Abstract
Free full text

Tumor cells engineered to codisplay on their surface 4-1BBL and LIGHT costimulatory proteins as a novel vaccine approach for cancer immunotherapy
Abstract
Primary tumor cells genetically modified to express on their surface a collection of immunological ligands may have utility as therapeutic autologous cancer vaccines. However, genetic modification of primary tumor cells is not only cost, labor, and time intensive, but also has safety repercussions. As an alternative, we developed the ProtEx™ technology that involves generation of immunological ligands with core streptavidin (SA) and their display on biotinylated cells in a rapid and efficient manner. We herein demonstrate that TC-1 tumor cells can be rapidly and efficiently engineered to codisplay on their surface two costimulatory proteins, SA-4-1BBL and SA-LIGHT, simultaneously. Vaccination with irradiated TC-1 cells codisplaying both chimeric proteins showed 100% efficacy in a prophylactic and > 55% efficacy in a therapeutic tumor setting. In contrast, vaccination with TC-1 cells engineered with either protein alone showed significantly reduced efficacy in the prophylactic setting. Vaccine efficacy was associated with the generation of primary and memory T cell and antibody responses against the tumor without detectable signs of autoimmunity. Engineering tumor cells in a rapid and effective manner to simultaneously display on their surface a collection of immunostimulatory proteins with additive/synergistic functions presents a novel alternative approach to gene therapy with considerable potential for cancer immunotherapy.
Introduction
T cell responses against tumors are initiated by TCR recognition of tumor-associated antigens (TAAs) in the context of MHC molecules. This recognition process requires costimulatory signals delivered through CD28 family for full T cell activation. Following activation, T cells upregulate various other costimulatory receptors, particularly those that belong to the TNFR family on their surface, which derive T cell expansion and differentiation to acquire effector and memory functions.1 T cell either respond to TAAs directly presented by tumor cells or professional APCs that take up such antigens, process, and present on their surface in the context of either class I or class II MHC molecules. Because of the lack of expression of costimulatory molecules on a significant number of tumors of hematopoietic origin and almost all nonhematopoietic solid tumors, the direct presentation of TAAs by tumor cells lacking costimulation results in T cell anergy or immune nonresponsiveness to the progressing tumor.2–4 Therefore, the provision of signal 2 by tumor cells is perceived to be an effective means of inducing tumor-specific immune responses. Consistent with this notion, syngeneic tumor cells genetically modified to express the B7 costimulatory ligand on their surface underwent rejection.5 This initial observation set the stage for intense studies using genetically modified tumor cells expressing various costimulatory molecules as cancer vaccines with reported efficacy in preclinical settings.6–8
Genetic manipulation of tumor cells to express costimulatory molecules using various vectors is inefficient, time-consuming, and bears safety concerns.9–11 To overcome these obstacles, we recently developed a novel protein display technology designated as ProtEx™.12 This technology allows for the generation of recombinant chimeric proteins with core streptavidin (SA) and the display of these proteins on the cell membrane modified with biotin taking the advantage of unmatched high noncovalent affinity (10−15 M) between streptavidin and biotin.12 Importantly, the chimeric immunological ligands have improved activities as compared with their native counterparts. This is potentially due to the ability of chimeric proteins to form tetramers and oligomers, owing to the structural features of the core streptavidin fusion partner,13 and as such crosslinking their receptors on immune cells for effective signal transduction.12,14–18 We have previously shown that cancer cells displaying a chimeric costimulatory protein, CD80-SA, were effective as vaccine in preventing tumor growth in a prophylactic setting using a mouse B cell lymphoma model.18 Furthermore, primary tumors form cancer patients engineered to display CD80-SA on their surface generated autologous T cell proliferative and cytotoxic responses when used as stimulators in ex vivo assays.17 In the present study, we asked if tumor cells can be engineered to simultaneously display on their surface more than one chimeric costimulatory molecule and if such engineered cells have efficacy as tumor vaccines. It is anticipated that the display of more than one costimulatory ligands having additive/synergistic functions on tumor cells may endow them with improved immunostimulatory activity than those expressing a single molecule. This notion is supported by studies demonstrating that tumor cells genetically modified to express more than one costimulatory ligand belonging to B7 and TNF families were rejected in syngeneic mice or had enhanced therapeutic efficacy when used as vaccine.19,20
4-1BBL (CD137) and LIGHT (TNFS14) are members of the TNF superfamily and play critical roles in the regulation of various immune responses. The 4-1BB receptor is constitutively expressed on a subpopulation of resting dendritic cells (DCs) and CD4+CD25+FoxP3+ T regulatory (Treg) cells14 and inducibly expressed on activated CD4+ and CD8+ T cells as well as various cells of innate immunity, such as macrophages, NK cells, and neutrophils1. As such, signaling through 4-1BB has pleiotropic effects and modulate innate, adaptive, and regulatory immunity.14 Signaling through 4-1BB receptor plays a critical role in the survival of T cells and CD8+ T cell memory responses.21 Similarly, signaling via 4-1BB on DCs was shown to result in their activation and secretion of cytokines necessary for T cell responses.14,22 Importantly, we have recently demonstrated that signaling via 4-1BB licenses the T effector cells to overcome the suppressive function of Treg cells.14,15 Consistent with the pleiotropic effects of 4-1BB signaling are a series of studies demonstrating that modulation of the 4-1BB signaling in tumor settings using either agonistic Abs to this receptor or ectopic expression of 4-1BBL in tumors had therapeutic efficacy.19,23–26 Therapeutic efficacy was correlated with the generation of primary and memory T cell responses as well as reversal of CD8+ T cell ignorance and anergy.25,27
