Abstract
Free full text
Conscious perception of errors and its relation to the anterior insula
Abstract
To detect erroneous action outcomes is necessary for flexible adjustments and therefore a prerequisite of adaptive, goal-directed behavior. While performance monitoring has been studied intensively over two decades and a vast amount of knowledge on its functional neuroanatomy has been gathered, much less is known about conscious error perception, often referred to as error awareness. Here, we review and discuss the conditions under which error awareness occurs, its neural correlates and underlying functional neuroanatomy. We focus specifically on the anterior insula, which has been shown to be (a) reliably activated during performance monitoring and (b) modulated by error awareness. Anterior insular activity appears to be closely related to autonomic responses associated with consciously perceived errors, although the causality and directions of these relationships still needs to be unraveled. We discuss the role of the anterior insula in generating versus perceiving autonomic responses and as a key player in balancing effortful task-related and resting-state activity. We suggest that errors elicit reactions highly reminiscent of an orienting response and may thus induce the autonomic arousal needed to recruit the required mental and physical resources. We discuss the role of norepinephrine activity in eliciting sufficiently strong central and autonomic nervous responses enabling the necessary adaptation as well as conscious error perception.
Introduction
Performance monitoring, i.e., the ability to monitor one’s own mistakes and to implement appropriate behavioral adjustments, has been intensively studied over the last two decades. A topic that has moved into the focus of research much more recently is conscious perception of errors. Why do we consciously detect some errors while others escape our attention? Which of the brain structures identified to contribute to performance monitoring are relevant for the awareness that an error has been made? Why are we able to consciously perceive almost all errors in certain tasks, while in other tasks more than 50% of the errors remain unnoticed? Here, we review the current knowledge on conscious perception of errors. For the sake of brevity, we will henceforth call conscious error perception “error awareness” and, accordingly, the failure to perceive errors “error blindness”.
After a brief introduction to the neural correlates of performance monitoring in general, we will elaborate on the methods of measuring error awareness and discuss the conditions under which subjects may become aware or remain unaware of an error. This will be followed by overviews of correlates of error awareness in the central nervous system (CNS) and autonomic nervous system (ANS), respectively. In a further section, we discuss the putative role of the neuromodulator norepinephrine (NE) in driving post-error brain and ANS responses. As the anterior insular cortex (AIC) appears to be particularly relevant for error awareness, this review will focus on the role of this cortical area in performance monitoring and its potential links to accompanying autonomic responses. In the subsequent sections, we hypothesize that errors are salient events eliciting an orienting response and activating a “salience network” centered around the AIC. We propose that the orienting/salience response in the AIC and associated areas enable flexible recruitment of resources to react to any upcoming problem in task performance. A sufficiently strong orienting response appears to be associated with error awareness, but the direction of causality between central and autonomic responses and awareness remain to be determined. We will conclude with a number of open questions and suggest approaches to address them in future research.
Correlates of performance monitoring
In speeded choice reaction time tasks, errors are typically associated with a specific event-related brain potential (ERP) signature, consisting of the error-related negativity (ERN, also error negativity, Ne) and the error positivity (Pe) (Falkenstein et al. 1990; Gehring et al. 1993; Overbeek et al. 2005). The ERN reaches its peak around 50–100 ms after erroneous actions and has a frontocentral distribution over the scalp. Source localization studies as well as single-trial EEG-informed functional magnetic resonance imaging (fMRI) suggest it to be generated in the posterior medial frontal cortex (pMFC), in particular in the rostral cingulate zone (RCZ) (Dehaene et al. 1994; Debener et al. 2005). The RCZ is the putative homologue of the monkey’s rostral and dorsal cingulate motor areas (Picard and Strick 2001) and is roughly equivalent to the midcingulate cortex according to Brent Vogt’s terminology (Vogt et al. 2004). In line with the EEG findings, neuroimaging studies consistently implicate the RCZ in performance monitoring (Ridderinkhof et al. 2004; Taylor et al. 2007). A currently predominating view on the function of the pMFC is that it is engaged in monitoring situations when the action outcome is worse than expected or when the outcome is at risk, and in signaling the need for adjustment (Ridderinkhof et al. 2004).
After the ERN, at a latency of about 300–500 ms, the more centroparietally distributed Pe occurs. While it seems to share morphological, topographical, temporal, and functional features with the P300 (P3b) component, its functional significance and its generators are far from clear. Interestingly, it seems to be most consistently modulated by awareness (Overbeek et al. 2005) and motivational salience of errors (Ridderinkhof et al. 2009), which will be discussed in more detail below (Fig. 1).
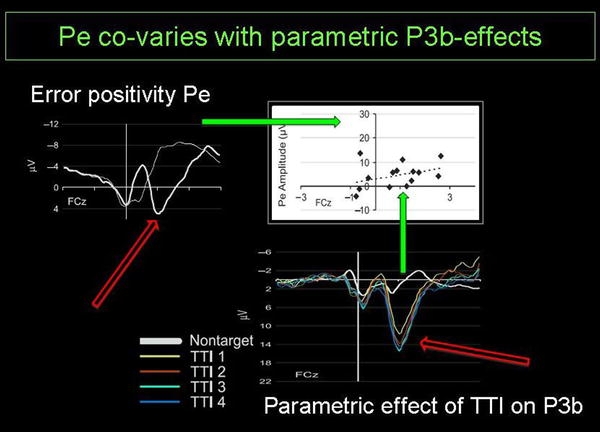
Correlation of error positivity (Pe) and P3b, suggestive of a common functional significance in terms of orienting to salient events (adapted from Ridderinkhof et al. 2009). In an oddball task, infrequent target stimuli trigger an orienting response reflected in the P3b. The orienting response is most evident in the effect of the interval between successive targets (expressed in terms of the number of intermittent nontargets) on P3b amplitude. Individual differences in this effect of target–target–interval (TTI) on the P3b amplitude were found to co-vary (r = 0.47) with individual differences in the amplitude of the Pe obtained from erroneous responses in a Simon task
In addition to the RCZ, the anterior insula is consistently activated during errors and other instances when performance monitoring becomes necessary (Klein et al. 2007). Here, we further broke down the metaanalysis using activation likelihood estimates (Turkeltaub et al. 2002) presented by Klein et al. (2007) according to the different conditions of performance monitoring. This metaanalysis included 55 functional magnetic resonance imaging (fMRI) studies on performance monitoring. Here, we focus particularly on the anterior insula, which—similar to the pMFC—was activated during all studied conditions calling for adjustments, specifically pre-response conflict, decision uncertainty, response errors, and negative feedback (Fig. 2). Pre-response conflict arises when several competing response tendencies are elicited by a task (Yeung et al. 2004); decision uncertainty refers to underdetermined responding in situations when information about the correct response is insufficient (Botvinick et al. 2001; Volz et al. 2003; Dayan and Yu 2006). Pre-response conflict and uncertainty both indicate an increased likelihood to fail, which can still be countermanded by quickly recruiting additional control processes (solving the conflict or gathering the necessary information to reduce uncertainty). Errors and negative feedback occur after the response and call for remedial actions compensating the failure and/or subsequent adjustments improving future performance (Ridderinkhof et al. 2004; Ullsperger et al. 2004). In the metaanalysis, interesting differences are visible: pre-response conditions activate predominantly the superior AIC, whereas post-response conditions (errors and negative feedback) seem to activate the AIC to a larger extent with maxima in the inferior AIC (Table 1). Moreover, in post-response conditions, activity in the right AIC is larger then in the left AIC. In contrast, pre-response conflict seems not to activate the right AIC significantly. Note that by far most studies included in the metaanalysis studied pre-response conflict, such that this null finding is unlikely to result from insufficient power. In any case, similarly as in the pMFC (Ullsperger and von Cramon 2001; Garavan et al. 2003; Rushworth et al. 2004; Nachev et al. 2005) there seems to be a functional gradient in the localization of activity within the AIC. Interestingly, this subregional differentiation is highly reminiscent of the findings on risk processing (Preuschoff et al. 2008; Bossaerts 2010, this issue). Pre-response risk anticipation preferentially involves the superior AIC, whereas (post-response or post-feedback) risk prediction errors involve the inferior AIC. Similarly as in the performance monitoring conditions investigated in the metaanalysis, pre- and post-response risk processing would call for different adjustments. The different subregions of the AIC may play a role in recruiting the necessary effort to avoid (superior AIC: risk prediction, increased likelihood to fail) and compensate (inferior AIC: risk prediction error, negative reward prediction error) potential risks and failure. These findings and our resulting speculations strongly call for further fine-grained research on the functional topography within the AIC.
