Abstract
Free full text

Interaction between RasV12 and scribble clones induces tumour growth and invasion
Associated Data
Abstract
Human tumours exhibit a large degree of cellular and genetic heterogeneity 1. Complex cell interactions in the tumour and its microenvironment are thought to play a significant role in tumourigenesis and cancer progression 2. It is also known that cooperation between oncogenic genetic lesions is required for tumour development 3. However, it is not known how cell interactions contribute to oncogenic cooperation. The genetic techniques available in the fruit fly Drosophila melanogaster allow analysis of the behavior of cells with distinct mutations 4, giving this model organism a privileged position to study cell interactions and oncogenic cooperation. In Drosophila eye-antennal discs, cooperation between the oncogenic protein RasV12 5 and loss-of-function mutations in the conserved tumour suppressor scribble (scrib) 6,7 gives rise to metastatic tumours that display many characteristics observed in human cancers 8-11. Here we show that clones of cells bearing different mutations can cooperate to promote tumour growth and invasion in Drosophila. We found that the RasV12 and scrib− mutations can also cause tumours when they affect different adjacent epithelial cells. We show that this interaction between RasV12 and scrib− clones involves JNK signaling propagation and JNK-induced upregulation of JAK/STAT-activating cytokines, a compensatory growth mechanism for tissue homeostasis. The development of RasV12 tumours can also be triggered by tissue damage, a stress condition that activates JNK signaling. Given the conservation of the pathways examined here, similar cooperative mechanisms could play a role in the development of human cancers.
Clones of mutant cells marked with green fluorescent protein (GFP) can be generated in the eye-antenna imaginal discs of Drosophila larvae by mitotic recombination. Clones expressing the oncogenic protein RasV12 moderately overgrow 12 (Fig. 1a, b). Clones mutant for scrib lose apico-basal polarity and die 6,13 (Fig. 1c). In contrast, scrib clones simultaneously expressing RasV12 grow into large metastatic tumours (Fig. 1d) 8. To better understand cooperation between these two mutations, we produced animals in which cell division after a mitotic recombination event creates two daughter cells, one expressing RasV12 and the other mutant for scrib. Discs containing adjacent RasV12 (GFP-positive) and scrib− clones developed into large tumours, capable of invading the ventral nerve cord (VNC) (Fig. 1e). This shows that RasV12 and scrib cooperate for tumour induction also when they occur in different cells. We will refer to these tumours as RasV12//scrib− tumours, to denote interclonal oncogenic cooperation and distinguish them from RasV12scrib− tumours, in which cooperation occurs in the same cells intraclonally.
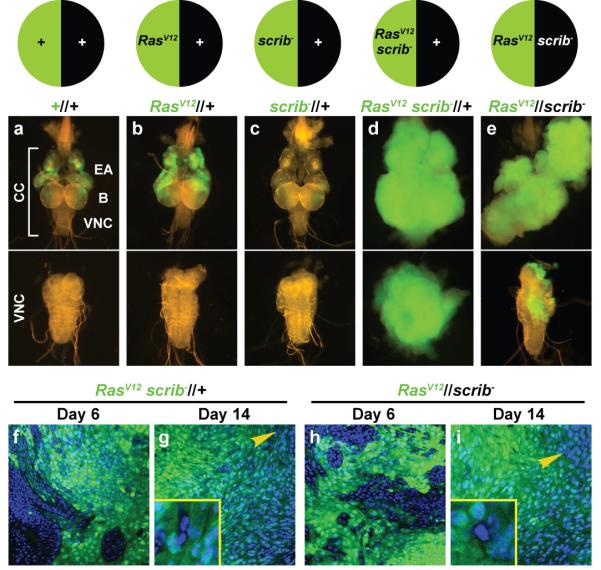
a-e, Clones of cells marked with GFP in the eye-antennal discs of third-instar larvae. Upper subpanels show the cephalic complex (CC), consisting of eye-antennal discs (EA), brain (B) and ventral nerve cord (VNC). Lower subpanels show the dissected VNC. Compared to wild-type clones (a), RasV12 clones overgrow moderately (b). scrib− clones are eliminated from the tissue (c). Double mutant RasV12scrib− clones (d, intraclonal cooperation), as well as RasV12 clones confronted with scrib− clones (e, interclonal cooperation), cause tumours that overgrow and invade the VNC. f-i, Confocal sections of the inner tumour mass in RasV12scrib− (f, g) and RasV12//scrib− tumours (h, i) at day 6 and 14 after egg laying (AEL). Non-RasV12 cells (absence of GFP) are progressively eliminated from the tissue. Cell nuclei labeled with DAPI. Yellow arrowheads point to scattered remaining GFP-negative cells (insets in g and i).