LIGHT, the second molecule of choice used in this study, has attracted considerable interest for its emerging roles in promoting tumor-specific T cell responses.28,29 LIGHT signals through three distinct receptors; herpes virus entry mediator (HVEM), lymphotoxin-β receptor (LTβR), and DcR3 with distinct pattern of tissue expression and effector functions. Engagement of LIGHT with HVEM on T cells results in T cell proliferation and cytokine production,29,30 whereas on NK cells stimulates these cells to potentiate the function of CD8+ T cells for the eradication of established tumors by secretion of IFN-γ.28 Similarly, LIGHT signaling into DCs induced IL-12 required for the generation of efficient Th1 response31. Importantly, engagement of LIGHT with LTβR expressed on tumor stroma results in the release of the chemokine CCL21 that attracts naïve T cells into the tumor, leading to tumor eradication.29
The importance of LIGHT and 4-1BBL in the modulation of immune responses, their distinct mechanisms of action, and demonstrated therapeutic efficacy in various preclinical tumor models led us to test if the codisplay of these two molecules on tumor cells will have efficacy as cell-based tumor vaccine. Using our ProtEx™ technology, we generated SA-LIGHT chimeric protein and codisplayed with our previously reported SA- 4-1BBL32 on the surface of TC-1 tumor cells in an efficient and rapid manner with equal efficiencies. Vaccination with irradiated TC-1 cells codisplaying on their surface both chimeric proteins demonstrated 100% efficacy in preventing tumor growth and over 55% efficacy in eradicating established tumors. In marked contrast, vaccination with TC-1 cells displaying either chimeric protein singly only prolonged survival in the prophylactic setting. Vaccine efficacy was associated with the generation of potent cellular and humoral immune responses. Taken together, these findings demonstrate that tumor cells can be engineered effectively to codisplay on their surface more than one immunostimulatory molecules and such modified tumors are effective in eradicating established tumors. This approach, therefore, serves as a rapid, safe, and clinically applicable practical alternative to gene therapy for immunomodulation and has significant potential for the development of effective cell based therapeutic cancer vaccines.
Materials and methods
Mice and cell lines
Male C57BL/6.SJL and C57BL/6 mice (6–8 wk old) were purchased from The Jackson Laboratory or bred in our animal facility at the University of Louisville. All animals were cared for in accordance with institutional and National Institutes of Health guidelines. TC-1 cell line were purchased from American Type Culture Collection (Manassas, VA) and maintained as previously described.14
Reagents
Construction, expression, purification, and characterization of SA-4-1BBL were recently described33. A construct encoding the extracellular portion of mouse LIGHT fused C-terminal to core streptavidin (SA) was generated by cloning the extracellular portion of the LIGHT molecule from mouse splenocytes using specific primers in RT-PCR. A functional cDNA was then subcloned in frame C-terminal to SA in the Drosophila expression vector PMT/BiP/V5-HisA. The chimeric SA-LIGHT construct was then stably transfected into S2 Drosophila cells and inducibly expressed using CuSO4 as previously described.32 Fluorochrome-conjugated Abs (anti-CD4-APC, anti-CD8-PerCP, anti-CD25-PE, anti-CD19-APC, anti-I-A/I-E-PE, anti-CD80-FITC, anti-CD40-FITC, anti-CD86-APC, anti-4-1BB-bio, anti-4-1BBL-PE, anti-IFN-γ-PE, anti-CD45.1-APC, anti-CD45.2-PE, and SA-PerCP) and isotype controls were purchased from BD PharMingen and eBioscience. Human Papillomavirus type 16 E7 peptide (E749–57 RAHYNIVTF, designated “P”) was purchased from CPC Scientific Inc. (San Jose, CA).
Engineering of tumor cells with SA-4-1BBL and SA-LIGHT proteins
TC-1 cells were biotinylated in either in low (15 μM) or high (50 μM) concentrations of EZ-Link Sulfo-NHS-LC-Biotin (Pierce) in PBS for 30 min at room temperature. Cells were then washed extensively and incubated for 30 min with either low (0.2 μg/106 cells) or high (2 μg/106 cells) concentrations of SA-4-1BBL or SA-LIGHT either singly or together in PBS. The presence of SA-LIGHT and SA-4-1BBL on the cell surface was assessed using anti-streptavidin Ab and anti-4-1BBL Ab in multiparameter flow cytometry or confocal microscopy.
Flow cytometry
For phenotyping and sorting, spleens, lymph nodes, and tumors were processed into single-cell suspensions, and cells were labeled with saturating concentrations of fluorochrome-conjugated Abs. Isotype matched Abs with the same fluorochrome were used as controls. For intracellular cytokine staining, lymph node cells were processed into single-cell suspensions. Cells were resuspended in complete MLR medium at 2×106/ml and stimulated with PMA (5 ng/ml, Sigma) and ionomycin (500 ng/ml, Sigma) for 2 hrs in a 37°C, 5% CO2 incubator. GolgiPlug (1 μl/ml, BD PharMingen) was added to the activation mixture and cells were incubated for additional 4 hrs. Cells were then stained with anti-CD4-APC and anti-CD8-PerCP, fixed with 4% paraformaldehyde, and stained with PE-conjugated anti-mouse IFN-γ or isotype control in permeabilization buffer containing saponin.