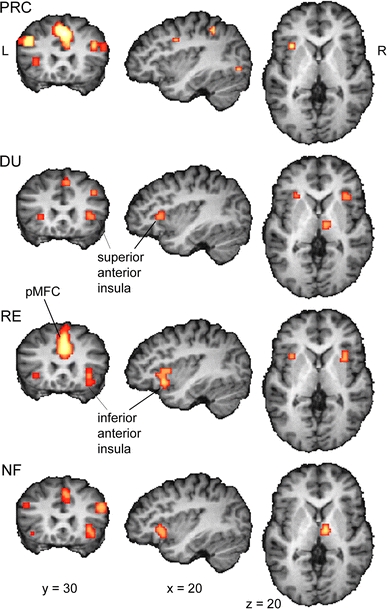
Results of metaanalysis performed separately for pre-response conflict (PRC), decision uncertainty (DU), response errors (RE), and negative feedback (NF), projected on coronal, right sagittal, and axial slices. Alpha level = 0.01. L/R left/right, pMFC posterior medial frontal cortex
Table 1
Significant activation likelihood maxima in the anterior insular cortex resulting from the metaanalysis at an alpha level of 0.01, determined separately for different performance monitoring conditions
| Condition | N | Side | Talairach coordinates | Volume (mm3) | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Pre-response conflict | 28 | L | −35 | 15 | 6 | 351 |
| R | – | – | – | – | ||
| Decision uncertainty | 6 | L | −29 | 21 | 3 | 162 |
| R | 34 | 21 | 6 | 513 | ||
| Response errors | 19 | L | −32 | 15 | 3 | 405 |
| R | 34 | 15 | −9 | 1,377 | ||
| Negative feedback | 9 | L | −38 | 15 | −6 | 108 |
| R | 34 | 18 | −6 | 756 | ||
L/R left/right, N number of studies included in metaanalysis
How to study error awareness?
Error signaling and retrospective accuracy judgement
Up to now, error awareness has been studied by asking participants whether they noticed that they had made an error. Classically, participants are instructed to signal errors by pressing an “error signaling button” (which usually is not used for the primary task) when they encountered an error (Rabbitt 1968, 2002; Nieuwenhuis et al. 2001). An error reported correctly by pressing this signaling button is considered consciously perceived (“aware error”), whereas an error not followed by a signaling response is considered unnoticed (“unaware error”). Notably, error correction responses (i.e., pressing the correct response immediately after the error) have no such indicative value as they do not necessarily require error awareness; they can occur even in the absence of error monitoring (Ullsperger and von Cramon 2006).
The classical method of error signaling has some limitations that need to be considered. It may well be that participants become aware of an error despite not having signaled it. For example, under time pressure induced by short inter-trial intervals they may consider it more important to concentrate on the upcoming trial than on error signaling. In a number of pilot studies participants reported that they became aware of errors but felt that they had no time to signal them (Danielmeier, Wessel, Ullsperger; unpublished observations). Furthermore, error signaling may be influenced by response bias depending on motivational and other factors. In a few studies, some of these limitations have been circumvented by asking participants to evaluate each button press by indicating whether the preceding response to the primary task was deemed correct or incorrect instead of merely signaling erroneous responses (Endrass et al. 2007; Klein et al. 2007; Wessel et al. 2010). However, any kind of signaling or performance classification response may interfere with trial-by-trial post-error adjustments. Finally, the signaling or performance classification response may be erroneous due to motor action slips. Thus, currently there is no optimal way of studying error awareness (a) as it is difficult to elicit optimal numbers of aware and unaware errors and (b) awareness is generally not measurable directly but only by introspection. The precision of assessing error awareness and error blindness could be improved by post-decision wagering procedures that have been used successfully to assess other kinds of awareness (Persaud et al. 2007). Here, participants are offered cash rewards for revealing conscious knowledge. After determining whether a response was correct or incorrect, they should make a bet of either a small or large amount of money on the accuracy judgment. This method as well as application of signal detection theory has great potential to reveal error blindness and may thus be helpful in future studies on error awareness.
Tasks associated with error blindness
In speeded forced manual choice tasks usually applied to study performance monitoring, for example, the Eriksen flanker task (Eriksen and Eriksen 1974), Simon task (Simon and Rudell 1967) and Go/NoGo tasks, most errors result from premature responding prior to completion of stimulus evaluation such that prepotent but incorrect reactions are issued. As stimulus evaluation continues after the response the information necessary to detect errors becomes available very soon (Coles et al. 2001). Therefore, almost all errors are signaled by the subjects preventing a comparison with errors that were missed (Ullsperger and von Cramon 2006; Maier et al. 2008), rendering these tasks poorly suited for the study of error awareness.
Figure 3a shows a schematic of the timing of different events and processes ongoing during erroneous trials and factors that may modulate error awareness at different stages. Early indicators of error processing such as the ERN are based on quickly available information [be it coded as post-response conflict (Yeung et al. 2004), as mismatch between efference copy and evolving correct response tendency (Coles et al. 2001) or as a reinforcement learning (reward prediction error) signal (Holroyd and Coles 2002)] and do not require other inputs such as proprioceptive feedback (Allain et al. 2004) or redundant sensory feedback (De Bruijn et al. 2004; Gentsch et al. 2009). In contrast, awareness about the error does not need to occur immediately. It is likely to instantiate when cumulating evidence about erroneous behavior exceeds some threshold and activates sufficiently large neural networks (Dehaene et al. 2006). In other words, a compound internal error signal based on numerous sources of information needs to become sufficiently strong compared to noise and counterevidence coded in the brain. Thus, error awareness can arise from later events, such as proprioceptive feedback from the erroneous action, sensory input (e.g., the “click” feeling and sound elicited by pressing the response button), and—as will be discussed below—from interoception of autonomic responses accompanying the error. Steinhauser et al. (2008) showed empirically and by computational modeling that in a flanker task the latency of the error signaling response (as an indicator of error awareness) is strongly influenced by the correct(ive) response tendency. In their model, post-response conflict is the major source of information inducing error detection (Yeung et al. 2004; Steinhauser et al. 2008). As tasks used to study error awareness (described in detail below) differ considerably from the flanker task with respect to stimulus uncertainty, task complexity, and availability of efference copy and proprioceptive feedback, other sources of information may be more important for error awareness in those cases.
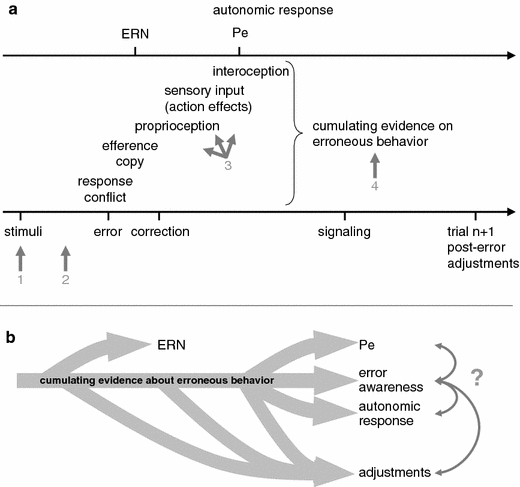
Schematic of timing and interaction of processes during an erroneous trial that may support error awareness. a Timing of external and internal events as well as psychophysiological measures associated with the accumulation of evidence that the response was erroneous (not to scale). The latency of conscious error perception may vary substantially and should result from accumulation of sufficient evidence that an error has occurred, independent of the source of information which may range from early pMFC-mediated error monitoring via proprioceptive and sensory input discordant with expectancies from forward modeling to interoception. The numbered arrows indicate different influences that may lead to error blindness: 1 ambiguous stimuli make it objectively impossible to detect errors. Also fluctuations in attention or eye blinks precluding the perception of short stimuli may result in errors that cannot be detected reliably. 2 failure to represent task sets or to activate complex task rules may lead to errors. 3 insufficient efference copy, proprioceptive feedback or sensory input on action effects may hinder conscious perception of an error. 4 interactions with ongoing fluctuations in brain activity may lower signal-to-noise ratio in the representation of accumulating evidence of erroneous behavior. Moreover, action slips in the signaling or accuracy classification response may occur. b Influence of accumulating evidence of an error on different processes and correlates during error monitoring. It is unclear whether processes are working in serial or—at least partly—parallel fashion and how much they depend on each other. In particular the causal relationship of error awareness, some post-error adjustments and autonomic responses is still unclear
Thus, theoretically, influences at many stages of information processing may influence whether an error enters awareness or not. This may start at the level of stimulus presentation and perception (Fig. 3a, arrow 1). In some experiments, stimulus detectability was modulated by increasing perceptual difficulty (Scheffers and Coles 2000; Oliveira et al. 2007) or masking (Maier et al. 2008; Cohen et al. 2009). Also fluctuations in ongoing brain activity may influence stimulus perception and thus task performance (Boly et al. 2007; Sadaghiani et al. 2009). In addition, quite trivially, eye blinks in tasks with very short stimulus presentation may prevent the necessary input on some trials. All these conditions have in common is that the high uncertainty about the stimuli results in underdetermined responding, i.e., without appropriate external feedback the brain has no chance to detect the error. In other words, while the subject may become aware of the high uncertainty, she/he cannot determine the valence of the response reliably but must guess and wait for external feedback (which, by itself, may induce error awareness at a later stage, of course). Hence, studies using masking or degradation of stimuli need to ensure that the dependent variables are deconfounded from the accompanying uncertainty.
