Abstract
Free full text

From bench to bedside: the growing use of translational research in cancer medicine
Abstract
Cancer is responsible for one in eight deaths worldwide, with more than twelve million new cases diagnosed yearly. A large percentage of patients die after developing cancer despite aggressive treatment, indicating a need for new approaches to cancer therapy. The push for development of novel diagnostic and therapeutic agents has allowed translational cancer research to flourish. Genomic and proteomic technologies have generated an enormous amount of information critical to expanding our understanding of cancer biology. New research on the differences between normal and malignant cell biology has paved the way for the development of drugs targeted to specific biological molecules, potentially increasing antitumor efficacy while minimizing the toxicity to the patient that is seen with conventional therapeutics. Current targets in include regulators of cell cycle, angiogenesis, apoptosis, DNA repair, and growth factors and their receptors. Collaboration among researchers, clinicians, and pharmaceutical companies is vital to conducting clinical trials to translate laboratory findings into clinically applicable therapeutics. In this review, we discuss current therapeutic approaches and present an introduction to a wide range of topics undergoing investigation in an effort to highlight the importance of translational research in the development of clinically relevant therapeutic strategies.
Introduction: what is translational research?
Research is traditionally broken into two categories: basic and applied research. Basic research is necessary to further our understanding of normal vs. disease states but does not directly translate this knowledge into clinically useful applications. Applied research advances the development of new diagnostic tests or drugs for patients based on our understanding of disease development and progression. The primary goal of “translational” research is to integrate advancements in molecular biology with clinical trials, taking research from the “bench-to-bedside” [1-5]. Understanding and interpreting the molecular information gained through various laboratory techniques, including microarray, genome sequencing, and proteomics, requires that information be shared between laboratories and clinics [6-7]. Clinical researchers' observations about the nature and progression of a disease drive basic science investigations. Researchers use clinical samples to study diagnosis, expression of disease biomarkers, differences between normal and disease states, and response to therapy. Basic scientists then provide clinicians with new treatment strategies based on laboratory data (Figure 1). This constant feedback promotes the discovery of disease biomarkers and drug targets, resulting in more rational drug design, improved efficacy of therapeutic agents, and faster optimization of lead compounds for clinical use.
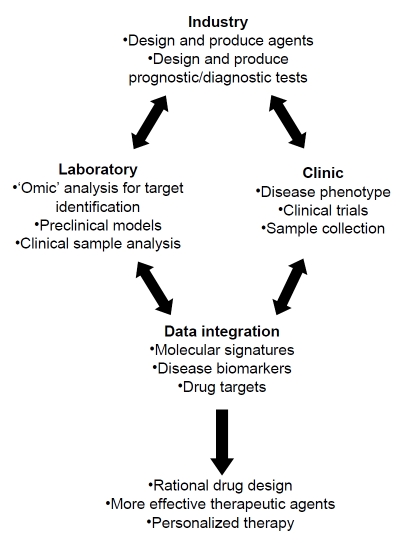
Translational research cycle. Laboratory researchers, clinicians, and the pharmaceutical industry must work together to design rational drugs for improved therapeutic efficacy.
One prime example of translational research in human disease is the study of cancer therapy. Extensive cooperation between basic researchers, clinicians, and industry has generated numerous new targeted compounds with enhanced efficacy and decreased toxicity. In addition to the development of new anti-cancer agents, translational research can also be applied to predicting response to therapy and resistance and sensitizing cancer cells to current therapeutics [1-2,6,8]. Translational research may also allow more rapid development of potential therapeutics, thus reducing the time between drug target identification and clinically relevant therapeutic options. Currently, completion of all phases of preclinical and clinical testing of a single drug can take 7-12 years, but the vast amount of translational research being conducted around the world bodes well for more rapid advancements in the near future. Here we provide an overview of current therapies made possible by translational research, as well as an overview of some recent prospects with a promising future.
Current approaches to cancer therapy
The conventional principle of chemotherapy is that, “The ideal anti-cancer drug should be one that has a specific affinity for cancer cells without affecting normal cells” [9]. However, prior to 1945, therapeutic agents were relatively nonexistent, despite intense research into drug development. The first chemotherapy agent was accidentally discovered during World War I by observations that patients exposed to mustard gas presented with lymphoid and myeloid suppression. Physicians hypothesized that mustard agents may therefore be able to treat leukemia, which is a disease caused by abnormal proliferation of myeloid or lymphoid precursor cells. The next several decades saw a rapid increase of new therapeutic agents, including anti-metabolites, DNA damaging agents, and taxanes, as well as the introduction of combination therapy. Indeed, most efforts at cancer therapy involve direct interference with cell proliferation by altering events that occur during the cell cycle, as cell growth is largely unregulated in cancer cells. Early chemotherapy focused on inhibiting cell growth through mitotic poisons to control tumor cell proliferation [10-12]. However, later research also exposed alterations in vasculature, growth regulation, and evasion of cell death as essential events in tumor growth [1,10,13]. Such changes present additional targets for anti-cancer strategies. We present here a brief overview of radiation and chemotherapy as cancer treatments and discuss how translational research has led to the development of targeted therapies.