In vivo cytotoxicity assay
A population of C57BL/6.SJL (CD45.1) spleen cells were labeled with 2.5 μM fluorescent dye CFSE (CFSEhigh) while a second population was labeled with 0.25 μM CFSE (CFSElow). CFSEhigh cells were then pulsed with either 2 μg/ml of E749–57 peptide representing the dominant CD8+ T cell epitope for HPV E7 or an irrelevant peptide (survivin) as an internal control for 90 min at 37°C in a 5% CO2 incubator. CFSEhigh and CFSElow cells were then extensively washed to remove free peptides, mixed at a 1:1 ratio, and injected i.v. into C57BL/6 (CD45.2) mice 5 days after another booster vaccination with irradiated TC-1 cells displaying SA-4-1BBL, SA-LIGHT or both. Spleens were removed 48 hrs later, processed into single cell suspension, stained with APC-labeled anti-mouse CD45.1 Ab and analyzed by multiparameter flow cytometry to determine the ratio of CFSElow/CFSEhigh target cells. The percentage of in vivo killing was calculated by the formula [1-((CFSElow/CFSEhigh for sample)/(CFSElow/CFSEhigh for naive))] × 100.
Tumor model and vaccination
To establish tumors, 1×105 live TC-1 cells were resuspended in 200 μl of PBS and injected s.c. into the right back flank of naive syngeneic C57BL/6 mice. Tumor growth was monitored 2–3 times per week and tumor size was measured in mm using a caliper. Average tumor size was calculated by measuring two perpendicular diameters. Under these conditions, all the mice develop palpable tumors within 9–12 days. Animals bearing tumors were euthanized when tumors reached a size of 15 mm in one of the two perpendicular diameters or earlier if tumors ulcerated or animal showed signs of discomfort. For preventive studies the TC-1 cells were first biotinylated then irradiated (15,000 cGy) and then engineered with 4-1BBL, LIGHT or both molecules together. Two million engineered tumor cells were then injected s.c. for prophylactic vaccination in right flank on days 7 and 3 prior to live TC-1 tumor challenge. For therapeutic studies, mice were immunized with irradiated TC-1 cells displaying both chimeric proteins simultaneously s.c. on the same day following the tumor challenge. In two vaccinations setting mice were injected on same days as well as four days post tumor challenge. Tumor growth was monitored twice a week using caliper.
Analysis of tumor infiltrating CD8+ T cells
Mice that did not eradicate the tumor after immunotherapy were euthanized when tumor reached 5–8 mm in diameter. Tumor was cut into 2-mm pieces after removal of blood vessels and connective tissue by dissection. To isolate T cells, tumors were incubated in an enzyme mixture consisting of 2 mg of collagenase-P/ml, 1 mg of DNase I/ml, 10 U of penicillin/ml, and 10 μg of streptomycin/ml in PBS for 2 hrs at 37°C with occasional shaking. The digested tissue was passed through a nylon mesh and the resultant cells were washed twice in PBS before being stained for flow cytometric analysis. Cells were stained with appropriate fluorochrome labeled anti-mouse CD3 and CD8 Abs and PE-conjugated anti-mouse CD45.2 Ab to selectively exclude CD45 negative tumor cells from analysis. Three million cells were analyzed using multiparameter flow cytometry.
Statistical analysis
Statistical analysis was done using the Student’s t-test or ANOVA. The survival assays were analyzed using long-rank test in SPSS software. For each test p value less than 0.05 and 0.001 were considered significant (*) and very significant (**), respectively. Error bars represent SD.
Results
Generation and characterization of the SA-LIGHT protein
Construction, expression, and functional characterization of SA-4-1BBL have previously been reported.14,15 A construct containing the extracellular portion of LIGHT fused C-terminal to SA was generated in a similar fashion to the SA-4-1BBL. This design was to facilitate proper folding because LIGHT is a type II protein.34 The chimeric SA-LIGHT construct was then stably transfected into S2 Drosophila cells and inducibly expressed using CuSO4 as previously described.32. The chimeric SA-LIGHT protein formed primarily dimers and tetramers (66–132 kDa) under denaturing PAGE conditions and heating at 60°C that was dissociated into monomers of ~ 33 kDa of the expected size after heating at 100°C. These structural features of SA-LIGHT are consistent with those of native SA, which forms tetramers and oligomers that are dissociated into monomers under denaturing conditions and heating at 100°C.13
Efficient engineering of TC-1 tumor cells to codisplay SA-4-1BBL and SA-LIGHT proteins on their surface
ProtEx™ technology has the potential to simultaneously display a collection of SA-chimeric proteins on the surface of biotinylated cells with equal efficiencies. Toward this end, we tested various biotinylation conditions followed by engineering with various concentrations of each molecule alone and in combination. Biotinylation at 15 μM and engineering with 0.2 μg protein/106 TC-1 cells resulted in effective codisplay of both proteins on almost all TC-1 cells (Figure 1a). There was almost a 10-fold increase in the level of each protein per cell when TC-1 cells were biotinylated in 50 μM biotin and engineered with 2 μg protein/106 cells (Figure 1b). However, 4-1BBL showed higher mean fluorescence intensity than LIGHT. It is unclear at this point if this is because of higher 4-1BBL molecule per cell or the affinity of Abs to the proteins of interest or the fluorescence molecules used to detect the chimeric proteins. These observations were further confirmed using TC-1 cells engineered with 4-1BBL and LIGHT for analysis under confocal microscopy (Figure 1c). All TC-1 cells shown in the field of view appeared positive for individual proteins used for engineering singly or in combination. Importantly, TC-1 cells biotinylated with 50 μM biotin and engineered with SA-4-1BBL and SA-LIGHT chimeric proteins at 2 μg protein/106 cells could further be engineered with SA, demonstrating the presence of a significant number of free biotin. These results demonstrate that at 50 μM biotinlytion, TC-1 cells can be engineered simultaneously with a minimum of 3 exogenous proteins with equal efficiencies when these proteins are used at similar molarities. Consistent with our previously published data,18 both SA-4-1BBL and SA-LIGHT proteins persisted on the surface of splenocytes in vitro with t1/2 of 7 and 9 days, respectively (data not shown).

