 in which this man, 85 y old at CDR 0 at baseline declined in episodic memory and in working memory at age 88 years and was diagnosed as CDR 0.5, DAT at age 89 y. Although PET PIB imaging at age 88.5 y was negative, at age 89.5 y he had low CSF Aβ42 and elevated CSF tau. Neuropathological examination at age 91 y showed numerous neocortical diffuse Aβ plaques; biochemical analysis showed that PIB binding was below the level needed for in vivo detection.
In the 159 participants, the mean interval (with standard deviation) between the clinical assessment and PET PIB scan was 0.56 y (0.42 y) and between the clinical assessment and MRI was 0.44 y (0.41 y). The mean interval between PET PIB and MRI scans was 0.40 y (0.40 y). Table 1 shows demographic information for the participants at the time of their PET PIB scan (age) or at their index clinical assessment (MMSE; years follow-up). Participants ranged in age from 51.2 to 88.9 years; the follow-up duration from PET PIB to most recent clinical assessment ranged from 0.8 to 5.5 y and the number of assessments (including the index assessment) ranged from 2 to 6. Nine participants, all of whom were CDR 0 at time of PET PIB scan, subsequently were diagnosed with DAT at the CDR 0.5 stage. As shown in Table 2, higher MCBP values for PIB and older age predicted progression from cognitive normality to DAT.
Table 1
Characteristics of 159 Cognitively Normal Individuals
| Mean (SD) | % | |
|---|---|---|
| Age (y) | 71.5 (8.6) | |
| Women | 69.8 | |
| African American | 10.7 | |
| Education (y) | 15.6 (2.5) | |
| APOE ε4 carrier | 31.5 | |
| MMSE score | 29.0 (1.2) | |
| Years follow-up | 2.4 (1.3) |
Legend: SD=standard deviation; y=years; APOE ε4 indicates the ε4 allele of APOE; MMSE=MiniMental State Examination, where the range of scores is from 30 (best) to 0 (worst).
Table 2
Cox Proportional Hazards Model Testing MCBP for PIB as a Predictor of Time to DAT
| HR | 95% CI | P-value | ||
|---|---|---|---|---|
| Lower | Upper | |||
| MCBP | 4.82 | 1.22 | 19.01 | .02 |
| Age (y) | 1.14 | 1.02 | 1.28 | .03 |
| Education (y) | 0.91 | 0.69 | 1.19 | .49 |
| APOE ε4 carrier | 0.98 | 0.20 | 4.90 | .98 |
| Male gender | 0.54 | 0.10 | 2.90 | .48 |
Legend: HR=hazards ratio; CI=confidence interval; y=years; APOE ε4=indicates the ε4 allele of APOE.
An additional 14 participants were staged at CDR 0.5 subsequent to PET PIB scan but were not diagnosed with DAT. In these individuals, 2 were diagnosed with vascular dementia and for the remaining 12 no etiological diagnosis could be made (“uncertain”). As shown in Table 3, for all 23 individuals with CDR 0.5 subsequent to PET PIB (9 diagnosed with DAT, 14 nonDAT), only age predicted progression to CDR 0.5.
Table 3
Cox Proportional Hazards Model Testing MCBP as a Predictor of Time to CDR 0.5
| HR | CI | P-value | ||
|---|---|---|---|---|
| Lower | Upper | |||
| MCBP | 2.74 | 0.59 | 12.78 | .20 |
| Age (y) | 1.11 | 1.04 | 1.18 | .002 |
| Education (y) | 0.99 | 0.84 | 1.16 | .88 |
| APOE ε4 carrier | 1.49 | 0.56 | 3.94 | .42 |
| Male gender | 1.26 | 0.50 | 3.15 | .62 |
Legend: HR=hazards ratio; CI=confidence interval; y=years; APOE ε4=indicates the ε4 allele of APOE.