Ionizing radiation
Ionizing radiation (IR) is an important cancer therapy that has been widely used since its efficacy was first demonstrated over a century ago. IR utilizes high-energy radiation to kill cancer cells by inducing lethal DNA damage and is often used in conjunction with either surgery or chemotherapy [14-15]. Although radiation therapy is generally well tolerated, secondary cancers, skeletal complications, radiation-induced heart disease, and lung disease are common side effects [16-17]. Due to the toxicity of radiation, much focus has been placed on improving its cancer cell specificity. This includes research into agents that sensitize cancer cells to radiation or protect normal cells from damage induced by radiation [18-20].
Chemotherapy
Chemotherapy is an important treatment option that involves the use of systemic drugs targeting various aspects of cell growth. There are currently over 100 drugs available for use, with multiple agents typically used in conjunction with other drugs or treatment options. These agents vary widely in their chemical composition, function, specificity, and toxicity. While generally effective, chemotherapeutic agents are highly toxic and damage normal cells as well as cancer cells, causing severe side effects. New varieties of chemotherapy medicines to be used in both solo and combination therapy are being developed to increase treatment efficacy. As some chemotherapy drugs are delivered through solvents that are not easily taken up by the body, research into novel drug delivery methods and on improved drug solubility is ongoing. Because the side effects of chemotherapy can be devastating to a patient, chemoprotective and chemosensitizing agents are under development to increase drug efficacy without nonspecific toxicity [11-12].
Molecular targeted therapy
A key example of translating basic research into clinical applicability is the development of molecular targeted therapy, in which cancer cell growth inhibited by interfering with specific molecules that are necessary for tumor growth. Identification of specific molecular characteristics of tumors has facilitated rational drug development, and recognizing suitable targets has led to the generation of countless compounds that have been highly effective in both preclinical and clinical trials. These compounds are screened based on their effects on specific targets in tumor cells and the overall effect on cancer cell growth [4]. Numerous such molecular targets exist for cancer therapy, including epidermal growth factor receptor (EGFR), vascular endothelial growth factor (VEGF), mesenchymal-epithelial transition factor (C-MET), Src, insulin-like growth factor-1 receptor (IGF-1R), and human epidermal growth factor receptor 2 (HER2). Amplification of these genes and proteins is associated with increased cell proliferation, angiogenesis, invasion, metastasis, and decreased cell death- all hallmarks of cancer development. Furthermore, these proteins are associated with more aggressive disease, decreased survival, and drug resistance, making them ideal targets for cancer cell specific therapy. Most compounds that inhibit these targets are small molecule inhibitors or monoclonal antibodies against a particular gene or protein. Additionally, kinase inhibitors (imatinib, erlotinib, lapatinib) are widely used in the clinic to inhibit inappropriate signaling in a number of cancers. These targeted compounds bind to the intracellular or extracellular domain of growth factor receptors to inhibit receptor dimerization or phosphorylation, which blocks the signal transduction events and tumorigenic effects associated with receptor activation. Over thirty antibody and kinase inhibitors are currently in various stages of preclinical development [21]. The past several decades have seen a dramatic increase in the clinical use of molecular targeted therapeutics, as these agents may be more specific to cancer cells than current chemotherapeutic treatments and less harmful to normal cells.
The ErbB/EGFR family of transmembrane receptor tyrosine kinases plays an important role in tumor cell growth, invasion, and metastasis in multiple types of cancer [22-25]. EGFR was the first receptor to be proposed for targeted cancer therapy, as it is frequently overexpressed in a variety of epithelial tumors. Erlotinib (Tarceva®) is currently the primary EGFR tyrosine kinase inhibitor in use [26-28]. Cetuximab is a monoclonal antibody that inhibits EGFR signaling and has been shown to mediate antibody dependent cellular cytotoxicity. However, its clinical benefit remains unknown [28]. Trastuzumab (Herceptin™) is a monoclonal antibody against HER2 that induces antibody-dependent cellular cytotoxicity, prevents receptor activation and signaling, inhibits angiogenesis, and induces apoptosis [29-30]. These types of agents have become important therapeutic options for patients whose cancers overexpress growth factor receptors and are widely used for treating advanced metastatic disease. Importantly, EGFR and HER2 inhibitors work well in combination with many chemotherapeutic agents; however, not all cells respond to treatment. Furthermore, resistance develops rapidly in a large number of patients [24,31-34]. In an effort to improve the efficacy of anti-EGFR/HER2 therapy, lapatinib (Tykerb®) was created. This antibody interferes with both HER2 and EGFR intracellular signaling and has been shown to be highly effective in clinical trials [35-36]. Additional compounds are in development to improve efficacy of inhibitors of growth factor receptors.