Engineering TC-1 tumor cells to simultaneously display on their surface SA-4-1BBL and LIGHT proteins. (a) Flow cytometry analysis of engineered TC-1 cells. The TC-1 cells were biotinylated using 15 μM EZ-Link Sulfo-NHS-SS-biotin followed by incubation with 0.2 μg of proteins either individually or in combination per 106 cells. The presence of SA-4-1BBL and SA-LIGHT on the cell surface was assessed using antibodies against 4-1BBL and SA, respectively. (b) The TC-1 cells were biotinylated using 50 μM EZ-Link Sulfo-NHS-SS-biotin followed by incubation with 2 μg of proteins either individually or in combination per 106 cells. (c) Confocal microscope analysis of TC-1 tumor cells for the cell surface presence of chimeric proteins. TC-1 cells were engineered with SA-4-1BBL, SA-LIGHT, or the two proteins in combination with the conditions stated in (b) and stained using Abs against 4-1BBL and SA. Fluorochrome-labeled SA was used as a third protein to assess the presence of free biotin remaining on the cell surface after engineering with the chimeric proteins.
Vaccination with TC-1 cells engineered to codisplay SA-4-1BBL and SA-LIGHT proteins on their surface is effective in generating antigen-specific killing responses in vivo
To test if tumor cells engineered to simultaneously display both SA-4-1BBL and SA-LIGHT proteins on their surface generate augmented immune responses as compared with cells displaying single molecules, we used the dominant CD8+ T cell epitope (E749–57 RAHYNIVTF E7) for human papilloma virus 16 (HPV16) E7 molecule expressed by TC-1 cells as an in vivo model system to test antigen-specific killing. C57BL/6 mice that were vaccinated twice with TC-1 cells displaying both SA-4-1BBL and SA-LIGHT proteins simultaneously generated a more vigorous in vivo killing response as compared with cells displaying individual proteins (Figure 2a and b). Taken together, these data provided proof-of-principle for enhanced immune efficacy of tumor cells engineered to codisplay both chimeric proteins on their surface as vaccine.
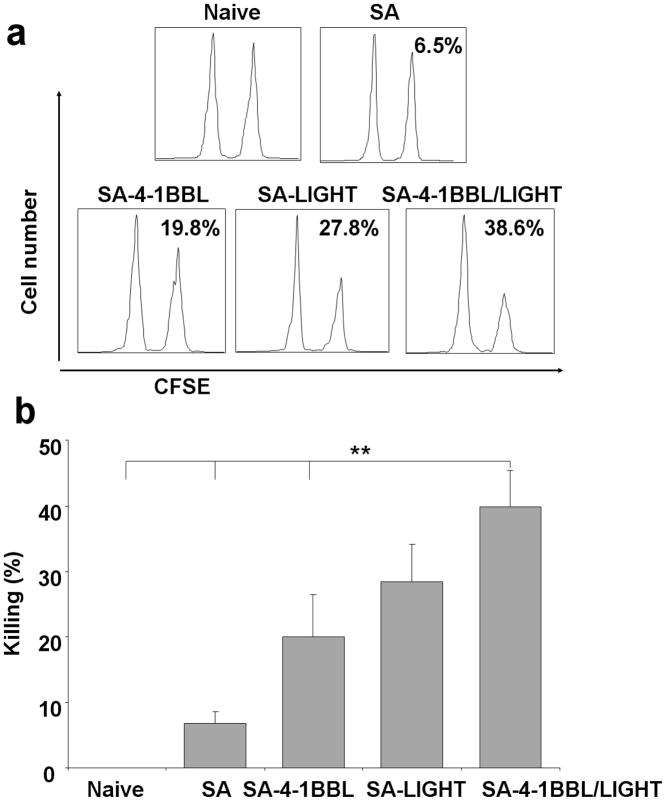
Vaccination with TC-1 cells engineered to display both SA-LIGHT and SA-4-1BBL generates potent in vivo killing responses. Two million of irradiated TC-1 cells engineered with SA-4-1BBL, SA-LIGHT, or both molecules simultaneously were used to vaccinate C57BL/6 (CD45.1) mice s.c. on days 7 and 3 prior to i.v. injection of target syngeneic splenocytes (CD45.2) labeled with CFSE and pulsed with the E749–57 CD8+ T cell dominant epitope. Two days after target injection, total splenocytes were harvested and analyzed for specific in vivo killing using multiparameter flow cytometry by gating on CD45.2 cells. TC-1 cells engineered with control core streptavidin (SA) served as control. (a) Shows a representative in vivo killing histogram and (b) Tabulation of killing response for three animals per group.