In the late stages of the development of RasV12//scrib− tumours, most cells in the tumour mass are RasV12 cells (Fig. 1h, i). scrib− cells, as well as residual wild-type cells, are almost completely absent from the tissue, similar to the absence of wild-type cells in late RasV12scrib− tumours (Fig. 1f, g). To test the possibility that RasV12//scrib− tumours are caused by unopposed growth of RasV12 cells, we examined eye-antennal discs where all cells expressed RasV12. Dramatic overgrowth or invasion did not occur (Supplementary Fig. 1), showing that interaction between RasV12 and scrib− cells is required for tumour development. Interclonal cooperation between RasV12 and lethal giant larvae (lgl) also produced tumours (Supplementary Fig. 2), suggesting that other polarity mutations can cooperate interclonally with RasV12. Intrigued by these findings, we decided to investigate the mechanisms underlying non-autonomous oncogenic cooperation and sustained growth in RasV12//scrib− tumours.
JAK/STAT signaling promotes cell proliferation in different contexts in mammals and flies 14, including the overgrowth caused by mutation of several tumour suppressors 15. In a cDNA microarray analysis of RasV12scrib− tumours, we discovered upregulation of the unpaired genes (upd, upd2 and upd3; data not shown), which encode JAK/STAT-activating cytokines related to Interleukin 6 16-18. We confirmed the upregulation of the unpaired genes in RasV12scrib− and RasV12//scrib− tumours by real-time RT-PCR (Fig. 2a). Furthermore, we observed elevated expression of the JAK/STAT reporter STAT-GFP 19 in both RasV12scrib− and RasV12//scrib− tumours (Fig. 2b-e), thus correlating high expression of Upd cytokines with increased JAK/STAT activity.
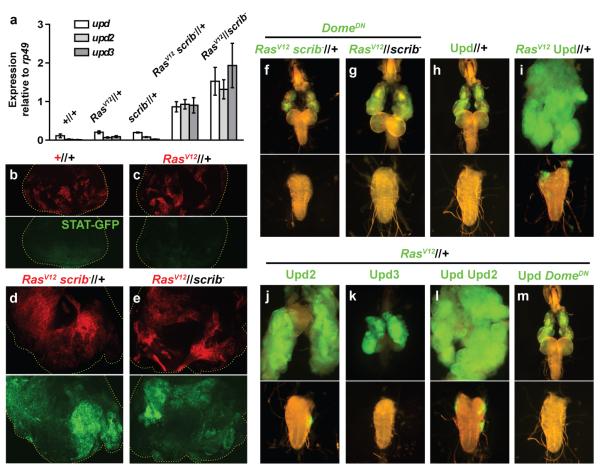
a, Quantification by real-time RT-PCR of expression of the upd genes, encoding the JAK/STAT-activating cytokines Upd, Upd2 and Upd3, in eye-antennal discs containing wild-type clones, RasV12-expressing clones, scrib− clones (day 6 AEL), RasV12scrib− tumours and RasV12//scrib− tumours (day 10 AEL). Expression is normalized to the housekeeping gene rp49. Error bars depict 95% confidence intervals (1.96 × s.e.m., n=3). b-e, Expression of the JAK/STAT reporter STAT-GFP (green) in day 6 AEL eye-antennal discs containing wild-type clones (b, red), RasV12 clones (c), RasV12scrib− (d) and RasV12//scrib− tumours (e). f, g, Suppression of RasV12scrib− (f) and RasV12//scrib− (g) tumours by expression of a dominant negative form of the JAK/STAT receptor Domeless (DomeDN). h, Clones overexpressing Upd. i-l, Tumours caused by RasV12 clones co-overexpressing Upd (i), Upd2 (j), Upd3 (k) and both Upd and Upd2 (l). m, RasV12Upd tumours are suppressed by DomeDN.