There were significant differences in parahippocampal volume (p=.008) across the three groups. [Note: 40 participants did not have volume data.] The cognitively normal group (N = 101) had larger parahippocampal volumes compared with individuals (N = 7) who progressed to DAT (p=.03 for adjusted means) and individuals (N = 10) who progressed to nonDAT (p=.05 for adjusted means). The two dementia groups did not differ (p>.63). Group differences in hippocampal volume did not reach significance (p=.09), and there were no significant group differences in any other regional volumes (p >.22).
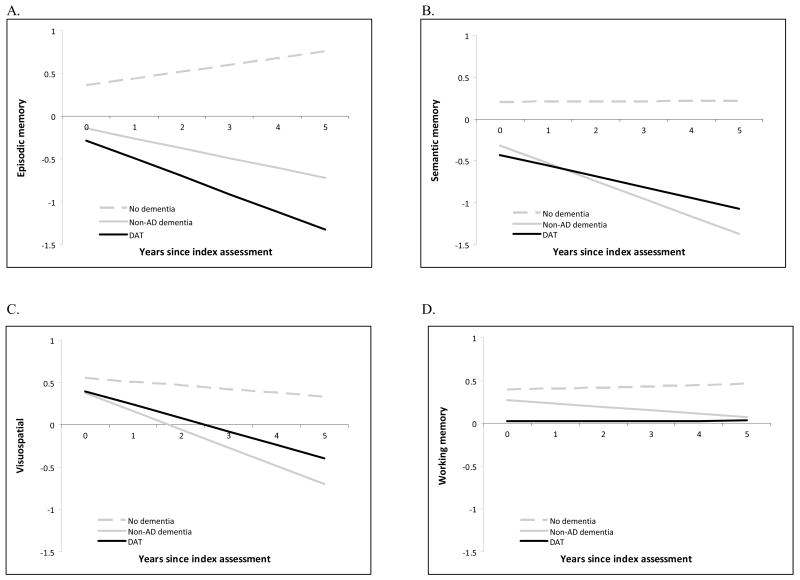
Data from participants in the Adult Children Study who received a different set of psychometric tests and for whom cognitive composite scores could therefore not be generated were not included in the mixed linear models, leaving a sample of 116 participants for the slope analyses. There were significant differences in longitudinal cognitive performance across the three groups (N=97 who maintained nondemented cognition, N=11 who developed nonDAT, and N=8 who developed DAT) in the slope of episodic memory (p=.01), semantic memory (p<.0001), and visuospatial (p=.004) composite scores over the follow-up period. There was no difference in the slope of working memory composite scores across the groups (p=.84). The adjusted mean slopes and intercepts for each group on each composite score are plotted in Figure 1. The decline across three cognitive domains for both CDR 0.5 groups (DAT and nonDAT) is consistent with the independent clinical determination of very mild dementia.
DISCUSSION
Although interpretative caution is imposed by the small number of individuals who progressed to CDR 0.5 and the smaller number of individuals who also were diagnosed with DAT, this study provides initial evidence that cognitively normal individuals with elevated MCBP for PIB are at significantly greater risk for developing the symptomatic stages of AD than individuals with less or no PIB retention. This study also suggests that the PIB predictive effect is restricted to symptomatic AD and does not encompass nonDAT causes of CDR 0.5. These results would be expected if elevated MCBP for PIB is an in vivo indicator of preclinical AD.