The growth of new blood vessels is a critical factor in primary tumor growth and metastasis, thus sparking interest in developing anti-angiogenesis agents [37-39]. Bevacizumab (Avastin®) was the first compound approved by the U.S. Food and Drug Administration (FDA) approved for use in lung, colorectal, prostate and breast cancer [40]. Bevacizumab is a humanized monoclonal antibody directed against the activity of vascular endothelial growth factor (VEGF). Like trastuzumab and erlotinib, bevacizumab works well in combination with many chemotherapeutic agents. While bevacizumab is the most advanced anti-angiogenesis drug currently available, many new compounds are currently under development, including inhibitors of the tyrosine kinase activity of the VEGF receptor and other receptor-like molecules that might mediate angiogenesis [37,41].
Imatinib mesylate (Gleevec®) is a drug specifically designed to inhibit the Bcr-abl fusion protein, or the Philadelphia chromosome, a hallmark of chronic myelogenous leukemia (CML). Bcr-abl is a specific tyrosine kinase that results from the fusion of the Bcr and Abl genes through chromosomal translocation. Bcr-abl initiates a number of signal transduction pathways that influence the growth and survival of hematopoietic cells, can induce leukemic transformation, and induce resistance to apoptosis [42-43]. This is the first example of a compound that specifically interacts with abnormal signaling in cancer cells while largely sparing normal cells from harm. Imatinib is currently the standard front-line therapy for CML in chronic phase, but the drug presents two major drawbacks. First, Imatinib does not completely eradicate residual leukemic stem cells and progenitors, thus presenting a risk of disease relapse [43]. In addition, drug resistance is common, prompting the development of superior inhibitors such as dasatinib (Sprycel®) and nilotinib. These compounds inhibit Bcr-abl and other receptor tyrosine kinases, including c-Kit and PDGF-R. Dasatinib also inhibits Src, which is hypothesized to be involved in disease progression through mechanisms distinct from Bcr-abl [44]. These compounds are significantly more effective than imatinib, with additional compounds undergoing development.
Why current therapeutics are not enough
The ultimate goal of any cancer treatment is to remove or destroy all cancer cells in the body, though some abnormal cells often remain after treatment. These cells could locate at the site of tumor origin, or they could be in another part of the body. These cancer cells may be dormant for a period of time, but eventually will begin to multiply, resulting in relapse. Conventional therapeutics have proven to be initially effective, although many patients relapse over time [43]. Tumor re-growth and disease relapse may be due to the development of therapeutic resistance, insufficient primary therapy, or to a population of intrinsically resistant cancer cells. However, the risk of recurrence for cancer survivors is different for each person and depends on the type of cancer, initial therapy, and time elapsed since initial treatment, among other factors. Recurrent cancer can usually be successfully eliminated from the body through additional treatments. If elimination is not possible, however, treatment goals include controlling tumor growth, managing pain and side effects, and helping the patient to lead a normal, active life for as long as possible [45-46].
Metastatic cancer is largely incurable with a low number of patients achieving long-term survival after standard treatments [47-48]. Although a large number of therapeutic agents are available, overall survival has changed little during the last half century, demonstrating the need for novel treatments that target drug resistant cells, as well as cells capable of causing tumor recurrence and metastasis. Furthermore, understanding the mechanisms underlying the response to therapeutics is vital to devising treatments with superior efficacy and specificity. Importantly for successful treatment, several genes have been discovered that confer drug resistance to cancer cells through drug efflux pumps (P-glycoprotein/MDR1, ABCG2/BCRP) [49-51]. Many new agents are under development to inhibit these multi-drug-resistant pumps, allowing chemotherapy drugs to remain in the cancer cells longer. Additionally, many cancer cells develop resistance through further genetic mutations, resulting in a compensatory response to one particular type of treatment [35]. For example, HER2-positive breast cancer cells often become resistant to trastuzumab, which may be due, at least in part, to upregulation or enhanced activation of EGFR or IGF-1R [52-54].
Cancer cells often develop ways to avoid death normally caused by oxygen or growth factor deprivation, genetic mutations, or exposure to toxic agents, thus limiting successful therapy [55-56]. Mutations in genes associated with the induction of death give cancer cells a growth advantage, allowing them to become invasive, often resulting in metastatic disease or therapeutic resistance. For example, a gene involved in autophagy, one type of cell death, has been found to be deleted in a high percentage of epithelial cancers [56-57]. p53 inactivation is one of the most common mutations found in cancer and provides a mechanism to avoid cell death in response to conventional therapy. Despite deregulated cell death, cancer cells are driven to initiate death pathways in response to cellular damage, leading to the hypothesis that reactivating apoptotic pathways may render cancer cells more susceptible to therapeutic agents [58]. Understanding how cancer cells evade cell death is important for developing longer-lasting treatments with higher specificity to cancer cells, and extensive research has been devoted to characterizing therapeutically relevant proapoptotic targets (see discussion below).