Vaccination with TC-1 cells engineered to codisplay SA-4-1BBL and SA-LIGHT on their surface has complete efficacy as prophylactic cancer vaccine
We next tested if the immune response generated by vaccination with TC-1 cells displaying both SA-4-1BBL and SA-LIGHT proteins on their surface translates into effective anti-tumor immune responses with prophylactic efficacy. Naive C57BL/6 mice were vaccinated twice s.c. with 2×106 irradiated TC-1 cells engineered with SA-4-1BBL and SA-LIGHT alone, together, or a mixture of cells engineered with individual molecules 7 and 3 days prior to challenge with 1 × 105 live tumor cells. Vaccination with TC-1 cells displaying both molecules resulted in the prevention of tumor growth in 100% of mice (Figure 3a). In marked contrast, immunization with the mixture of tumor cells engineered with individual molecules resulted in the prevention of tumor growth only in ~ 66% of mice (Figure 3a). Vaccination with SA-4-1BBL-enginered TC-1 cells although prolonged survival, all mice expired from tumor burden. SA-LIGHT had improved efficacy as ~30% of mice showed palpable tumor-free survival over a 60-day observation period. Importantly, tumor-free mice vaccinated with TC-1 cells engineered with both costimulatory molecules generated significant E7-peptide specific in vivo killing response (Figure 3b).

Vaccination with TC-1 cells engineered with both SA-LIGHT and SA-4-1BBL prevents tumor growth in a prophylactic setting. (a) Naïve C57BL/6 mice were vaccinated twice with 2 × 106 irradiated TC-1 cells engineered to display on their surface control SA, SA-4-1BBL, SA-LIGHT, or both SA-4-1BBL and SA-LIGHT proteins together 7 and 3 days prior to challenge with live 1 × 105 TC-1 cells. One million of TC-1 cells engineered with the individual molecules were mixed and used for vaccination to test if the presence of both molecules on the same cell affects vaccine efficacy. (b) The long term (over 60 days) tumor free animals from (a) were boosted with another dose of the same vaccine formulation and in vivo E749–57 peptide-specific killing was assed one week later. Tabulation of killing response for three animals per group.
Vaccination with TC-1 cells engineered to codisplay SA-4-1BBL and SA-LIGHT on their surface has efficacy as therapeutic cancer vaccine
Given the robust efficacy of TC-1 tumors engineered with both SA-4-1BBL and SA-LIGHT costimulatory proteins in preventing tumor growth, we next tested this vaccine in a therapeutic setting. Naïve C57BL/6 mice were challenged with 1 × 105 live TC-1 tumor cells followed by vaccination with 2×106 irradiated TC-1 cells engineered with both chimeric molecules on the same day. Mice vaccinated with control SA engineered TC-1 cells served as control. All mice developed tumor irrespective of the vaccination schemes (data not shown). These results were surprising given the efficacy of the vaccine in the prophylactic setting. We, therefore, tested if increasing the protein levels on the surface of tumor cells had a beneficial effect on the therapeutic efficacy of the vaccine. Vaccination once with 2×106 irradiated TC-1 cells modified with 3.3-fold more biotin (from 15 μM to 50 μM) and 10-fold more chimeric proteins (200 ng to 2 μg protein/106 cells) resulted in prolonged survival with >55% mice showing tumor-free survival over the 60-day observation period. In contrast, all the control mice vaccinated with SA-engineered TC-1 cells developed tumor and expired within 45 days (Figure 4a). One booster injection with irradiated TC-1 cells engineered with both SA-4-1BBL+SA-LIGHT proteins 4 days later did not further increase the efficacy of the vaccine as only 3 out of 5 mice (60 % tumor free survival) remained tumor free for the 60 days of observation period (data not shown). Therapeutic efficacy was specific to the TC-1 tumors as vaccination with TC-1 tumors engineered with the costimulatory molecules did not alter the progression of 3LL lung carcinoma (data not shown).