Molecular and fluid biomarkers identify cognitively normal individuals with preclinical AD. For example, we recently reported that elevated MCBP for PIB increases with age and the presence of APOE ε4 such that 30% of cognitively normal adults age 80–88 years have the PIB phenotype for AD.37 Similar observations of PIB-positive nondemented older adults have been reported,31,39–41 although with a lower frequency (about 20%) that likely reflects younger samples. The frequency of preclinical AD may be even greater when detected by CSF biomarkers. Where PIB detected 30% of cognitively normal individuals age 80–88 years as PIB-positive, we found that 50% of these same individuals had reduced CSF levels of Aβ4237 suggesting that PIB may be less sensitive in identifying cerebral Aβ deposits (including those that are nonfibrillar) than CSF biomarkers. The well established CSF signature of AD is reduced Aβ42 and elevated tau or phosphorylated tau.42–45 There is a very strong in vivo inverse relationship of CSF Aβ42 and PIB amyloid burden in cognitively normal individuals46 but some individuals have low CSF Aβ42 without detectable cortical binding of PIB.38,47 These findings suggest that, at least in some individuals, levels of CSF Aβ42 may decline with the initial appearance in the cerebral cortex of diffuse amyloid deposits and prior to sufficient fibrillar changes to permit adequate PIB binding for detection by PET.38
Increasing evidence suggests that preclinical AD has deleterious consequences for brain structure and function. Reduced levels of CSF Aβ42 in cognitively normal older adults are associated with whole brain atrophy36 and with hypometabolism in the medial temporal lobe.48 Nondemented older individuals with elevated PIB binding levels also demonstrate regional brain atrophy28 and cerebral cortical thinning,49 as well as episodic memory deficits40 and longitudinal cognitive decline.28 Higher educational attainment may permit individuals with preclinical AD, as ascertained by PET PIB, to better tolerate AD pathology without obvious cognitive deterioration than those individuals with less education,50 suggesting greater reserve against the clinical expression of AD. Similarly, nondemented older adults with high occupational status and who also have preclinical AD have accelerated rates of brain atrophy.51
At least two studies suggest that preclinical AD, as detected by the CSF signature for AD in cognitively normal individuals, predicts development of symptomatic AD. Skoog and colleagues reported that 7 of 35 individuals, nondemented at their baseline assessment at age 85 years when CSF was obtained, developed dementia by age 88 years and had lower levels of CSF Aβ42.52 In 61 cognitively normal individuals with a mean age of 73 years, our group found that the ratio of CSF tau/Aβ42 predicted the 13 who became demented within 3 to 4 years of follow-up after CSF was obtained.9 The findings from these two studies are consistent with reports that progression from MCI or early symptomatic AD to more overt DAT can be predicted by CSF biomarkers6,8,53–55 and by PET PIB.56,57
We now extend these findings by providing, to our knowledge, the first demonstration that elevated MCBP for PIB in cognitively normal older adults predicts the development of symptomatic AD (DAT). This predictive effect appears to be specific to the diagnosis of DAT and does not extend to nonDAT causes of dementia. The diagnosis of DAT was made by experienced clinicians who successfully diagnose the disorder at earlier stages than is typical14,58 and was independently supported by decline in multiple cognitive domains and by parahippocampal volume loss. (The parahippocampal region includes the entorhinal cortex, a region that is a sensitive indicator of AD.)59,60
Our study is limited by several factors, including the small number of individuals who developed DAT, the relatively short follow-up period, and the confirmation of the diagnosis of DAT by postmortem examination in only 1 of the 9 cases38 (the other 8 DAT individuals remain alive). Another limitation is that the nonDAT group likely has heterogeneous etiologies and it is possible that some of these individuals later will be recognized to have AD. Finally, our psychometric battery was developed in 1979 at the inauguration of our studies on memory and aging and has been maintained to allow longitudinal comparisons, but may lack more sensitive measures now available for some domains. Many more individuals, studied for longer intervals and ideally through to autopsy, will be needed to confirm or refute our observations. Nonetheless, this study provides support for the premise that preclinical AD, detected either by the CSF signature for AD9,52 or here by elevated PIB retention, predicts symptomatic AD.