If task representations are weak, this may also lead to errors as well as reduced error awareness. A number of studies on error awareness made use of a Go/NoGo task in which subjects were instructed to withhold their response in two different conditions (Hester et al. 2005; O’Connell et al. 2007; Shalgi et al. 2007, 2009; O’Connell et al. 2009b). For example, Stroop-like stimuli were presented (color words in colored ink). Each stimulus should be responded to, except for repetitions of the same stimulus or incongruent stimulus configurations (e.g., when the color of the ink differs from the meaning of the presented word). This task, termed error-awareness task (EAT) by Hester and colleagues, has proven to yield sufficiently large numbers of both perceived and unperceived errors. It seems conceivable that subjects do not keep both task instructions in mind with equal strength, which may produce slips due to “forgetting” of the relevant rule. It might be speculated that lapses in retrieval of one of the two rules may be the reason of the high number of errors remaining unconscious. However, as total number and error awareness seem to be dissociated between the two NoGo conditions (O’Connell et al. 2007), the sources of information inducing error awareness as well as the sources of errors may differ between the two conditions (O’Connell et al. 2009a).
The first study explicitly addressing error awareness made use of an oculomotor task (Nieuwenhuis et al. 2001) and inspired a number of follow-up studies using this effector modality (Endrass et al. 2005, 2007; Klein et al. 2007; Harsay et al. 2010; Wessel et al. 2010). In an antisaccade task (AST) in which subjects are instructed to shift their gaze in the direction opposite to a briefly displayed peripheral cue, erroneous saccades occur when subjects look initially in the direction of the peripheral cue before redirecting their gaze to the correct, opposing side of the screen. These erroneous prosaccades remain unconscious in about 50% of the cases. The reason might be that gaze changes differ in their control as well as proprioceptive and sensory feedback quite significantly from manual responses. In addition, untrained subjects are not familiar with using gaze as a response effector. It is therefore conceivable that information sources such as efference copy and proprioception have less access to the general goal-oriented performance monitoring system (Fig. 3a, arrow 3).1 Notably, visual input is not processed during saccades. Compared to the broad range of information on a manual error, brief errors in eye motion (i.e., a prosaccade usually immediately followed by a corrective antisaccade) seem to be much less detectable.
Central nervous correlates of error awareness
In the seminal study by Nieuwenhuis et al. (2001) using the AST, the ERN did not differ between aware and unaware errors, whereas the Pe was present only for aware errors. This result has been replicated numerous times with oculomotor tasks and the manual EAT for visual and auditory stimuli (Endrass et al. 2005, 2007; O’Connell et al. 2007; Shalgi et al. 2009). This has led to the interpretation that the ERN is a preconscious correlate of performance monitoring, whereas the Pe is a more likely correlate of error awareness (Overbeek et al. 2005).
Neuroimaging studies hint at a role for the AIC, the RCZ, and somatosensory cortex (SI, SII) in error awareness (Hester et al. 2005; Klein et al. 2007). The findings suggest that the RCZ and the AIC, respectively, show a modulation by error blindness and error awareness reminiscent of the patterns observed for the ERN and the Pe, respectively. AIC and adjacent orbitofrontal/frontoopercular cortex has been found to be engaged selectively to aware errors, whereas the error-related RZC activity shows no significant difference between unaware and aware errors (Hester et al. 2005; Klein et al. 2007). In contrast to the direct role of the pMFC in generating the electrophysiological signal captured in the ERN (Debener et al. 2005), Klein et al. (2007) deemed it implausible that the AIC is the immediate generator of the widely distributed fronto-parietal Pe. They suggested that AIC might be involved more indirectly in generating the Pe through its functional connections with frontal and parietal cortices. Interestingly, Hester et al. (2005) found parietal somatosensory areas more active for aware than for unaware errors and speculated that these regions may contribute to Pe generation. As of yet, however, no concurrent ERP and fMRI data are available to put these hypotheses to a test.
Thus, it is yet unclear how AIC, RCZ and somatosensory cortex relate to each other during error awareness. They may simply co-activate, however the rich anatomical connections of AIC with the anterior cingulate cortex and pMFC and with somatosensory cortex (Augustine 1996) suggest functionally coordinated activity among these key players.
Note that in some studies modulations of the ERN and RCZ activity by error awareness was found (Maier et al. 2008; Harsay et al. 2010; Wessel et al. 2010), which contrasts with the common notion that the preconscious early correlates of error processing are not related to error awareness. However, as shown in Fig. 1a, it is not implausible that error awareness is modulated by factors that take effect already prior to error-related pMFC activity and may thus influence these early correlates as well (Fig. 1a). The discordant findings regarding ERN modulation may relate to statistical power. Almost all reported studies have very small sample sizes, increasing the type-II-error probability. This is especially true given the small amplitudes of the ERN in those paradigms.
In a number of studies, error awareness was associated with stronger post-error adjustments such as post-error slowing or post-error improvement of accuracy (Nieuwenhuis et al. 2001; Klein et al. 2007). Note that in the EAT with widely spaced NoGo trials performance adjustment is reflected in speeding on subsequent trials; accordingly aware but not unaware errors were followed by post-error speeding (Hester et al. 2005). It is also conceivable that other trial-by-trial and long-term post-error adjustments and maladjustments may be influenced by error awareness, for example, in task switching (Steinhauser and Hubner 2008) and errorful learning.
Pathologic alterations of error awareness in autism, ADHD and addiction have been observed and suggested to relate to both hypo- and hyper-activity of AIC (Paulus and Stein 2006; Hester et al. 2007; Silani et al. 2008; Vlamings et al. 2008; O’Connell et al. 2009b; Paulus and Stein 2010, this issue). In a group of patients with focal lesions of the thalamus we found significantly impaired error awareness in a flanker task (signaling rate, patients, 39.1 vs. controls 85.1%) accompanied by amplitude reduction of the ERN and Pe (Seifert et al. 2010). However, the majority of patient studies published so far did not address error awareness directly. Given the putative relationship of error awareness and some post-error adjustments, error awareness research in patients with neuropsychological deficits in cognitive control and flexible adjustments is needed.
Autonomic nervous correlates of error awareness
The finding of the differential role of the AIC in neuroimaging experiments of error processing and error awareness is of particular interest, given the evidence of the AIC’s association with autonomic signals of the body periphery (Craig 2002, 2009). Autonomic responses prepare the organism to respond to changed internal and external requirements and to recruit the necessary mental as well as physical efforts. Thus, they occur as adjustments to errors and external events (e.g., pain; Paine et al. 2009), and they accompany numerous cognitive tasks already in the preparatory phase.
The autonomic nervous system (ANS) has been shown to be sensitive to errors on many accounts. One of the most regularly reported ANS correlates sensitive to performance accuracy is heart-rate (HR) variability measured by means of electrocardiography (ECG). As early as in 1971, heart rate has been shown to decelerate as a consequence of error commission (Danev and de Winter 1971). This effect has been replicated many times in the following decades (Crone et al. 2003; Hajcak et al. 2003; Fiehler et al. 2004; van der Veen et al. 2004; Wessel et al. 2010) and can be seen as the most reliable known ANS correlate of error processing. Furthermore, pupil diameter (PD), an index of autonomic arousal with faster latencies than heart-rate deceleration, has been investigated in relation to action accuracy. Pupil dilation subsequent to the offset of the (contractory) pupillary light reflex (evoked by the onset of a luminant imperative stimulus) has been shown to be enlarged in case the stimulus has led to an error (Critchley et al. 2005a). Importantly, in this study, these PD changes indexing autonomic arousal were correlated with activity in both the AIC and the pMFC (amongst other brain areas) by means of an fMRI conjunction analysis, suggesting a possible link between AIC activation and autonomic activity during error processing.
However, none of these studies have explicitly paid attention to the question of error awareness, allowing no immediate insights into the question of why the AIC should be significantly more active on consciously perceived errors.
Recently, there have been two studies focusing on exactly this question: Does the ANS reaction differ depending on subjective error awareness? In a first experiment, using the EAT, O’Connell and colleagues were able to demonstrate that the skin-conductance response (SCR) to subjects’ reactions, another correlate of (mostly sympathetic) ANS activity, is highly sensitive to subjective error awareness: The SCR was enlarged towards errors (in comparison to corrects), yet this only held true for consciously perceived errors. Unperceived errors did, in fact, not yield any significant SCR at all whereas correct trials did, to lesser extend than perceived errors (O’Connell et al. 2007).
In a later study, using the AST, we contrasted both heart-rate deceleration and pupil diameter with respect to error awareness (Wessel et al. 2010). We found that the well-established post-error heart-rate deceleration is indeed only present on consciously perceived errors, paralleling the results by O’Connell et al. (2007) for electrodermal activity. For PD, a more complex finding ensued.
Extending earlier studies on stimulus-related pupillary changes (Critchley et al. 2005a), we focused on response-locked PD, showing that it is sensitive to performance (accuracy) already in very early latency ranges (immediately subsequent to the response saccade), yet this measurement is only in later latency ranges modulated by error awareness. The very early difference between errors and corrects gives rise to speculations about the role of neuronal systems associated with PD already in pre-error processes, leading to or influencing error commission.