To test the involvement of JAK/STAT signaling in the growth of RasV12scrib− and RasV12//scrib− tumours, we used a dominant negative form of the JAK/STAT receptor Domeless (DomeDN) 20. Expression of DomeDN achieved suppression of overgrowth and invasion of the VNC in RasV12scrib− tumours (Fig. 2f). Also RasV12//scrib− tumours were suppressed by expression of DomeDN in RasV12 cells (Fig. 2g). A loss-of-function mutation in stat92E, encoding the Drosophila STAT transcriptional activator, reduced growth and invasiveness of RasV12scrib− and RasV12//scrib− tumours (Supplementary Fig. 3). From these experiments, we conclude that JAK/STAT signaling is required for the development of RasV12scrib− and RasV12//scrib− tumours.
The suppression of RasV12scrib− and RasV12//scrib− tumours by reducing JAK/STAT activity in RasV12 cells points to cooperation between Ras and JAK/STAT signaling as a cause of tumour growth. To confirm this, we generated clones of cells co-expressing Upd cytokines and RasV12. While Upd overexpression in wild-type cells (Fig. 2h), in scrib− cells or in wild-type cells adjacent to scrib− cells (Supplementary Fig. 4) did not cause tumours, co-expression of RasV12 and Upd produced large invasive tumours (Fig. 2i). Similar results were obtained co-expressing RasV12 and Upd2 (Fig. 2j), whereas co-expression of RasV12 and Upd3 caused smaller, non-invasive tumours (Fig. 2k). Finally, RasV12Upd Upd2 tumours were larger than RasV12Upd and RasV12Upd2 tumours (Fig. 2l), suggesting an additive effect of the expression of different Upd cytokines (see also Supplementary Fig. 5).
Prevention of actual cell death in cells apoptotically stimulated has been shown to potently promote overgrowth 21. To assess a possible involvement of apoptosis prevention in the synergy between Ras and JAK/STAT signaling, we coexpressed the apoptosis inhibitor p35 with RasV12 or Upd. Neither conditions produced tumours (Supplementary Fig. 6), suggesting that cooperation between Ras and JAK/STAT involves a mechanism other than apoptosis prevention. RasV12Upd tumours were suppressed by expression of DomeDN (Fig. 2m), thus confirming that their development requires JAK/STAT activity. In all, both loss- and gain-of-function experiments lead us to conclude that Ras and JAK/STAT signaling exhibit a strong synergistic tumour-promoting interaction, responsible for the development of RasV12scrib− and RasV12//scrib− tumours.
Having established the involvement of JAK/STAT signaling in the growth of RasV12scrib− and RasV12//scrib− tumours, we decided to investigate how expression of the Upd cytokines is upregulated. We previously showed that expression of the unpaired genes is elevated in wounds in a JNK-dependent manner 22. It has been shown as well that JNK signaling can induce non-autonomous overgrowth 23,24 and that JNK signaling is upregulated in scrib− clones 13,25 and scrib− discs 22, which develop as tumours in scrib− larvae 6. To test the possibility that JNK activation causes ectopic JAK/STAT signaling in scrib− cells, we monitored STAT-GFP expression in discs double mutant for scrib and hep, coding for the Drosophila JNK-kinase Hemipterous. In these discs, STAT-GFP expression was reduced and overgrowth suppressed (Fig. 3a-c), showing that JAK/STAT elevation in scrib− cells depends on JNK activity.