Current evidence indicates that a large number of cancers arise from a single cell that has undergone malignant transformation. There is increasing evidence that there exists a subset of cancer cells that are capable of forming new tumors, called cancer stem cells (CSCs). CSCs have all the characteristics of normal stem cells: they are immortal, pluripotent cells with self-renewal capability and the ability to give rise to multiple different cell lines. To date, cancer stem cells have been identified in many solid and non-solid cancers, including breast, prostate, brain, multiple myeloma, and leukemia [59-64]. However, defining CSCs has proven challenging due to the lack of universal morphologic characteristics and marker expression among cancer types. While some dispute remains about their reliability as CSC identifiers, there are several relatively well-accepted CSC markers [60,63,65-66]. Moreover, it is has been demonstrated that there is heterogeneity regarding stem cell markers within cancer types, highlighting the need for ongoing research in this relatively new field [67-68]. As most therapeutics target rapidly dividing cells, quiescent CSCs may have the ability to withstand initial treatment, thus repopulating a tumor once treatment has ended. Current therapeutic strategies fail to account for potential differences in drug sensitivity between normal cells and CSCs, which may help to explain the inability of current therapeutic regimens to consistently eradicate solid tumors [59,62].
Current and future prospective
Advancements in biotechnology have created vast opportunities for developing new ways to prevent, diagnose, and treat cancer. Ideal therapeutic targets are those that are specifically expressed in cancer cells and are critical for maintaining malignancy. Approaches to understanding cancer prevention, diagnosis, and treatment have changed considerably over time [4,8]. Much effort is geared toward developing drugs that target cancer-specific molecules so as to minimize damage to normal tissues. This method also aims to create drugs that are useful in a wide variety of cancers rather than those located in a particular tissue. The process for designing anti-cancer strategies also leads to promising advancements in developing personalized therapy. To date, most new therapeutic strategies are still in preclinical studies, with few new agents in clinical trials. While this review is not all-inclusive, we present here several examples of promising advancements that translate our understanding of cancer biology into the development of clinically relevant therapeutics.
‘-Omics’
Genomic and proteomic studies are useful in identifying genes and proteins that may have a role in cancer biology by determining significant changes in gene activation and expression in normal versus disease states. Functional proteomics evaluates the activation state of proteins, protein interactions, and the role of aberrantly expressed proteins, helping to map signaling pathways in a cell and develop therapeutics that target particular proteins. Importantly, the response to molecular targeted therapy could be monitored through proteomic methods to determine the efficacy of the targeted therapy, as well as potential future therapies involving the same protein pathway [4,8,69].
One prevalent area of research is the discovery of biomarkers that have the potential to guide treatment and significantly improve survival. Biomarkers are molecular characteristics of precancerous or cancerous cells that can aid in predicting cancer development, behavior, and prognosis [41,70]. Genomics and proteomics have been vital to the discovery and validation of disease biomarkers. Biomarkers can be grouped into three major categories: diagnostic, prognostic, and predictive. Diagnostic markers aid in diagnosing disease, such as through PSA levels in prostate cancer or CA-125 in ovarian cancer [71]. Prognostic markers, such as hormone receptors, angiogenic markers, and proliferation markers, provide information about the likely clinical course of a disease. Predictive markers can help anticipate the course of a disease and how a patient may respond to particular types of therapy. Together with diagnostic and prognostic markers, predictive markers can help physicians formulate a treatment plan.
Despite their usefulness, there are still significant issues in understanding how to properly interpret information obtained through genomic and proteomic approaches. Consistency and stability of proteomic markers is an ongoing issue, as proteins can be rapidly degraded or lose modifications during sample collection and preparation. Microarray results are also subject to considerable variability due to inconsistent methods of DNA extraction, probe labeling and hybridization, the type of microarray platform used, number and type of samples analyzed, and analysis methods. Due to tumor heterogeneity, the resulting gene and protein expression profiles of any tumor will most likely reflect the predominant cell population throughout the section sampled. Genomic and proteomic studies must be meticulously designed and carried out so that the information gained from these technologies can have maximum impact on the progress of translational research [71-73].