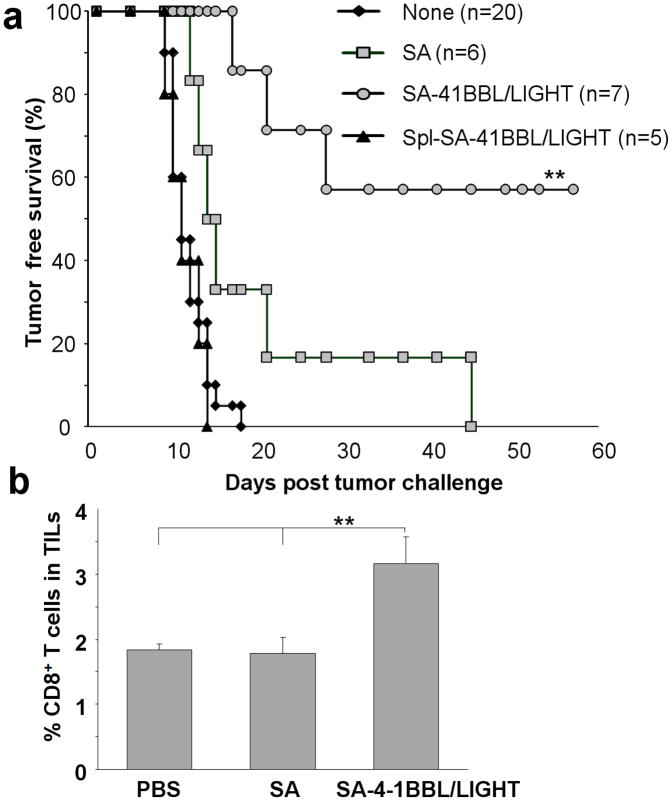
Vaccination with TC-1 cells engineered with both SA-LIGHT and SA-4-1BBL proteins prevents tumor growth in a therapeutic setting. (a) Naïve C57BL/6 mice were challenged with 1 × 105 live TC-1 cells followed by vaccination s.c. with 2 × 106 irradiated TC-1 cells engineered using 50 μM biotin and 2 μg/protein/106 cells on the same day. Mice without vaccine or those vaccinated with TC-1 cells engineered with equimolar SA or syngeneic splenocytes codisplaying both molecules were used as controls (p <0.001). (b) Mice vaccinated with TC-1 tumors engineered with costimulatory proteins that failed immunotherapy had higher number of intratumoral CD8+ T cells as compared with controls.
Inasmuch as we envision using surgically extracted tumors as the source of vaccine for clinical trials, it is important to address if the presence of normal cells within the tumor has any effect on the efficacy of the vaccine as well as generation of autoimmunity. Vaccination with syngeneic splenocytes co-displaying both SA-41BBL and SA-LIGHT proteins had no detectable effects on the progression of TC-1 tumors (Figure 4a). Importantly, mice vaccinated with engineered splenocytes did not generate detectable titers of autoantibodies against dsDNA (data not shown).
Mice that failed immunotherapy using TC-1 cells engineered with chimeric proteins had severe retardation of tumor growth. LIGHT costimulatory molecule is shown to induce the expression of various T cell chemokines by affecting tumor stroma or mast cells, thereby enhancing the infiltration of CD8+ T cells in to the tumor. 35 To test this notion, slow growing tumors in mice vaccinated with the SA-4-1BBL and SA-LIGHT-engineered TC-1 cells were harvested, processed into single cell suspension, and stained with Abs against various cell surface markers, and analyzed using multiparameter flow cytometry. As shown in Figure 4b, tumors from vaccinated mice had significantly higher levels of CD8+ T cell infiltration as compared with controls. Although these data provide evidence for the efficacy of our vaccine regimen to facilitate the infiltration of CD8+ T cells into the tumor, the molecular basis of inability of such cells to eradicate tumors is not known. One possibility is that this level of CD8+ T cell infiltration for tumors of this size is not sufficient to eradicate the tumor or these cells may not be tumor specific or functionally inactive. Further studies will be required to address this issue.
Efficacy of engineered tumor vaccine is associated with long-term T and B cell responses
Mice that underwent effective immunotherapy were boosted with TC-1 tumor cells engineered with both SA-4-1BBL and SA-LIGHT proteins 60 days after the initial tumor challenge and analyzed for E749–57 peptide-specific killing response a week later. Naïve animals vaccinated with the same regimen were used as control. As expected, long term animals generated enhanced E749–57 peptide-specific killing response as compared with the control group (Figure 5a). To test if long term mice have augmented antigen specific T cell proliferative responses, splenocytes from long term boosted and naive primed animals were pulsed with recombinant E7 protein and the proliferative response was assessed using thymidine incorporation assay. Long term tumor-free mice generated higher E7 protein specific T cell proliferative response as compared with the control groups (Figure 5b). T cells from long-term mice also generated higher IFN-γ response (Figure 5c) as compared with primed naïve mice.