Acknowledgments
This study was supported by grants to JCM from the National Institute on Aging (P50 AG05681, P01 AG03991, and P01 AG026276), an anonymous foundation, and the Charles and Joanne Knight Alzheimer’s Research Initiative. CMR is enrolled in the Postdoctoral Program of 1UL1RR024992 from the National Center for Research Resources. The authors thank investigators and staff from the Alzheimer’s Disease Research Center’s Clinical and Genetics Cores for clinical and cognitive assessments and genotyping and from the Imaging Core, particularly Lindsay Casmaer and Tessa Mazzocco, for data processing. Finally, the authors thank our dedicated participants and their families for their contributions.
Reference List
Full text links
Read article at publisher's site: https://doi.org/10.1001/archneurol.2009.269
Read article for free, from open access legal sources, via Unpaywall:
https://jamanetwork.com/journals/jamaneurology/articlepdf/798780/noc90065_1469_1475.pdf
Subscription required at archneur.ama-assn.org
http://archneur.ama-assn.org/cgi/content/full/66/12/1469
Subscription required at archneur.ama-assn.org
http://archneur.ama-assn.org/cgi/reprint/66/12/1469.pdf
Free to read at archneur.ama-assn.org
http://archneur.ama-assn.org/cgi/content/abstract/66/12/1469
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1001/archneurol.2009.269
Article citations
Quantitative Brain Amyloid PET.
J Nucl Med, 65(5):670-678, 01 May 2024
Cited by: 0 articles | PMID: 38514082 | PMCID: PMC11064834
Mitochondria-sequestered Aβ renders synaptic mitochondria vulnerable in the elderly with a risk of Alzheimer disease.
JCI Insight, 8(22):e174290, 22 Nov 2023
Cited by: 1 article | PMID: 37991017 | PMCID: PMC10721326
Neuropsychiatric Symptoms and Alzheimer Disease Biomarkers Independently Predict Progression to Incident Cognitive Impairment.
Am J Geriatr Psychiatry, 31(12):1190-1199, 26 Jul 2023
Cited by: 0 articles | PMID: 37544835
Gut microbiome composition may be an indicator of preclinical Alzheimer's disease.
Sci Transl Med, 15(700):eabo2984, 14 Jun 2023
Cited by: 68 articles | PMID: 37315112 | PMCID: PMC10680783
Detection and treatment of Alzheimer's disease in its preclinical stage.
Nat Aging, 3(5):520-531, 18 May 2023
Cited by: 19 articles | PMID: 37202518
Review
Go to all (277) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Absence of Pittsburgh compound B detection of cerebral amyloid beta in a patient with clinical, cognitive, and cerebrospinal fluid markers of Alzheimer disease: a case report.
Arch Neurol, 66(12):1557-1562, 01 Dec 2009
Cited by: 132 articles | PMID: 20008664 | PMCID: PMC2796200
Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer's disease: implications for sequence of pathological events in Alzheimer's disease.
Brain, 132(pt 5):1355-1365, 31 Mar 2009
Cited by: 649 articles | PMID: 19339253 | PMCID: PMC2677798
Brain volume decline in aging: evidence for a relation between socioeconomic status, preclinical Alzheimer disease, and reserve.
Arch Neurol, 65(1):113-120, 01 Jan 2008
Cited by: 140 articles | PMID: 18195148
Application of pet imaging to diagnosis of Alzheimer's disease and mild cognitive impairment.
Int Rev Neurobiol, 84:133-149, 01 Jan 2009
Cited by: 14 articles | PMID: 19501716 | PMCID: PMC3647230
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NCATS NIH HHS (1)
Grant ID: UL1 TR000448
NCRR NIH HHS (3)
Grant ID: 1UL1RR024992
Grant ID: UL1 RR024992
Grant ID: UL1 RR024992-03
NIA NIH HHS (9)
Grant ID: P50AG05681
Grant ID: P50 AG005681
Grant ID: P01 AG003991-26
Grant ID: P50 AG005681-26
Grant ID: P01 AG026276-05
Grant ID: P01AG026276
Grant ID: P01 AG003991
Grant ID: P01 AG026276
Grant ID: P01AG03991