Pupil diameter is regulated complementarily via the interplay between the sympathetic and parasympathetic branches of the ANS: Agonistic pupillary dilation is controlled by the sympathetic branch via the ciliospinal center. It projects to the superior cervical ganglion, from which fibers (along the truncus sympathicus) project directly to the pupil dilator muscle. Agonistic pupillary constriction is parasympathetically regulated via (pretectal) inputs to the nucleus Edinger–Westphal (nucleus accessorius nervi occulomotorii). Efferences of this nucleus reach the iris sphincter muscle by means of the ciliary ganglion. Pupil diameter is regulated by an interaction of the two pathways, and, in effect, by synergetic effects of both the dilator and sphincter muscles of the iris (Qiyuan et al. 1985).
The putative role of norepinephrine in post-error CNS and ANS responses
Although the exact neurophysiological underpinnings are not yet fully understood, it has been argued that pupil diameter responsivity constitutes an indirect index for the locus coeruleus–norepinephrine (LC/NE) function (Gilzenrat et al. 2010). The LC is the main norepinephrine-generating nucleus in the brainstem, and is central to regulating the sympathetic discharge and the inhibition of parasympathetic tone in arousal responses. Fluctuations in tonic and phasic LC firing modes have been found to index variability in performance efficiency that can be linked to lapses of task engagement in rodents (Usher et al. 1999; Rajkowski et al. 2004). Thus, recently it has been hypothesized that the LC/NE plays a role in higher-order cognitive functioning and task engagement (Aston-Jones and Cohen 2005). Phasic increases in firing have been proposed to be associated with unexpected events eliciting unexpected uncertainty (Yu and Dayan 2005; Dayan and Yu 2006), which by itself seems to involve, among other brain regions, the AIC. Notably, the LC has been argued to be anatomically connected with the AIC (among other frontal structures) (Aston-Jones et al. 1986, 1991). Based on these anatomical connections it has been suggested that signals from frontal structures (including AIC) vary with LC firing and interestingly, pupil responsivity. Studies of pupil diameter responses during cognitive control tasks show that baseline pupil diameter (before a stimulus) and evoked pupil diameter (after a stimulus) may serve as indices for tonic and phasic modes of LC/NE function (Gilzenrat et al. 2010). Larger baseline pupil diameter before a task-relevant event and reduced task-evoked pupil dilations thus may provide overt indications of task disengagement and poorer performance.
In a recent fMRI experiment, we found that baseline PD did indeed predict subsequent activity in areas previously found to be associated with error awareness (Harsay et al. 2010). Specifically, PD prior to and after aware errors covaried with activity increases in the pMFC, AIC, somatosensory cortex and oculomotor areas, and the concurrent deactivation in the default mode network (DMN) (Fig. (Fig.4).4).
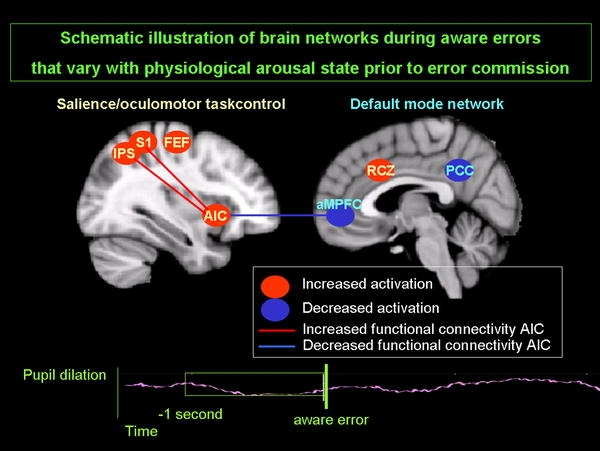
Pupil diameter just prior to errors predicts opposing neural network dynamics that are supported by changes in functional connectivity of anterior insular cortex (AIC), but only when subjects become aware of the error (adapted from Harsay et al. 2010). Preparatory pupil diameter predicts increased activation of AIC and salience/control structures (RCZ rostral cingulate zone, S primary sensory cortex, and task control oculomotor structures IPS intraparietal sulcus, FEF frontal eye field) and decreased activation in the default mode network (aMPFC anterior medial prefrontal cortex, and PCC posterior cingulate cortex). Likewise, preparatory pupil diameter predicts functional connectivity of AIC with nodes of the attention/control networks (S, IPS) increased at the expense of functional connectivity with nodes of the default mode network (aMPFC)
Taken together, many different measures of ANS activity have been shown to be differentially sensitive towards error awareness and error blindness. Importantly, these indices of ANS have earlier been demonstrated to be associated with activity in both the RCZ and the AIC (Critchley et al. 2005a, b; Medford and Critchley 2010, this issue). Possible consequences of this association for the role of the AIC in emerging error awareness are discussed in the following section.
A perspective on integrating the AIC, the ANS, and error awareness: the orienting response account
Alongside the AICs role in performance monitoring, a great deal of evidence has been provided implicating it as a correlate of autonomic nervous system (ANS) activity, ranging from heart beat to indices of pupil diameter (Craig 2002, 2009; Critchley 2005). It is far from clear, however, what precise association the AIC and the ANS share. Some authors that have reported such associations primarily attribute the role of insular activity to the process of the generation of autonomic activity, whereas others emphasize a possible key role of the insula in the perception, rather than the generation, of ANS state and responses. Due to the correlative nature of most neuroimaging techniques, causal ties are hard to establish without the use of patient studies.
As a least common denominator, it seems safe to say, though, that there is a reliable association between the activity of large parts of the ANS and the AIC. As reported earlier, erroneous actions are accompanied by changes in ongoing ANS activity, among reflected in HR, PD, and SCR. Importantly, the response of the ANS is stronger for perceived than for unperceived errors.
Accounts trying to explain the involvement of the ANS in error processing have not always provided clear explanations of the role of the ANS engagement as a consequence of errors. In our aforementioned EEG-ANS study (Wessel et al. 2010) we interpret our results in the light of recent behavioral experiments (Notebaert et al. 2009), which suggested strong behavioral parallels between processing of errors and other rare, potentially significant events (e.g., deviant and/or novel stimuli). According to their account, error-related ANS activity is a manifestation of an orienting response (OR, Sokolov 1963) towards an erroneous outcome. The OR is a reflex-like reaction of the organism to improbable changes in its environment (which are potentially motivationally relevant) and is accompanied by a cascade of CNS and ANS reactions, including HR, PD, and SCR associated with increased arousal. We are aware that the term orienting has been used in many contexts and use it here to indicate the complex arousal response to motivationally salient events rather than orienting attention in space or time. Such OR-related interpretations of error-related ANS activity have been provided already in the 1970s of the twentieth century (Danev and de Winter 1971), but have not been followed up on in later studies demonstrating ANS activity as a consequence of errors.
The central argument is that an error, much like a rare stimulus, elicits an OR-like reaction. The emergence of an OR towards an infrequent stimulus is heavily dependent on the realization of the potential significance (information content) of the stimulus. For an error to elicit an OR, then, it is reasonable to assume that the emergence of the OR after an error is also associated with its detection. That is, error awareness should be crucial to the emergence of an error-related OR, or vice versa. The larger OR-like ANS activity after perceived errors seems to support this account. Moreover, there are striking parallels between the association of the Pe with error awareness and the association of the P3b with the OR (Ridderinkhof et al. 2009). Notably, the P3b has been suggested to be related to phasic activity of the LC/NE system (Nieuwenhuis et al. 2005), as has the OR. The autonomic response to an error may serve to increase autonomic arousal and thus facilitate necessary adjustments. Note that strong responses and associated arousal may also lead to maladaptation. For example, post-error slowing [suggested to be at least in part driven by the OR (Notebaert et al. 2009)] may be associated with improvements in accuracy (=adaptive) or with decreases in accuracy (=maladaptive) (Rabbitt 1966; Rabbitt and Rodgers 1977; Fiehler et al. 2005). It may be speculated that the adaptiveness of the adjustment depends not only on external factors such as time pressure but also on the pre-trial baseline state of the LC/NE system (see above).
Independently of the arguments as to why the ANS response is different for perceived and unperceived errors, this mere observational fact alone offers a simple possible explanation for the differential effects of error awareness on AIC activity: if the anterior insula serves either as an active agent in eliciting the ANS response, or as a crucial monitor for the milieu interne (on a proprioceptive/interoceptive level with regard to ANS activity) of the body, the effects of error awareness and error blindness on insular activity might be the same that are responsible for the effects of error awareness on the ANS. As already discussed in Klein et al. (2007), it is unclear whether (a) AIC activity precedes and causes the ANS response, (b) the ANS response elicits the AIC engagement by interoception, or (c) both occur simultaneously. In other words, it is of great interest whether AIC activity is causal to the ANS activity (and possibly to components of the OR) or whether it serves more for monitoring or interoceptive awareness-related functions. In consequence, it is also of large interest whether the presence of the ANS reaction itself is a mere consequence of error awareness or whether it provides additional input for potential other systems decisive for the conscious recognition of erroneous actions (see Fig. 1b). This, however, once again underlines the need for lesion studies to establish a causal relationship and its directionality.