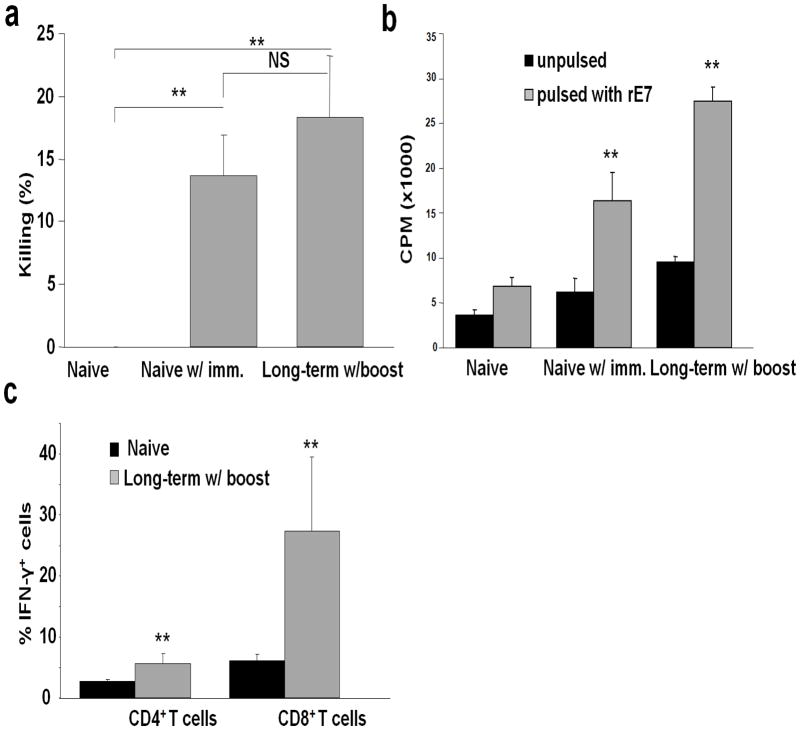
Tumor immunotherapy is associated with robust long-term T cell responses. (a) Long term tumor free animals have tumor specific killing responses. Tumor-free mice were vaccinated s.c. with one dose of TC-1 cells engineered with both SA-4-1BBL and SA-LIGHT proteins on day 60 post tumor challenge. Naïve C57BL/6 mice vaccinated with the same regimen were used as control. E749–57 peptide-specific in vivo killing response was assessed one week later. (b) Long term tumor free animals retain tumor specific T cell proliferative responses. Splenocytes from vaccinated mice in (a) were pulsed with a recombinant E7 protein in a 5-day thymidine incorporation assay to assess T cell proliferation. (c) Long term tumor free animals have higher percentages of CD8+ T cells expressing IFN-γ. Draining lymph nodes from boosted long term tumor free animals and naïve mice were treated with PMA/ionomycine for 6 hr and analyzed for intracellular cytokines using multiparameter flow cytometry.
Signaling induced by 4-1BBL and LIGHT was shown to play a critical role in enhancing and maintaining T cell memory pool. Therefore, we analyzed T cells from the long term and boosted mice for memory phenotype. As shown in Figure 6, long-term mice had higher frequency of CD44high memory T cell pool as compared with control groups. Importantly, long term tumor-free mice also had significantly higher titers of antibodies against E7 in their sera (Figure 7), demonstrating that vaccination with TC-1 cells engineered with the costimulatory molecules not only generate potent cellular, but also humoral immune responses to the tumor antigens.
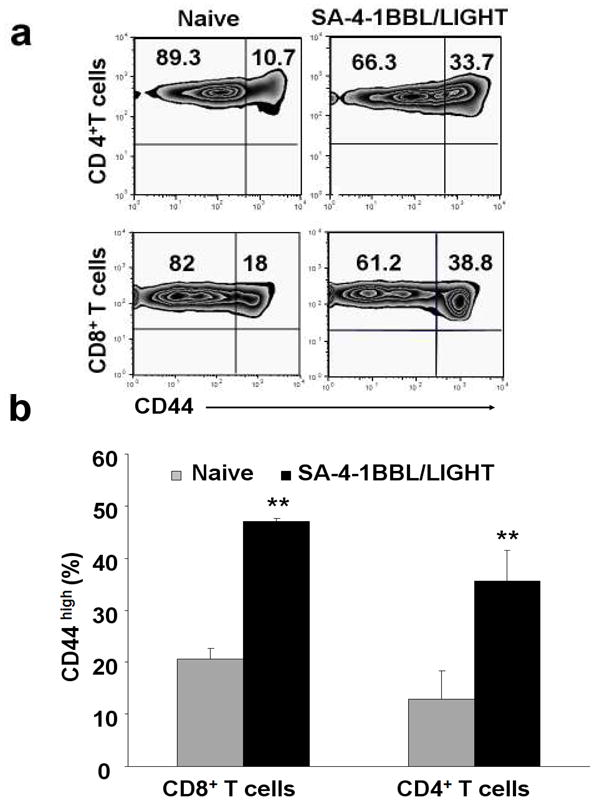
Long term tumor free mice retain higher CD44high memory T cell pool. Splenocytes from the boosted long term tumor free animals were stained with various cell surface markers and analyzed in multiparameter flow cytometry to assess memory CD4+ and CD8+ T cells. (a) A representative flow cytometry histogram and (b) Tabulated data for a minimum of 3 animals per group.
Discussion
Autologous whole tumor cell-based vaccines have the potential to generate effective immune responses by presenting a diverse repertoire of TAAs to T cells either in a direct fashion serving as antigen-presenting cells or indirectly via cross-priming.36–38 The diverse pool of TAAs in cell based vaccines also have the potential to overcome antigenic drift of dominant TAA epitopes in response to immunological pressure, which is an important limitation of subunit vaccines based on TAAs.38–40 Autologous whole tumor cell based vaccines, however, suffer from inefficacy plausibly because of the lack of immunostimulatory, such as costimulatory, molecules expressed by tumor cells. Consistent with this notion are a series of published studies demonstrating that tumor cells genetically modified to express costimulatory molecules serve effective vaccines against cancer.5,36,39,41 However, the application of genetically modified tumor cell-based vaccines to the clinic suffers from various limitations. These include most often lack of sufficient tumor mass for genetic modification, difficulties associated with the transfer of foreign DNA into primary tumor cells using various approaches, the inability to expand the genetically modified tumor cells for vaccine preparation, and safety concerns associated with the introduction of foreign genetic material into the patient.