Gene/protein regulation
Despite advancements in cancer biology, determining how to utilize potential targets remains difficult, and many compounds remain in pre-clinical testing. Targeted therapy remains one of the most promising areas of cancer therapy, with many specific compounds being explored in both preclinical and clinical settings. Targeted therapy can also be aimed at cancer prevention with highly tolerable drugs, such as newer generations of selective estrogen receptor modulators (SERMs), 5α-reductase inhibitors, and inhibitors of cyclooxygenase 2 [2]. The importance of ubiquitin in cancer led to the development of the FDA-approved proteasome inhibitor Velcade in 2003, with additional compounds currently under investigation [74-75]. Indeed, ubiquitin-mediated protein modification and degradation is important in the regulation of a wide array of cellular processes, including regulation of p53 stability, cell cycle, gene transcription, and the response to DNA damage. Mitotic inhibitors have proven to be effective anti-cancer agents; however, these agents are highly toxic due to nonspecific effects on non-malignant cells. Inhibitors of proteins involved in deregulated mitosis in cancer cells have been developed to restrict cancer cell growth while minimizing toxicity to normal cells [76-78]. In addition, enhanced expression of multiple DNA repair proteins has been detected in a number of cancers, leading to the development of small compounds that inhibit DNA repair proteins [79-81]. Transcriptional inhibitors may be useful in cancer therapies as well, through inhibition of cell migration, angiogenesis, and induction of cell death. Transcriptional inhibitors have also been shown to synergize with other cytotoxic agents [82-84]. Furthermore, new generations of growth factor receptor inhibitors are undergoing testing to overcome issues discovered with earlier formulations (see above). Inhibitors of important receptors such as the EGFR family, IGFR, PDGFR, c-KIT, and FLT3 have been developed with promising results [85-88]. In addition to targeting the receptor signaling, inhibitors of downstream effectors are currently under development. Targets under development include the Ras signaling cascade, PI3K, AKT, Src, and components of the Jak/STAT pathway. These targeted agents have been shown to be effective in both preclinical and clinical studies [89-96]. As shown in Figure 2, these pathways are highly interconnected, and inhibition of one molecule alone is likely not enough to reduce tumor growth. Molecules such as dasatinib, which inhibits Bcr-Abl, c-KIT, PDGFR, and Src, are likely to become more prominent in cancer therapy.

Sites of action of molecularly targeted drugs in cancer cells. Some of the intermediates between growth factor receptor/ ligand binding and downstream effects are illustrated. Molecular targeted agents against representative proteins are indicated. See text for details.
Altered gene expression is present in all tumors, allowing altered regulation of genes controlling proliferation, cell motility, matrix degradation, immune system evasion, and metastasis. Enzymes that modify histones through acetylation or methylation regulate chromatin organization and affect a wide range of DNA-based events, including transcription, replication, stem cell maintenance and differentiation, genome integrity, tissue development, and differentiation. Importantly, imbalances of both DNA methylation and histone acetylation may play an important role in cancer development and progression. Furthermore, genes that inhibit apoptosis and promote drug resistance, or those that promote apoptosis and enhance drug sensitivity, have been found to be epigenetically modified in cancer [97-100]. Translocation, amplification, overexpression, or mutations of histone acetyltransferase and histone methyltransferase genes occur in a variety of cancers, leading to the hypothesis that compounds targeting DNA methylation and/or acetylation regulators are a potential therapeutic strategy for restoring normal gene regulation. For example, 5-aza-dC (decitabine or Dacogen) has been shown to induce demethylation in numerous cancer cell lines and restore the expression of several tumor suppressors, and is currently in clinical trials. Additionally, histone deacetylase inhibitors show anti-tumor activity both in vitro and in vivo, suggesting their usefulness as novel cancer therapeutic agents. Several of these agents are currently in phase I/II clinical trials both in hematological malignancies and in solid tumors. When used in combination with conventional chemotherapeutic agents, epigenetic-based therapies may provide a means to resensitize drug-resistant tumors to established treatments [101-102].
Apoptosis is a complex pathway in cells, offering many opportunities for defects in proper cell death regulation, as well as targets for therapeutic use. Defects in apoptosis permit inappropriate cell survival, providing opportunities for selection of potentially malignant cells. Anti-apoptotic signals play a major role in chemo- and radio- resistance, which significantly reduces the efficacy of cancer therapies. Inhibitors of apoptosis proteins (IAPs) are preferentially expressed in malignant cells and are associated with poor prognosis, making them attractive therapeutic targets [103-105]. IAP inhibitors have been found to enhance apoptosis induced by ionizing radiation and chemotherapy, and efforts are under way to develop IAP inhibitors for clinical use. One important IAP called survivin may be a useful diagnostic marker, as there is significant differential expression between malignant and normal adult cells. Measuring the level of survivin in cancer patients can help diagnose disease as well as monitor disease progression [106-107]. Additionally, tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) is a member of the TNF family of cytokines that promotes apoptosis. In mouse xenograft models, TRAIL and agonistic antibodies directed at TRAIL receptors have demonstrated potent antitumor activity. Importantly, a Phase I trial in humans was recently completed using an agonistic antibody directed against TRAIL-receptor-1 (TRAIL-R1), revealing little toxicity. Unfortunately, many tumor cell lines display intrinsic resistance to TRAIL, despite expressing the necessary cell-surface receptors [57,103-104,108]. Furthermore, antisense oligonucleotides targeting Bcl-2 have advanced to Phase III clinical trials for melanoma, myeloma, chronic lymphocytic leukemia, and acute mylogenous leukemia, with Phase II trials underway for solid tumors. These results indicate the potential for the use of apoptosis-inducing agents in the clinical management of cancers [104,109].