a-c, STAT-GFP expression in wing discs of wild-type larvae (a) and scrib− larvae heterozygous (b) or hemizygous (male, c) for the JNK-kinase loss-of-function mutation hepr75. Overgrowth and STAT-GFP upregulation are suppressed by hepr75. Arrowheads point to normal STAT-GFP expression in the wing hinge. Discs stained with phalloidin (red). d-g, Expression of a dominant negative form of the Jun-kinase Basket (BskDN) suppresses RasV12scrib− tumours (d, e), but not RasV12Upd tumours (f, g). h, i, Expression of BskDN in RasV12 cells partially suppresses RasV12//scrib− tumours. j, Quantification by real-time RT-PCR of expression of the upd genes in RasV12//scrib− and RasV12BskDN//scrib− tumours (day 6 AEL). Error bars represent 95% confidence intervals (n=3). k, Propagation of JNK activity (puc-lacZ reporter, green) into the posterior (P) compartment (arrowhead) in wing discs wounded in the anterior (A) compartment (asterisk) 24 hours after wounding. l, puc-lacZ expression in discs expressing the JNK-phosphatase Puc under control of ptc-GAL4 (red cells, expressing RFP), wounded as in k. Puc is both a downstream target and a negative regulator of JNK. Propagation of puc-lacZ expression into the P compartment is not observed (hollow arrowhead).
The induction of Upd cytokines by JNK in scrib− cells can explain the growth of RasV12scrib− tumours, placing JAK-STAT signaling downstream of JNK. In support of this, a dominant negative form of the Jun-kinase Basket (BskDN) suppressed RasV12scrib− tumours 9 (Fig. 3d, e), but not RasV12Upd tumours (Fig. 3f, g). In the case of RasV12//scrib− tumours, few scrib− cells remain in the tissue at late stages (Fig. 1i). Therefore, Upd induction in scrib− cells cannot fully account for tumour development. Indeed, expression of BskDN in RasV12 cells partially suppressed the growth of RasV12//scrib− tumours (Fig. 3h, i) and expression of the unpaired genes (Fig. 3j). This shows that in RasV12//scrib− tumours expression of Upd cytokines downstream of JNK signaling also occurs in RasV12 cells.
scrib− clones cause JNK activation both autonomously and non-autonomously 25. Furthermore, in wing discs, wounding induces JNK activation away from the site of wounding 22,26, suggesting that JNK activity can propagate. To investigate this, we wounded wing discs and examined the expression of puckered (puc), a JNK downstream gene encoding a JNK-phosphatase that negatively regulates the pathway 27. When wounds were induced in the anterior or posterior wing regions, JNK activation, revealed by puc-lacZ expression, was observed across the disc in the opposite compartment (Fig. 3k). In contrast, overexpression of Puc in a central stripe of cells prevented expansion of JNK to the opposite compartment. (Fig. 3l, Supplemental Fig. 7). This indicates that JNK activity propagates through a feed-forward loop and, together with previous findings, suggests that in RasV12//scrib− tumours, scrib− cells trigger JNK activation and that this activation propagates to adjacent RasV12 cells. JNK-dependent upregulation of Upd cytokines in RasV12 cells, thus, can sustain tumour growth when the original source of JNK activity, the scrib− cells, is no longer present.
The previous experiments reveal a central role for JNK in the cooperation of RasV12 and scrib−. Since both wounds and scrib− induce JNK activation, we tested the possibility that tissue damage could cooperate with RasV12 to promote tumour overgrowth. We wounded larval right wing discs and examined them 48 hours later. In wild-type discs, compared to the unwounded left disc, wounding resulted in size reduction (Fig 4a, b; Supplementary Fig. 8; wounded/unwounded size ratio ±SD=0.70±0.18). In contrast, wounding of RasV12-expressing discs caused a marked increase in RasV12-induced overgrowth (Fig. 4c, d; Supplementary Fig. 8; 1.46±0.31). No metastasis was detected in this experiment (not shown). Finally, the wounded/unwounded ratio in p35-expressing discs (1.09±0.14, Supplementary Fig. 8) shows that apoptosis prevention by RasV12 cannot completely account for its cooperation with mechanically-induced damage.