The AIC as part of a “salience network”
During performance across tasks as well as during rest and rumination, the AIC has been argued to regulate competitive dynamics between large-scale networks (Sridharan et al. 2008). Functional connectivity studies suggest the existence of several large-scale networks that appear to underlie different task-related and task-unrelated functions (Dosenbach et al. 2006, 2007; Nelson et al. 2010, this issue). While the functional concepts regarding these networks are still rather vague and the engagement of certain brain regions varies in a task-specific manner, three major and relatively invariant networks seem to emerge: the default mode network (DMN) typically most active during rest and deactivated during cognitive effort, a fronto-parietal “control network” assumed to be engaged in top–down shifts of attention, and a “salience network” encompassing the pMFC, bilateral AIC and the adjacent frontal operculum/orbitofrontal cortex (Seeley et al. 2007). This putative salience network strongly overlaps with activity patterns found in performance monitoring research (see metaanalysis reported above and in Klein et al. (2007)). Interestingly, it is strongly connected to the LC/NE system (Aston-Jones et al. 1986, 1991). Phasic NE release has been proposed to act as an interrupt signal (Dayan and Yu 2006) allowing to (re-)orient to any kind of upcoming salient event and thus to flexibly react to unexpected problems. Functionally, activation of structures of the salience network has been associated with the processing of personally and motivationally important “salient” information, in the broad spectrum of nociceptive (Peyron et al. 2000), emotional and social (Bartels and Zeki 2004; Singer et al. 2004; Lamm and Singer 2010, this issue), cognitive (Ramautar et al. 2006), homeostatic and sympathetic efferent and interoceptive autonomic domains (Craig 2002, 2003; Critchley 2004; Critchley et al. 2000, 2005a, b). The salience network appears to be central to monitoring for motivationally important changes that require autonomic regulation (Downar et al. 2000, 2002, 2003). Interestingly, the pattern of differential network activation, perhaps reflecting a direct competition for processing resources, has been found to be predictive for variability in performance (Kelly et al. 2008). Tendencies toward improved performance appear to be foreshadowed in the activation of the salience and task-related control networks, whereas increased activation in the DMN often presage performance lapses (Weissman et al. 2006; Boly et al. 2007; Eichele et al. 2008). That the AIC might be involved in these performance fluctuations in terms of preparatory engagement (e.g., through the allocation of cognitive and physical resources) of salience processing and deliberate adaptive control networks while simultaneously disengaging the DMN (Sridharan et al. 2008).
Similarly, the AIC and the salience network may help recruiting cognitive and physical effort reactively, in response to an error, or more generally, to OR. Initial evidence for this hypothesis comes from a recent analysis of the functional connectivity of AIC during error awareness in the previously described AST (Harsay et al. 2010). AIC displayed increased functional connections with (other) salience network structures (primary somatosensory cortex) and with oculomotor areas (intraparietal sulcus) during error awareness, suggesting coordinated activity of AIC with distant brain regions presumably in an effort to amplify the neural salience-signal of the detected error and to preset task-relevant oculomotor structures. Interestingly, the functional connectivity of AIC varied with individual’s baseline pupil dilation before the aware error. Pupil dilation before the aware error predicted increased connectivity with primary somatosensory cortex and oculomotor areas, together with decreased functional connectivity of AIC with areas of the DMN (anterior medial frontal cortex, frontal pole). Thus, the autonomic activity before the aware error seems to translate centrally into an enhanced neural control state during error awareness that is characterized by inverse connectivity-patterns of AIC.
A potential limitation of the salience network account is that there is little that seems to differentiate the roles of the pMFC and the AIC. In fact, except for the differential responsivity to error awareness (Hester et al. 2005; Klein et al. 2007) we know of only one neuroimaging study on performance monitoring in which AIC and pMFC activity are dissociated (Magno et al. 2006). Here, the AIC and pMFC are again coactivated on errors. However, when subjects avoided trials associated with strong effort and high error likelihood by pressing an “escape” button, only the pMFC was active, whereas the insula showed no signal change. Human and animal research indicate that the pMFC is involved in cost–benefit calculations related to whether or not it is worth taking effort for a certain reward (Walton et al. 2003, 2007; Croxson et al. 2009; Hauber and Sommer 2009). Reconciling the findings by Magno et al. (2006) with these results it appears that pMFC activity is associated with deciding about taking effort whereas the AIC activity is associated with recruiting the resources needed for mental and physical efforts (Deary et al. 2004; Jansma et al. 2007; Eichele et al. 2008; see also Sterzer and Kleinschmidt 2010, this issue). These hypotheses should be tested in future experiments.
Discussion and outlook
To summarize, error awareness is a difficult-to-study phenomenon that is of high interest as it may substantially modify flexible adjustments, in particular the recruitment of resources for effortful behavior and long-term strategy changes. Error awareness certainly impacts general motivation and affective responses to one’s own performance. It appears to result when a compound error signal emerges from numerous inputs (Fig. 1a). The weighting and timing of these inputs determines the time when errors become consciously perceived.
Regarding the neural correlates we are still at the very beginning of this research. The RCZ, AIC, and somatosensory cortex appear to be the most relevant structures. Error awareness is clearly associated with the occurrence of the Pe and autonomic responses at a latency of 300 ms and later. In contrast, earlier correlates of performance monitoring such as the ERN and baseline changes in ANS activity may vary with and even predict error awareness but are unlikely to reflect the entry of the error signal into consciousness.
The AIC and its relation to the autonomic response appear to be crucial for error awareness. Their exact causal interactions, however, are yet to be determined. Errors may elicit an OR, associated with burst firing of LC neurons and release of NE. It may be speculated that the OR is a prerequisite of error awareness as it increases arousal and recruits large-scale brain networks sufficient to cause conscious experience (Dehaene et al. 2006). In other words, instead of actually becoming aware of the error itself, we first may become aware of the OR. Furthermore, it may be hypothesized that the AIC and pMFC as members of a salience network are well-connected key players in regulating the allocation of mental and physical effort prior to important tasks and in response to unexpected action outcomes. Recent functional connectivity studies seem to suggest that this salience network is crucial for balancing the engagement of task-related, rest-related activity and ongoing activity in the human brain. In any case, we should always keep in mind that cognitive and autonomic responses are coupled in order to allow optimal and most flexible adjustments; and the AIC and pMFC appear to mediate this coupling.
A number of open questions remain to be addressed:
Is there any causal relationship between AIC activity, autonomic responses, and error awareness? If yes, what is the directionality of the causal interactions? To address these questions, the autonomic responses to errors and error awareness in patients and non-human primates with focal insular lesions should be examined. Also invasive electrophysiology (e.g., in the context of epilepsy diagnostics with depth electrodes) could provide valuable information.
Is there any functional subregionalization of the AIC? If yes, what are the functional principles and anatomical underpinnings of this spatial differentiation? It appears that more superior AIC activity occurs in situations when risks and failures can still be avoided, whereas the inferior AIC is involved when a negative event (error, increase in risk) has already occurred. These situations may call for different types of responses and require the recruitment of different resources. Diffusion-weighted tractography studies should focus on the differential connectivities of the putative superior and inferior AIC subregions, specifically with respect to other brain structures involved in performance monitoring. This may provide hints on the functional significance of the observed subregional activity differences.
How much is error awareness related to awareness of response conflict, decision uncertainty or generally “difficulty” and the effort one is willing to put into a task? So far, no study has investigated whether response conflict—according to dominant theories one of the major forces in recruiting cognitive effort (Botvinick et al. 2001, 2004; Ridderinkhof et al. 2004; Botvinick 2007)—becomes aware and whether such “conflict awareness” is needed for typical conflict-driven adjustments (Egner and Hirsch 2005; Ullsperger et al. 2005). Perhaps, a unifying concept could be that these kinds of awareness relate to salient, motivationally relevant, information-laden conditions, calling for adjustments. They may already lead to some adjustments in the absence of awareness, but the stronger the signals and the accompanying autonomic response, the more likely they enter awareness. The orienting response may be at the top level of these responses.
What is the relationship of uncertainty, LC/NE function, and AIC activity? While phasic NE release seems associated with unexpected uncertainty (Dayan and Yu 2006), and the AIC has been shown to be involved in uncertainty processing, it remains to be determined when and how uncertainty changes in relation to errors. Detecting errors may reduce uncertainty and thus help learning to choose favorable actions and to avoid unfavorable actions, new uncertainties may arise at the same time. To choose appropriate remedial actions and subsequent performance adjustments it is helpful to determine the cause of the failure to reach the goal in the first place. Uncertainty about the source of an error and the appropriate adjustment has been shown to increase pMFC and AIC activity (Ullsperger et al. 2007).
Which post-error adjustments depend on error awareness, and which do not? Is error awareness an all-or-nothing prerequisite for some adjustments, or is there a gradual correlation of the adjustments with the strength of the error signal resulting in awareness?
What are the interactions between error awareness and emotion and motivation? Particularly aware errors should have impact on motivation. Vice versa, affective and motivational state may influence to what extent errors are consciously perceived.
Which patient groups have difficulties with error awareness? What is the problem with patients who become aware of their errors but still cannot implement necessary strategy changes (thereby exhibiting knowing–doing dissociations)? In a number of brain diseases, for example, in diffuse axonal injury resulting from traumatic brain injury and in frontotemporal dementia, patients are not aware of inappropriate behavior. To what extent is their pathology related to the processes involved in error awareness as reviewed here?