Nanotechnology
New technologies promote innovative, integrated approaches to cancer treatment that previously would have been considered impossible fantasy. An area currently under investigation is the use of radionuclides that can be specifically targeted to cancer cells. Radioactive atoms are bound to cancer cell-specific antibodies and injected into the bloodstream, bringing radiation directly to the cancer site. Several factors need to be considered in radionuclide-based therapies, including the choice of antibody/antigen, radionuclide, and delivery system/schedule. Tumor location, shape, size, and vasculature also play an important role in targeted therapy. Currently, two radiolabeled anti-CD20 antibodies are available for radioimmunotherapy in lymphoma and have been shown to increase remission rates as compared to the unlabeled antibody [110]. Radiolabled antibodies have been less successful in patients with solid tumors than in patients with malignant lymphoma, as solid tumors are less sensitive to radiation, have limited vascularization, and do not have uniform uptake of the radiolabeled antibody. Ongoing studies are investigating a variety of techniques to increase radionuclide efficacy, including improved uptake, better clearance, and easier labeling for enhanced specificity [19,110-112].
Drug delivery is important for optimizing drug efficacy and reducing toxic side effects, and most current therapeutic options are largely non-specific and highly toxic to the vast majority of cells in the human body. Nanoparticles have been developed as an important strategy to overcome several problems in the delivery of conventional drugs, recombinant proteins, and vaccines for disease treatment. The use of nanoparticles improves the kinetics, distribution, and drug release of an associated drug [113-114]. Furthermore, nanotechnology can be used to improve cancer-cell specific drug targeting to minimize toxicity to patients. For instance, tumor necrosis factor (TNF) can be bound to nanoparticles and delivered safely and effectively to tumor-burdened animals. Albumin, which transports nutrients to cells, has been shown to accumulate in rapidly growing tumors. Binding paclitaxel to albumin (Abraxane, Abraxis BioScience) has been shown to improve drug efficacy, and higher doses can be administered as compared to conventional formulations of paclitaxel. In a randomized Phase III trial, the response rate of Abraxane was almost twice that of traditional preparations of taxol [113-114].
Detection of cancer biomarkers is important for diagnostics as well as development of new cancer therapeutics. Nanotechnology is also useful in the development of high-throughput, highly sensitive assays for the identification of new cancer biomarkers. Quantum dots (QDs) are inorganic fluorophores that offer significant advantages over conventionally used fluorescent markers. Quantum dots are highly specific, brighter, and more stable than other fluorescent markers. QDs can be attached to a molecule that will target a specific type of cancer, and will accumulate in the tumor and emit light. This method of biomarker assessment can assist clinicians in diagnosis and tumor imaging without performing biopsies and help improve the rate of accurate diagnosis [115-118]. These bioconjugates open up new possibilities for studying genes, proteins and drug targets in single cells, tissue specimens, and even in live animals [115]. One limitation to this method is that light penetration into the body is limited. Tumors located close to the surface of the body can be screened for, but cancers located deep within the body are much more difficult to reach. However, early studies with QDs demonstrate that these nanoparticles can be used for biomarker profiling and may ultimately prove to be useful for correlation with disease progression and response to therapy. These applications will increase the clinician's ability to predict the likely outcomes of drug therapy in a personalized approach to disease management [115,119-121].
Telomerase
Telomerase activity can be detected in 85-90% of human tumors, but not in most somatic cells, making telomerase an attractive and specific target for cancer therapy [122]. Additionally, telomerase activity increases during cancer progression, suggesting that telomerase is a potential cancer biomarker [123-124]. Telomerase inhibition induces telomere shortening, leading to apoptosis or senescence due to genomic instability [125-128]. Importantly for cancer therapy, telomerase inhibition is expected to be relatively specific to cancer cells, as somatic and stem cells are largely quiescent [128-129]. In vivo studies have demonstrated that telomerase inhibition slows the growth of primary tumors and reduces metastases to the lung [130-131]. Furthermore, telomerase inhibition has been shown to sensitize cells to chemotherapy, irradiation, and molecular targeted therapy [129,132-139]. The most widely studied telomerase inhibitors are agents that inhibit telomerase proteins (hTERT, hTR, or telomerase-associated proteins), and the accessibility of the active telomerase complex to telomeres. Despite an abundance of targets for enzyme inhibition, few telomerase inhibitors have reached clinical trials. Because telomerase is almost universally expressed in cancer cells, reactivation of the hTERT gene allows the use of vaccines that signal the destruction of cells expressing hTERT antigens (discussed below). GRN163L, a lipid-conjugated telomerase template antagonist, has been in clinical trials for patients with chronic lymphocytic leukemia since 2005, for solid tumor malignancies since 2006, and patients with resistant or recurrent multiple myeloma since 2007. GRN163L is well tolerated by patients with no dose-limiting toxicities or serious adverse side effects, and beneficial effects of treatment have been reported. Furthermore, a Phase I/II trial with GRN163L in combination with paclitaxel and bevacizumab was initiated in 2008 for patients with locally recurrent or metastatic breast cancer.