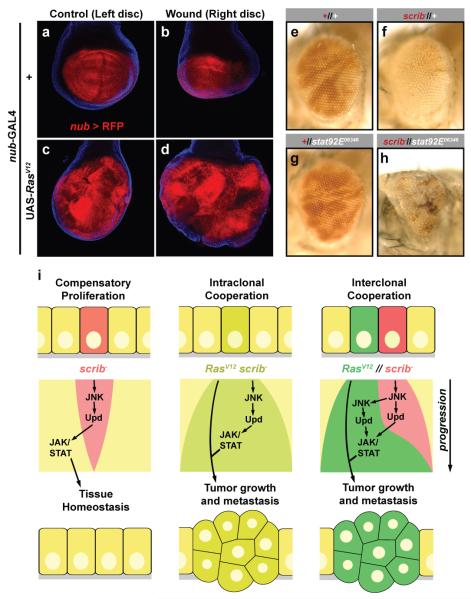
a-d, Cooperation between RasV12 and tissue damage. Right (wounded) and left (unwounded) wing discs of a wild-type larva (a, b) and a larva expressing RasV12 under control of nub-GAL4 (c, d). Discs were wounded by repeated pinching and dissected 48h later. Expression of RFP driven by nub-GAL4 marks the wing blade region (red). Cell nuclei stained with DAPI (blue). e-h, Requirement of JAK/STAT signaling in compensatory proliferation. Wild-type (e) and stat92E− (g) clones in adult eyes, marked by absence of red pigment. In eyes containing scrib− clones confronted with wild-type cells (f), scrib− cells (red) are mostly absent and size of the eye is largely normal. In eyes containing scrib− clones (red) confronted with stat92E− cells (h), the size of the eye is reduced. i, Model for the involvement of JNK and JAK/STAT signaling in intraclonal and interclonal cooperation between RasV12 and scrib−. See text for details.
The fact that both scrib− clones and tissue damage induce overgrowth of RasV12 tissue suggests that compensatory proliferation in response to scrib− cells could underlie cooperation in RasV12//scrib− tumours. To test this, we studied the effect of confronting scrib− cells with cells mutant for stat92E. When scrib− clones are generated in eye-antennal discs, scrib− cells in the adult eye are mostly absent 13 and the eye appears normal in size (Fig. 4e, f). When stat92E− cells confront scrib− cells, in contrast, the eye is greatly reduced (Fig. 4g, h; Supplemental Fig. 9), showing that stat92E− cells cannot compensate for the loss of scrib− cells. These data indicate a role for JAK/STAT signaling in tissue homeostasis through compensatory proliferation (see also Supplemental Figs. 9 and 10). Therefore, a mechanism to ensure recovery after damage explains the development of RasV12scrib− and RasV12//scrib− tumours and can mediate interclonal oncogenic cooperation (Fig. 4i).
We have used Drosophila to investigate how oncogenic cooperation between different cells can promote tumour growth and invasion. Our experiments, addressed to understanding interclonal cooperation in RasV12//scrib− tumours, uncovered a two-tier mechanism by which scrib− cells promote neoplastic development of RasV12 cells: (1) propagation of stress-induced JNK activity from scrib− cells to RasV12 cells and (2) expression of the JAK/STAT-activating Unpaired cytokines downstream of JNK. Our findings, therefore, highlight the importance of cell interactions in oncogenic cooperation and tumour development. We also show that stress-induced JNK signaling and epigenetic factors such as tissue damage can contribute to tumour development in flies. Interestingly, tissue damage caused by conditions such as chronic inflammation has been linked to tumourigenesis in humans 28,29. Furthermore, expression of the Unpaired cytokines promotes tumour growth (this study) as well as an antitumoural immune response 22, which parallels the situation in mice and humans 30. Future research into phenomena such as compensatory growth and interclonal cooperation in Drosophila will provide valuable insights into the biology of cancer.
METHODS SUMMARY
Clones of mutant cells in the eye-antennal discs were generated as previously described 8. Detailed genotypes of the experimental individuals are described in Supplemental Information. The following antibodies and dyes were used: mouse monoclonal anti-βgal (1:500, Sigma), goat Alexa-488-conjugated anti-mouse IgG (1:200, Molecular Probes), phalloidin Texas Red (Molecular Probes). Wounds were performed with forceps in mid third-instar larvae as described previously 22.