The research on how we become aware of our own performance is still in its infancy. It is an important and fascinating topic. The ultimate goal of this research with respect to the function of the AIC should be to come up with a general concept that also takes into account the numerous other conditions engaging AIC as discussed in this special issue.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
1Error monitoring is likely to be organized hierarchically and to occur at many levels of the central nervous system. It appears that errors that interfere with achievement of rewards and abstract high-level goals are processed by the pMFC-centered performance monitoring system, independently of stimulus and effector modality. More low-level motor deviations and adjustments seem to be more related to posterior cortical and subcortical motor control areas [e.g., posterior parietal cortex and cerebellum, for manual responses (Desmurget et al. 1999; Krigolson et al. 2008)]. As action execution and on-line motor control networks differ considerably between manual and oculomotor actions, it is likely that the influence of efference copies and proprioceptive feedback from these “low level” systems on the pMFC-centered “high-level” performance monitoring system and the structures underlying error awareness differ as well.
References
- Allain S, Hasbroucq T, Burle B, Grapperon J, Vidal F. Response monitoring without sensory feedback. Clin Neurophysiol. 2004;115:2014–2020. [Abstract] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus–norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–450. [Abstract] [Google Scholar]
- Aston-Jones G, Ennis M, Pieribone VA, Nickell WT, Shipley MT. The brain nucleus locus coeruleus: restricted afferent control of a broad efferent network. Science. 1986;234:734–737. [Abstract] [Google Scholar]
- Aston-Jones G, Shipley MT, Chouvet G, Ennis M, van Bockstaele E, Pieribone V, Shiekhattar R, Akaoka H, Drolet G, Astier B, et al. Afferent regulation of locus coeruleus neurons: anatomy, physiology and pharmacology. Prog Brain Res. 1991;88:47–75. [Abstract] [Google Scholar]
- Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev. 1996;22:229–244. [Abstract] [Google Scholar]
- Bartels A, Zeki S. The neural correlates of maternal and romantic love. Neuroimage. 2004;21:1155–1166. [Abstract] [Google Scholar]
- Boly M, Balteau E, Schnakers C, Degueldre C, Moonen G, Luxen A, Phillips C, Peigneux P, Maquet P, Laureys S. Baseline brain activity fluctuations predict somatosensory perception in humans. Proc Natl Acad Sci USA. 2007;104:12187–12192. [Europe PMC free article] [Abstract] [Google Scholar]
- Bossaerts P (2010) Risk and risk prediction error signals in anterior insula. Brain Struc Func 214(5–6). 10.1007/s00429-010-0253-1 (this issue) [Abstract]
- Botvinick MM. Conflict monitoring and decision making: reconciling two perspectives on anterior cingulate function. Cogn Affect Behav Neurosci. 2007;7:356–366. [Abstract] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. [Abstract] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci. 2004;8:539–546. [Abstract] [Google Scholar]
- Cohen MX, van Gaal S, Ridderinkhof KR, Lamme VA. Unconscious errors enhance prefrontal-occipital oscillatory synchrony. Front Hum Neurosci. 2009;3:54. [Europe PMC free article] [Abstract] [Google Scholar]
- Coles MG, Scheffers MK, Holroyd CB. Why is there an ERN/Ne on correct trials? Response representations, stimulus-related components, and the theory of error-processing. Biol Psychol. 2001;56:173–189. [Abstract] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. [Abstract] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13:500–505. [Abstract] [Google Scholar]
- Craig AD. How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. [Abstract] [Google Scholar]
- Critchley HD. The human cortex responds to an interoceptive challenge. Proc Natl Acad Sci USA. 2004;101:6333–6334. [Europe PMC free article] [Abstract] [Google Scholar]
- Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol. 2005;493:154–166. [Abstract] [Google Scholar]
- Critchley HD, Corfield DR, Chandler MP, Mathias CJ, Dolan RJ. Cerebral correlates of autonomic cardiovascular arousal: a functional neuroimaging investigation in humans. J Physiol. 2000;523(Pt 1):259–270. [Abstract] [Google Scholar]
- Critchley HD, Tang J, Glaser D, Butterworth B, Dolan RJ. Anterior cingulate activity during error and autonomic response. Neuroimage. 2005;27:885–895. [Abstract] [Google Scholar]
- Critchley HD, Rotshtein P, Nagai Y, O’Doherty J, Mathias CJ, Dolan RJ. Activity in the human brain predicting differential heart rate responses to emotional facial expressions. Neuroimage. 2005;24:751–762. [Abstract] [Google Scholar]
- Crone EA, van der Veen FM, van der Molen MW, Somsen RJM, van Beek B, Jennings JR. Cardiac concomitants of feedback processing. Biol Psychol. 2003;64(1–2):143–156. [Abstract] [Google Scholar]
- Croxson PL, Walton ME, O’Reilly JX, Behrens TE, Rushworth MF. Effort-based cost–benefit valuation and the human brain. J Neurosci. 2009;29:4531–4541. [Europe PMC free article] [Abstract] [Google Scholar]
- Danev SG, de Winter CR. Heart rate deceleration after erroneous responses. A phenomenon complicating the use of heart rate variability for assessing mental load. Psychol Forsch. 1971;35:27–34. [Abstract] [Google Scholar]
- Dayan P, Yu AJ. Phasic norepinephrine: a neural interrupt signal for unexpected events. Network. 2006;17:335–350. [Abstract] [Google Scholar]
- De Bruijn ERA, Mars RB, Hulstijn W. It wasn’t me…or was it? How false feedback affects performance. In: Ullsperger M, Falkenstein M, editors. Errors, conflicts, and the brain. Current opinions on performance monitoring. Leipzig: MPI for Human Cognitive and Brain Sciences; 2004. pp. 118–124. [Google Scholar]
- Deary IJ, Simonotto E, Meyer M, Marshall A, Marshall I, Goddard N, Wardlaw JM. The functional anatomy of inspection time: an event-related fMRI study. Neuroimage. 2004;22:1466–1479. [Abstract] [Google Scholar]
- Debener S, Ullsperger M, Siegel M, Fiehler K, von Cramon DY, Engel AK. Trial-by-trial coupling of concurrent electroencephalogram and functional magnetic resonance imaging identifies the dynamics of performance monitoring. J Neurosci. 2005;25:11730–11737. [Europe PMC free article] [Abstract] [Google Scholar]
- Dehaene S, Posner MI, Tucker DM. Localization of a neural system for error detection and compensation. Psychol Sci. 1994;5:303–305. [Google Scholar]
- Dehaene S, Changeux JP, Naccache L, Sackur J, Sergent C. Conscious, preconscious, and subliminal processing: a testable taxonomy. Trends Cogn Sci. 2006;10:204–211. [Abstract] [Google Scholar]
- Desmurget M, Epstein CM, Turner RS, Prablanc C, Alexander GE, Grafton ST. Role of the posterior parietal cortex in updating reaching movements to a visual target. Nat Neurosci. 1999;2:563–567. [Abstract] [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50:799–812. [Europe PMC free article] [Abstract] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA. 2007;104:11073–11078. [Europe PMC free article] [Abstract] [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, Davis KD. A multimodal cortical network for the detection of changes in the sensory environment. Nat Neurosci. 2000;3:277–283. [Abstract] [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, Davis KD. A cortical network sensitive to stimulus salience in a neutral behavioral context across multiple sensory modalities. J Neurophysiol. 2002;87:615–620. [Abstract] [Google Scholar]
- Downar J, Mikulis DJ, Davis KD. Neural correlates of the prolonged salience of painful stimulation. Neuroimage. 2003;20:1540–1551. [Abstract] [Google Scholar]
- Egner T, Hirsch J. Cognitive control mechanisms resolve conflict through cortical amplification of task-relevant information. Nat Neurosci. 2005;8:1784–1790. [Abstract] [Google Scholar]
- Eichele T, Debener S, Calhoun VD, Specht K, Engel AK, Hugdahl K, von Cramon DY, Ullsperger M. Prediction of human errors by maladaptive changes in event-related brain networks. Proc Natl Acad Sci USA. 2008;105:6173–6178. [Europe PMC free article] [Abstract] [Google Scholar]
- Endrass T, Franke C, Kathmann N. Error awareness in a saccade countermanding task. J Psychophysiol. 2005;19:275–280. [Google Scholar]
- Endrass T, Reuter B, Kathmann N. ERP correlates of conscious error recognition: aware and unaware errors in an antisaccade task. Eur J Neurosci. 2007;26:1714–1720. [Abstract] [Google Scholar]
- Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept Psychophys. 1974;16:143–149. [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L (1990) Effects of errors in choice reaction tasks on the ERP under focused and divided attention. In: Brunia CHM, Gaillard AWK, Kok A (eds) Psychophysiological brain research. Tilburg University Press, pp 192–195
- Fiehler K, Ullsperger M, Grigutsch M, von Cramon D. Cardiac responses to error processing and response conflict. In: Ullsperger M, Falkenstein M, editors. Errors, conflicts, and the brain. Current opinions on performance monitoring. Leipzig: MPI for Human Cognitive and Brain Sciences; 2004. pp. 135–140. [Google Scholar]
- Fiehler K, Ullsperger M, von Cramon DY. Electrophysiological correlates of error correction. Psychophysiology. 2005;42:72–82. [Abstract] [Google Scholar]
- Garavan H, Ross TJ, Kaufman J, Stein EA. A midline dissociation between error-processing and response-conflict monitoring. Neuroimage. 2003;20:1132–1139. [Abstract] [Google Scholar]
- Gehring WJ, Goss B, Coles MG, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychol Sci. 1993;4:385–390. [Google Scholar]
- Gentsch A, Ullsperger P, Ullsperger M. Dissociable medial frontal negativities from a common monitoring system for self- and externally caused failure of goal achievement. Neuroimage. 2009;47:2023–2030. [Abstract] [Google Scholar]
- Gilzenrat MS, Nieuwenhuis S, Jepma M, Cohen JD (2010) Pupil diameter tracks changes in control state predicted by the adaptive gain theory of locus coeruleus function. Cogn Affect Behav Neurosci (in press) [Europe PMC free article] [Abstract]
- Hajcak G, McDonald N, Simons RF. To err is autonomic: error-related brain potentials, ANS activity, and post-error compensatory behavior. Psychophysiology. 2003;40:895–903. [Abstract] [Google Scholar]
- Harsay HA, Cohen MX, Spaan M, Weeda WD, Nieuwenhuis S, Ridderinkhof KR (2010) Error awareness: pupil dilation predicts opposing brain network dynamics during error awareness (submitted) [Abstract]
- Hauber W, Sommer S. Prefrontostriatal circuitry regulates effort-related decision making. Cereb Cortex. 2009;19:2240–2247. [Abstract] [Google Scholar]
- Hester R, Foxe JJ, Molholm S, Shpaner M, Garavan H. Neural mechanisms involved in error processing: a comparison of errors made with and without awareness. Neuroimage. 2005;27:602–608. [Abstract] [Google Scholar]
- Hester R, Simoes-Franklin C, Garavan H. Post-error behavior in active cocaine users: poor awareness of errors in the presence of intact performance adjustments. Neuropsychopharmacology. 2007;32:1974–1984. [Abstract] [Google Scholar]
- Holroyd CB, Coles MG. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol Rev. 2002;109:679–709. [Abstract] [Google Scholar]
- Jansma JM, Ramsey NF, de Zwart JA, van Gelderen P, Duyn JH. fMRI study of effort and information processing in a working memory task. Hum Brain Mapp. 2007;28:431–440. [Europe PMC free article] [Abstract] [Google Scholar]
- Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–537. [Abstract] [Google Scholar]
- Klein TA, Endrass T, Kathmann N, Neumann J, von Cramon DY, Ullsperger M. Neural correlates of error awareness. Neuroimage. 2007;34:1774–1781. [Abstract] [Google Scholar]
- Krigolson OE, Holroyd CB, Van Gyn G, Heath M. Electroencephalographic correlates of target and outcome errors. Exp Brain Res. 2008;190(4):401–411. [Abstract] [Google Scholar]
- Lamm C, Singer T (2010) The role of the anterior insular cortex in social emotions. Brain Struc Func 214(5–6). 10.1007/s00429-010-0251-3 (this issue) [Abstract]
- Magno E, Foxe JJ, Molholm S, Robertson IH, Garavan H. The anterior cingulate and error avoidance. J Neurosci. 2006;26:4769–4773. [Europe PMC free article] [Abstract] [Google Scholar]
- Maier M, Steinhauser M, Hubner R. Is the error-related negativity amplitude related to error detectability? Evidence from effects of different error types. J Cogn Neurosci. 2008;20:2263–2273. [Abstract] [Google Scholar]
- Medford N, Critchley HD (2010) Conjoint activity of anterior insular and anterior cingulate cortex: awareness and response. Brain Struc Func 214(5–6). 10.1007/s00429-010-0265-x (this issue) [Europe PMC free article] [Abstract]
- Nachev P, Rees G, Parton A, Kennard C, Husain M. Volition and conflict in human medial frontal cortex. Curr Biol. 2005;15:122–128. [Europe PMC free article] [Abstract] [Google Scholar]
- Nelson SM, Dosenbach NUF, Cohen AL, Schlaggar BL, Petersen SE (2010) Role of the anterior insula in task-level control and focal attention. Brain Struc Func 214(5–6). 10.1007/s00429-010-0260-2 (this issue) [Europe PMC free article] [Abstract]
- Nieuwenhuis S, Ridderinkhof KR, Blom J, Band GP, Kok A. Error-related brain potentials are differentially related to awareness of response errors: evidence from an antisaccade task. Psychophysiology. 2001;38:752–760. [Abstract] [Google Scholar]
- Nieuwenhuis S, Aston-Jones G, Cohen JD. Decision making, the P3, and the locus coeruleus–norepinephrine system. Psychol Bull. 2005;131:510–532. [Abstract] [Google Scholar]
- Notebaert W, Houtman F, Opstal FV, Gevers W, Fias W, Verguts T. Post-error slowing: an orienting account. Cognition. 2009;111:275–279. [Abstract] [Google Scholar]
- O’Connell RG, Dockree PM, Bellgrove MA, Kelly SP, Hester R, Garavan H, Robertson IH, Foxe JJ. The role of cingulate cortex in the detection of errors with and without awareness: a high-density electrical mapping study. Eur J Neurosci. 2007;25:2571–2579. [Abstract] [Google Scholar]
- O’Connell RG, Dockree PM, Bellgrove MA, Turin A, Ward S, Foxe JJ, Robertson IH. Two types of action error: electrophysiological evidence for separable inhibitory and sustained attention neural mechanisms producing error on go/no-go tasks. J Cogn Neurosci. 2009;21:93–104. [Abstract] [Google Scholar]
- O’Connell RG, Bellgrove MA, Dockree PM, Lau A, Hester R, Garavan H, Fitzgerald M, Foxe JJ, Robertson IH. The neural correlates of deficient error awareness in attention-deficit hyperactivity disorder (ADHD) Neuropsychologia. 2009;47:1149–1159. [Abstract] [Google Scholar]
- Oliveira FT, McDonald JJ, Goodman D. Performance monitoring in the anterior cingulate is not all error related: expectancy deviation and the representation of action–outcome associations. J Cogn Neurosci. 2007;19:1994–2004. [Abstract] [Google Scholar]
- Overbeek TJM, Nieuwenhuis S, Ridderinkhof KR. Dissociable components of error processing: on the functional significance of the Pe vis-à-vis the ERN/Ne. J Psychophysiol. 2005;19:319–329. [Google Scholar]
- Paine P, Kishor J, Worthen SF, Gregory LJ, Aziz Q. Exploring relationships for visceral and somatic pain with autonomic control and personality. Pain. 2009;144:236–244. [Abstract] [Google Scholar]
- Paulus MP, Stein MB. An insular view of anxiety. Biol Psychiatry. 2006;60:383–387. [Abstract] [Google Scholar]
- Paulus MP, Stein MB (2010) Interception in anxiety and depression. Brain Struc Func 214(5–6). 10.1007/s00429-010-0258-9 (this issue) [Europe PMC free article] [Abstract]
- Persaud N, McLeod P, Cowey A. Post-decision wagering objectively measures awareness. Nat Neurosci. 2007;10:257–261. [Abstract] [Google Scholar]
- Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain. A review, meta-analysis (2000) Neurophysiol Clin. 2000;30:263–288. [Abstract] [Google Scholar]
- Picard N, Strick PL. Imaging the premotor areas. Curr Opin Neurobiol. 2001;11:663–672. [Abstract] [Google Scholar]
- Preuschoff K, Quartz SR, Bossaerts P. Human insula activation reflects risk prediction errors as well as risk. J Neurosci. 2008;28:2745–2752. [Europe PMC free article] [Abstract] [Google Scholar]
- Qiyuan J, Richer F, Wagoner BL, Beatty J. The pupil and stimulus probability. Psychophysiology. 1985;22:530–534. [Abstract] [Google Scholar]
- Rabbitt PM. Errors and error correction in choice–response tasks. J Exp Psychol. 1966;71:264–272. [Abstract] [Google Scholar]
- Rabbitt PM. Three kinds of error-signalling responses in a serial choice task. Q J Exp Psychol. 1968;20:179–188. [Abstract] [Google Scholar]
- Rabbitt P. Consciousness is slower than you think. Q J Exp Psychol A. 2002;55:1081–1092. [Abstract] [Google Scholar]
- Rabbitt P, Rodgers B. What does a man do after he makes an error? An analysis of response programming. Q J Exp Psychol. 1977;29:727–743. [Google Scholar]
- Rajkowski J, Majczynski H, Clayton E, Aston-Jones G. Activation of monkey locus coeruleus neurons varies with difficulty and performance in a target detection task. J Neurophysiol. 2004;92:361–371. [Abstract] [Google Scholar]