Immunotherapy
In addition to the six classical hallmarks of cancer [13], evasion of the immune response is considered an essential characteristic of cancer cells. Avoiding detection by the immune system allows tumor cells to escape anti-cancer immune responses or to actively suppress them. The benefit of tumor immunology in the management of most cancers is still under investigation; however, there is increasing evidence that anti-cancer immune responses may contribute to the control of cancer after conventional chemotherapy. There are currently many methods for involving the immune system in cancer therapy, including immunostimulatory and inhibitory antibodies and cancer vaccines. Many therapies target tumor antigens, inducing an immune response that results in immune system stimulation and subsequent cancer cell death [140]. Tumor antigens can be any protein produced in a tumor cell that has an abnormal structure due to mutation, such as mutated proto-oncogene and tumor suppressor genes. The use of tumor-specific antigens remains an area of intense interest, and a great deal of research is aimed at identifying these antigens and determining how to specifically target them.
Several methods for developing therapeutic vaccines have shown promise in preclinical testing; however most have either not moved into clinical trials or have not shown a significant patient response [141]. One major limitation of cancer immunotherapy is identifying a gene that is critically involved in causing or maintaining tumor growth. hTERT appears to be one such antigen due to near universal expression and function in most tumors, and has been clinically tested in several types of cancer. Patients treated with hTERT vaccines alone or in combination with GM-CSF or antigen-presenting dendritic cells present with hTERT-specific cytotoxic Tlymphocyte production [128,142-149]. Anti-cancer vaccines have proven effective in a variety of other cancers as well. For example, several antigens present on leukemia cells have been identified, including the Bcr-abl fusion protein, proteinase-3, and Wilms tumor 1 protein. Vaccines against these antigens have been tested clinically to treat patients with a variety of myeloid malignancies, demonstrating a positive response in patients [150-152]. Prostate cancer is also easy to target with cancer vaccines, and several are currently undergoing clinical trials. Early results have demonstrated a positive immune response, decreased PSA levels, and increased overall survival [152]. In addition to their benefit in treating cancer, vaccines are also useful in preventing cancer development. Human papillomaviruses (HPVs) are involved in 99.7% of cervical cancer cases. Recent clinical trials have shown that the prophylactic HPV vaccine is nearly 100% effective at preventing HPV-induced precancerous lesions; however, longer studies are needed to determine the effect on actual cancer rates and duration of protection. Furthermore, the HPV vaccine does not have a therapeutic effect against established HPV infections or HPV-associated lesions [153-155]. These examples indicate the enormous potential of cancer vaccines, and numerous other studies are investigating additional targets in multiple types of cancer as well as how to improve vaccination protocols.
Increasing immune responses with immune-stimulatory monoclonal antibodies (mAbs) is a new and exciting strategy in cancer therapy. This method either antagonizes receptors that suppress immune responses or activates others that increase immune responses. Several mAbs demonstrate antitumor activity in mouse models; however, only the anti-CTLA-4 (CD152) mAb has entered clinical trials. Despite lingering complications, clinical trials have revealed beneficial outcomes in the use of monoclonal antibodies in cancer therapy [156-158]. An intrabody is an antibody that has been designed to be expressed inside a cell and can be targeted to specific antigens. Intrabody targets include knockdown of growth-factor receptors, angiogenesis-related receptors, oncogenes, transcription factors, and cancer resistance related proteins. Intrabodies can also inhibit enzymatic function by blocking an active site, sequestering a substrate, or by changing the conformation of the catalytic site. Current antibody-based drugs are injected and transported to their site of action via the blood stream. However, intrabodies must be taken up by the cell to be effective, and therefore require an effective vehicle that will transport them to their site of action and then allow them to access to the interior of the target cell. Due to lack of optimal delivery, few intrabody based therapeutics have been introduced into the clinic [159-162].
Conclusions and discussion
Over the past twenty to thirty years, there have been significant advances in diagnosing and treating cancer, with early diagnosis one of the most important factors for successful treatment. The promise of new targeted therapies developed from an improved understanding of cancer biology has been realized, with additional strategies constantly undergoing development. As with traditional cytotoxic drugs, learning how to derive and effectively utilize biologically significant agents introduces a lag in how quickly they can be clinically useful. Introduction of imatinib into the clinic took forty years beyond the discovery of the Philadelphia chromosome. Although the HER2 gene was identified in 1979 and its role in cancer was elucidated shortly thereafter, trastuzumab was not clinically available for another twenty years and faced considerable difficulties throughout its development and testing process. In addition to challenges to introducing agents into the clinic, many molecular targeted agents do not perform as well in patients as expected from preclinical testing. For example, despite preclinical efficacy of gefitinib alone or in combination with chemotherapy, phase III trials in non-small-cell lung cancer failed to demonstrate improved survival in patients who had not previously received chemotherapy. One possible explanation for the discrepancy between preclinical and clinical results is antagonistic interactions between molecular targeted and cytotoxic therapies targeting the same tumor cell population. Alternatively, gefitinib may block cell-cycle progression, thereby antagonizing the effects of cytotoxic therapy. In addition, imatinib is highly effective in the early stages of chronic myeloid leukemia, but elicits short-term responses in advanced stages. Furthermore, acquired resistance to imatinib is seen in chronic-phase patients, often due to additional mutations in Bcr-abl that render imatinib ineffective. These examples demonstrate the continued need for ongoing research into both drug development and drug utilization. It is also clear that enthusiasm for new therapeutic techniques should not outweigh the need for thorough preclinical testing.