METHODS
Strains and culture
Cultures were maintained at 25°C on standard medium. Whenever staging of larvae was required, parental flies were placed in a fresh culture vial and left there to lay eggs for 1 day; we considered the time of removing the flies from the vial 12h (±12) AEL (after egg laying). The following strains were used in this study:
y w; FRT82B
y w; FRT82B,scrib1/TM6B
w; UAS-RasV12 (II)
w; UAS-RasV12 (III)
y w,ey-Flp1;act>y+>Gal4,UAS-GFP.S65T;FRT82B,tub-Gal80
w; FRT82B, tub-Gal80,scrib1/TM6B
y w,ey-Flp1;act>y+>Gal4,UAS-myrRFP;FRT82B,tub-Gal80
tub-Gal80,FRT19A;eyFLP5,act>y+>Gal4,UAS-GFP
y w,ey-Flp1;tub-Gal80,FRT40A;act>y+>Gal4,UAS-GFP.S65T
y w,ey-Flp1;FRT82B,ubi-GFP
w;10XSTAT-GFP.1 (II)
w; ey-Flp6 (III)
w; UAS-upd (II)
w; UAS-upd2 (III)
w; UAS-upd3 (II)
w; UAS-upd-IR(R-1) (III)
w,UAS-domeΔcyt1.1
w; UAS-domeΔcyt2.1 (II)
y w upd2Δ3-62
w,UAS-bskDN
w; UAS-bskDN (III)
y w hepr75/FM7i,act-GFP
w; ptc-GAL
w; UAS-myr-RFP (II)
pucE69-lacZ,ry/TM3,Sb
w; UAS-puc (III)
w; nub-GAL4.K
w; UAS-p35 (II)
w; UAS-p35 (III)
w; FRT82B,stat92E06346/TM6B
w; FRT82B,stat92E397/TM3,Sb
w; FRT82B,stat92E85C9/TM3,Sb
w; FRT82B,ubi-GFP,RpS3Plac92/TM6C, Sb
y w; ey-GAL4,UAS-Flp; FRT82B,GMR-hid,CL3R/TM6B.
Real-time RT-PCR
Total RNA from wild-type and tumour discs was isolated using Trizol (Invitrogen). cDNA was synthesized from 2 μg of RNA with the SuperScriptIII First-Strand Synthesis System (Invitrogen). Resulting DNA was subjected to real-time PCR with SYBR green fast kit (Applied Biosystems) according to manufacturers instructions. Relative gene expression was compared to rp49 as an internal control. Three experiments for each condition were averaged. The following primers were used: upd: 5 ′ TCCACACGCACAACTACAAGTTC 3′ and 5′ CCAGCGCTTTAGGGCAATC 3′; upd2: 5 ′ AGTGCGGTGAAGCTAAAGACTTG 3 ′ and 5 ′ GCCCGTCCCAGATATGAGAA 3′; upd3: 5′ TGCCCCGTCTGAATCTCACT 3′ and 5′ GTGAAGGCGCCCACGTAA 3′; rp49: 5′ GGCCCAAGATCGTGAAGAAG 3′ and 5′ ATTTGTGCGACAGCTTAGCATATC 3′.
Stainings and imaging
Images documenting tumour size and VNC invasion were taken in a Leica MZ FLIII fluorescence stereomicroscope with an Optronics Magnafire camera. Antibody staining was performed according to standard procedures for imaginal discs. The following antibodies and dyes were used: mouse monoclonal anti-βgal (1:500, Sigma), goat Alexa-488-conjugated anti-mouse IgG (1:200, Molecular Probes), phalloidin Texas Red (Molecular Probes). Samples imaged through confocal microscopy were mounted in DAPI-Vectashield (Vector Labs). Confocal images were taken in a Zeiss LSM510 Meta confocal microscope. Adult eyes were imaged with a Leica DFC300FX camera in a Leica MZ FLIII stereomicroscope. Measurements of wing blade size were performed from confocal pictures using NIH Image-J software. Adult eye size measurements were performed for each genotype from pictures of at least ten female flies collected 1-3 days after hatching using NIH Image-J software.