- Ramautar JR, Slagter HA, Kok A, Ridderinkhof KR. Probability effects in the stop-signal paradigm: the insula and the significance of failed inhibition. Brain Res. 2006;1105:143–154. [Abstract] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. [Abstract] [Google Scholar]
- Ridderinkhof KR, Ramautar JR, Wijnen JG. To P(E) or not to P(E): a P3-like ERP component reflecting the processing of response errors. Psychophysiology. 2009;46:531–538. [Abstract] [Google Scholar]
- Rushworth MFS, Walton ME, Kennerley SW, Bannerman DM. Action sets and decisions in the medial frontal cortex. Trends Cogn Sci. 2004;8:410–417. [Abstract] [Google Scholar]
- Sadaghiani S, Hesselmann G, Kleinschmidt A. Distributed and antagonistic contributions of ongoing activity fluctuations to auditory stimulus detection. J Neurosci. 2009;29:13410–13417. [Europe PMC free article] [Abstract] [Google Scholar]
- Scheffers MK, Coles MG. Performance monitoring in a confusing world: error-related brain activity, judgments of response accuracy, and types of errors. J Exp Psychol Hum Percept Perform. 2000;26:141–151. [Abstract] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. [Europe PMC free article] [Abstract] [Google Scholar]
- Seifert S, von Cramon DY, Imperati D, Tittgemeyer M, Ullsperger M (2010) Thalamo-cingulate interactions in performance monitoring (submitted) [Europe PMC free article] [Abstract]
- Shalgi S, O’Connell RG, Deouell LY, Robertson IH. Absent minded but accurate: delaying responses increases accuracy but decreases error awareness. Exp Brain Res. 2007;182:119–124. [Abstract] [Google Scholar]
- Shalgi S, Barkan I, Deouell LY. On the positive side of error processing: error-awareness positivity revisited. Eur J Neurosci. 2009;29:1522–1532. [Abstract] [Google Scholar]
- Silani G, Bird G, Brindley R, Singer T, Frith C, Frith U. Levels of emotional awareness and autism: an fMRI study. Soc Neurosci. 2008;3:97–112. [Abstract] [Google Scholar]
- Simon JR, Rudell AP. Auditory S-R compatibility: the effect of an irrelevant cue on information processing. J Appl Psychol. 1967;51:300–304. [Abstract] [Google Scholar]
- Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–1162. [Abstract] [Google Scholar]
- Sokolov EN. Higher nervous functions: the orienting reflex. Annu Rev Physiol. 1963;25:545–580. [Abstract] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci USA. 2008;105:12569–12574. [Europe PMC free article] [Abstract] [Google Scholar]
- Steinhauser M, Hubner R. How task errors affect subsequent behavior: evidence from distributional analyses of task-switching effects. Mem Cognit. 2008;36:979–990. [Abstract] [Google Scholar]
- Steinhauser M, Maier M, Hubner R. Modeling behavioral measures of error detection in choice tasks: response monitoring versus conflict monitoring. J Exp Psychol Hum Percept Perform. 2008;34:158–176. [Abstract] [Google Scholar]
- Sterzer P, Kleinschmidt A (2010) Anterior insula activations in perceptual paradigms – often observed but barely understood. Brain Struc Func 214(5–6). 10.1007/s00429-010-0252-2 (this issue) [Abstract]
- Taylor SF, Stern ER, Gehring WJ. Neural systems for error monitoring: recent findings and theoretical perspectives. Neuroscientist. 2007;13:160–172. [Abstract] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage. 2002;16:765–780. [Abstract] [Google Scholar]
- Ullsperger M, von Cramon DY. Subprocesses of performance monitoring: a dissociation of error processing and response competition revealed by event-related fMRI and ERPs. Neuroimage. 2001;14:1387–1401. [Abstract] [Google Scholar]
- Ullsperger M, von Cramon DY. How does error correction differ from error signaling? An event-related potential study. Brain Res. 2006;1105:102–109. [Abstract] [Google Scholar]
- Ullsperger M, Volz KG, von Cramon DY. A common neural system signaling the need for behavioral changes. Trends Cogn Sci. 2004;8:445–446. [Abstract] [Google Scholar]
- Ullsperger M, Bylsma LM, Botvinick MM. The conflict adaptation effect: It’s not just priming. Cogn Affect Behav Neurosci. 2005;5:467–472. [Abstract] [Google Scholar]
- Ullsperger M, Nittono H, von Cramon DY. When goals are missed: dealing with self-generated and externally induced failure. Neuroimage. 2007;35:1356–1364. [Abstract] [Google Scholar]
- Usher M, Cohen JD, Servan-Schreiber D, Rajkowski J, Aston-Jones G. The role of locus coeruleus in the regulation of cognitive performance. Science. 1999;283:549–554. [Abstract] [Google Scholar]
- van der Veen FM, Nieuwenhuis S, Crone EA, van der Molen MW. Cardiac and electro-cortical responses to performance feedback reflect different aspects of feedback processing. In: Ullsperger M, Falkenstein M, editors. Errors, conflicts, and the brain. Current opinions on performance monitoring. Leipzig: MPI for Human Cognitive and Brain Sciences; 2004. pp. 140–147. [Google Scholar]
- Vlamings PH, Jonkman LM, Hoeksma MR, van Engeland H, Kemner C. Reduced error monitoring in children with autism spectrum disorder: an ERP study. Eur J Neurosci. 2008;28:399–406. [Abstract] [Google Scholar]
- Vogt BA, Hof PR, Vogt LJ (2004) Cingulate cortex. In: Paxinos G, Mai JK (eds) The human nervous system. Elsevier, pp 915–948
- Volz KG, Schubotz RI, von Cramon DY. Predicting events of varying probability: uncertainty investigated by fMRI. Neuroimage. 2003;19:271–280. [Abstract] [Google Scholar]
- Walton ME, Bannerman DM, Alterescu K, Rushworth MF. Functional specialization within medial frontal cortex of the anterior cingulate for evaluating effort-related decisions. J Neurosci. 2003;23:6475–6479. [Europe PMC free article] [Abstract] [Google Scholar]
- Walton M, Rudebeck PH, Bannerman D, Rushworth M. Calculating the cost of acting in prefrontal cortex. Ann N Y Acad Sci. 2007;1104:340–356. [Europe PMC free article] [Abstract] [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–978. [Abstract] [Google Scholar]
- Wessel JR, Danielmeier C, Ullsperger M (2010) Error awareness revisited: the ERN, the Pe, and the autonomous nervous system (submitted)
- Yeung N, Cohen JD, Botvinick MM. The neural basis of error detection: conflict monitoring and the error-related negativity. Psychol Rev. 2004;111:931–959. [Abstract] [Google Scholar]
- Yu AJ, Dayan P. Uncertainty, neuromodulation, and attention. Neuron. 2005;46:681–692. [Abstract] [Google Scholar]
Full text links
Read article at publisher's site: https://doi.org/10.1007/s00429-010-0261-1
Read article for free, from open access legal sources, via Unpaywall:
https://link.springer.com/content/pdf/10.1007/s00429-010-0261-1.pdf
Citations & impact
Impact metrics
Article citations
Individualized functional magnetic resonance imaging neuromodulation enhances visuospatial perception: a proof-of-concept study.
Philos Trans R Soc Lond B Biol Sci, 379(1915):20230083, 21 Oct 2024
Cited by: 2 articles | PMID: 39428879 | PMCID: PMC11491853
Enhancing cognitive control of our decisions: Making the most of humor during the IGT in females and males.
Cogn Affect Behav Neurosci, 24(6):1031-1047, 05 Sep 2024
Cited by: 0 articles | PMID: 39237775 | PMCID: PMC11525253
The neural network of sensory attenuation: A neuroimaging meta-analysis.
Psychon Bull Rev, 01 Jul 2024
Cited by: 0 articles | PMID: 38954157
Review
Altered temporal awareness during Covid-19 pandemic.
Psychol Res, 88(8):2335-2345, 22 Jul 2024
Cited by: 0 articles | PMID: 39034344
Distinct neural markers of evidence accumulation index metacognitive processing before and after simple visual decisions.
Cereb Cortex, 34(5):bhae179, 01 May 2024
Cited by: 0 articles | PMID: 38706138 | PMCID: PMC11070453
Go to all (253) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Error blindness and motivational significance: Shifts in networks centering on anterior insula co-vary with error awareness and pupil dilation.
Behav Brain Res, 355:24-35, 26 Oct 2017
Cited by: 8 articles | PMID: 29107022
Conjoint activity of anterior insular and anterior cingulate cortex: awareness and response.
Brain Struct Funct, 214(5-6):535-549, 29 May 2010
Cited by: 341 articles | PMID: 20512367 | PMCID: PMC2886906
Review Free full text in Europe PMC
Risk and risk prediction error signals in anterior insula.
Brain Struct Funct, 214(5-6):645-653, 29 May 2010
Cited by: 88 articles | PMID: 20512378
Review
Fear conditioning in humans: the influence of awareness and autonomic arousal on functional neuroanatomy.
Neuron, 33(4):653-663, 01 Feb 2002
Cited by: 257 articles | PMID: 11856537

 1,2
1,2 