The ultimate goal for translational research is to design the best agents and therapeutic strategies that will achieve high efficacy with minimal toxicity. Despite gene and protein expression similarities between cancer types, response to therapy will differ based on the individual, indicating the need for individualized therapy. One of the most important characteristics that may limit the effectiveness of targeted therapy is the fact that most cancers are likely caused by multiple genetic abnormalities, suggesting the need for a cocktail of agents against multiple targets in cancer cells, or the use of agents that have a wide range of targets, such as dasatinib. Unfortunately, many agents are associated with toxicities that may be related to their mechanism of action or may be caused by off-target effects of the drug. Advances in both genomics and proteomics allow us to characterize a tumor to enable personalized treatment based on individual gene/protein signatures. In truth, clinicians have been practicing individual therapy for years when choosing a treatment based on tumor characteristics such as ER, PR, and HER2 status. However, additional differences in the genome of a particular tumor may be at least partially responsible for differential responses to anticancer agents. As discussed above, genes involved in chromatin remodeling, apoptosis, DNA repair, and telomere maintenance all contribute to genomic instability and subsequent malignancies. The biotechnology revolution has created undeniable opportunities for improving our ability to prevent, diagnose, and treat cancer. As discussed throughout this review, advances in molecular biology have led to a dramatic increase in the number of new agents undergoing preclinical investigation. The ability to create such compounds exemplifies the necessity for cooperation between basic researchers, clinicians, computational biologists, and the pharmaceutical industry. The collective knowledge from each sector will provide insights into how to overcome challenges that perpetually hinder therapeutic advancements. Past collaborations have yielded not only significant advances in cancer therapeutics but also improvements in the ability of physicians to predict the clinical course of a patient's disease based on individual tumor characteristics.
Acknowledgments
We apologize to authors whose work could not be thoroughly discussed due to space limitations. We thank E. Gentry, A. Powers, and A. Tapadia for critically reviewing this manuscript. EMG is supported by NIH Training grant (T32CA113265).
References
Articles from American Journal of Translational Research are provided here courtesy of e-Century Publishing Corporation
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/113092526
Article citations
Deciphering the multifaceted role of microRNAs in hepatocellular carcinoma: Integrating literature review and bioinformatics analysis for therapeutic insights.
Heliyon, 10(20):e39489, 18 Oct 2024
Cited by: 0 articles | PMID: 39498055 | PMCID: PMC11532857
Review Free full text in Europe PMC
Prediction of cell cycle distribution after drug exposure by high content imaging analysis using low-toxic DNA staining dye.
Pharmacol Res Perspect, 12(3):e1203, 01 Jun 2024
Cited by: 0 articles | PMID: 38682818 | PMCID: PMC11057241
Personalized medicine and nutrition in hepatology for preventing chronic liver disease in Mexico.
Front Nutr, 11:1379364, 09 May 2024
Cited by: 2 articles | PMID: 38784134 | PMCID: PMC11113077
Review Free full text in Europe PMC
"How is it going to help?": Exploring Black breast cancer patients' questions about biomarker testing to predict chemotherapy-induced peripheral neuropathy.
PEC Innov, 2:100118, 13 Dec 2022
Cited by: 1 article | PMID: 37214510 | PMCID: PMC10194344
Targeted Therapy and Personalized Medicine.
Cancer Treat Res, 185:177-205, 01 Jan 2023
Cited by: 5 articles | PMID: 37306910
Go to all (46) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The future of Cochrane Neonatal.
Early Hum Dev, 150:105191, 12 Sep 2020
Cited by: 5 articles | PMID: 33036834
[Development of antituberculous drugs: current status and future prospects].
Kekkaku, 81(12):753-774, 01 Dec 2006
Cited by: 27 articles | PMID: 17240921
Review
How well are we translating biofilm research from bench-side to bedside?
Biofilm, 2:100028, 05 Jun 2020
Cited by: 9 articles | PMID: 33447813 | PMCID: PMC7798461
Translational Metabolomics of Head Injury: Exploring Dysfunctional Cerebral Metabolism with Ex Vivo NMR Spectroscopy-Based Metabolite Quantification
CRC Press/Taylor & Francis, Boca Raton (FL), 14 Aug 2015
Cited by: 0 articles | PMID: 26269925
ReviewBooks & documents Free full text in Europe PMC