Supplementary Material
1
4
5
6
7
8
9
10
11
12
13
14
15
2
3
Acknowledgements
We thank E. Bach, D. Harrison, J. Castelli-Gair Hombria, M. Zeidler, T. Adachi-Yamada, M. Mlodzik, E. Matunis, D. Montell, H. Agaisse, the Bloomington Stock Center and the National Institute of Genetics (Kyoto) for fly strains, and T. Ni, S. Landrette and M. Rojas for comments. R. Pagliarini and S. Landrette helped with microarray analysis and RT-PCR. We thank T. Igaki for discussing the manuscript and providing FRT82B, tub-Gal80,scrib1/TM6B flies. We apologize for not being able to cite all the relevant references. M.W. is a Yale predoctoral fellow. J.C.P.-P. was funded by a Spanish Ministry of Education postdoctoral fellowship. This work was supported by a grant from NIH/NCI to T.X. T.X. is a Howard Hughes Medical Institute Investigator.
Footnotes
Competing interests statement The authors declare that they have no competing financial interest.
REFERENCES
Full text links
Read article at publisher's site: https://doi.org/10.1038/nature08702
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc2835536?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/102655053
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1038/nature08702
Article citations
An unscheduled switch to endocycles induces a reversible senescent arrest that impairs growth of the Drosophila wing disc.
PLoS Genet, 20(9):e1011387, 03 Sep 2024
Cited by: 1 article | PMID: 39226333 | PMCID: PMC11398662
Exclusion of HDAC1/2 complexes by oncogenic nuclear condensates.
Mol Cancer, 23(1):85, 27 Apr 2024
Cited by: 3 articles | PMID: 38678233 | PMCID: PMC11055323
A Tumor-Specific Molecular Network Promotes Tumor Growth in Drosophila by Enforcing a Jun N-Terminal Kinase-Yorkie Feedforward Loop.
Cancers (Basel), 16(9):1768, 02 May 2024
Cited by: 0 articles | PMID: 38730720 | PMCID: PMC11083887
Using Drosophila to uncover the role of organismal physiology and the tumor microenvironment in cancer.
Trends Cancer, 10(4):289-311, 13 Feb 2024
Cited by: 1 article | PMID: 38350736
Review
Cell competition and cancer from Drosophila to mammals.
Oncogenesis, 13(1):1, 03 Jan 2024
Cited by: 1 article | PMID: 38172609 | PMCID: PMC10764339
Review Free full text in Europe PMC
Go to all (258) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Interplay among Drosophila transcription factors Ets21c, Fos and Ftz-F1 drives JNK-mediated tumor malignancy.
Dis Model Mech, 8(10):1279-1293, 06 Aug 2015
Cited by: 49 articles | PMID: 26398940 | PMCID: PMC4610234
Loss of the Drosophila cell polarity regulator Scribbled promotes epithelial tissue overgrowth and cooperation with oncogenic Ras-Raf through impaired Hippo pathway signaling.
BMC Dev Biol, 11:57, 29 Sep 2011
Cited by: 74 articles | PMID: 21955824 | PMCID: PMC3206446
MRL proteins cooperate with activated Ras in glia to drive distinct oncogenic outcomes.
Oncogene, 36(30):4311-4322, 27 Mar 2017
Cited by: 6 articles | PMID: 28346426 | PMCID: PMC5537612
RasV12; scrib-/- Tumors: A Cooperative Oncogenesis Model Fueled by Tumor/Host Interactions.
Int J Mol Sci, 22(16):8873, 18 Aug 2021
Cited by: 5 articles | PMID: 34445578 | PMCID: PMC8396170
Review Free full text in Europe PMC
Funding
Funders who supported this work.
Howard Hughes Medical Institute
NCI NIH HHS (5)
Grant ID: R01 CA069408-13
Grant ID: R01 CA069408-11
Grant ID: R01 CA069408-12
Grant ID: R01 CA069408-13S1
Grant ID: R01 CA069408