As an alternative to gene transfer approaches for immunomodulation, we recently established a novel approach designated as ProtEx™ that allows for the generation of chimeric immunomodulatory molecules with core streptavidin and the display of these molecules on cell membrane that has been derivitized with biotin.12,17,18,42 The rationale for this approach is that the most critical immune decisions are the end result of cell surface receptor and ligand interactions and these interactions are short and transient in nature. Therefore, the transient display of exogenous proteins representing immunological ligands, such as costimulatory molecules, on the cell membrane in an effective and rapid manner under physiological conditions may have utility as a practical and safe approach to gene therapy-based immunomodulation. As a proof-of-principle study, we have recently shown that tumor cells engineered with a chimeric CD80-SA protein were effective as prophylactic vaccine in a mouse B cell lymphoma model.18 Based on this observation, we hypothesized that the display of more than one costimulatory molecule with distinct functions on tumor cells may improve their efficacy as whole cell-based autologous tumor vaccine.
We herein tested if tumor cells can be engineered to effectively display two costimulatory molecules, SA-4-1BBL and SA-LIGHT, on their surface and if such engineered cells have improved efficacy as cancer vaccine using HPV E7 expressing the TC-1 cervical cancer mouse model. Modification of tumor cells with 15 μM biotin and engineering with equal amounts (0.2 μg protein/1 × 106 cells) of SA-4-1BBL and SA-LIGHT proteins was sufficient for the codisplay of both proteins at similar levels on almost all the targeted cells. There was a direct correlation between the concentrations of biotin and proteins used to engineer the TC-1 cells and the levels of proteins displayed on the cell surface. For example, at 50 μM biotinylation conditions, a tenfold increase in each protein concentration resulted in almost a ten-fold increase in protein levels displayed on the cell surface. Importantly, under these engineering conditions there were sufficient free biotin molecules available on the cell surface that allowed for the co-display of streptavidin at similar levels to SA-LIGHT and SA-4-1BBL. Taken together, these data demonstrate the versatility and flexibility of this platform technology for the display of a single or a combination of proteins on the cell surface at desired levels for therapeutic purposes.
Tumor cells engineered with either single or a combination of both proteins generated effective E7-specific in vivo killing responses when used as vaccine in naive mice. However, vaccine efficacy was significantly enhanced when both proteins were co displayed on the surface of the same TC-1 cells as compared with individual proteins displayed on different cells, providing evidence for the utility of this combinatorial vaccine approach. Enhanced vaccine efficacy of TC-1 cells engineered with both proteins may be due to the distinct functions of these two molecules on the immune system. Unlike 4-1BBL that primarily enhances the function of antigen-activated T cells due to the lack of 4-1BB receptor on naive T cells,43 LIGHT was shown to activate naïve T cells independent of CD28 costimulation by engaging constitutively expressed HVEM receptor on their surface.35,44 Therefore, SA-LIGHT on tumor cells is anticipated to activate antigen-specific naive T cells which can then upregulate 4-1BB receptor and become responsive to SA-4-1BBL-mediated modulation, leading to improved immune responses as compared with individual molecules. In addition, LIGHT may also affect the innate arm of the immune system via interaction with LTβR receptor expressed on monocytes and HVEM receptor on DCs,35,45,46 further contributing to the T cell responses. This notion is consistent with the augmented efficacy of LIGHT only engineered TC-1 cells than those engineered with SA-4-1BBL in generating in vivo killing responses as well as preventing tumor growth. Importantly, LIGHT was shown to regulate the CD40 signaling on DCs for the generation of a CTL response,46 suggesting that similar cooperation/synergy may exists between LIGHT and 4-1BBL with respect to the activation of DCs and generation of killing responses.
The immune efficacy of TC-1 cells codisplaying both proteins on their surface was much more pronounced in a prophylactic tumor setting. Vaccination of mice with TC-1 cells co-displaying both SA-4-1BL and SA-LIGHT proteins on their surface resulted in the prevention of tumor growth in 100% of mice in a prophylactic setting and this effect was superior to vaccinations with tumor cells displaying the individual proteins. Mice vaccinated with TC-1 cells engineered with SA-4-1BBL alone although had retarded tumor growth; all animals succumbed to death within 60 days due to tumor burden. Vaccination with SA-LIGHT-engineered TC-1 cells had a efficacy than SA-4-1BBL-engineerd cells as the kinetics of tumor growth in these mice were substantially retarded with over twenty five percent of animals not developing detectable tumors over a 60-day observation period. Importantly, the simultaneous display of both molecules on the same tumor cell appeared to be critical for the maximum efficacy of the vaccine as vaccination of mice with a mixture of TC-1 cells displaying individual SA-4-1BBL and SA-LIGHT proteins was only effective in preventing tumor growth in ~65% of mice. The efficacy of vaccination with SA-LIGHT-engineered TC-1 tumor cells in the prophylactic tumor setting is consistent with our in vivo killing data. This may be due to the ability of LIGHT to directly activate DCs and monocytes and stimulate T cells in a CD28-independent fashion.47,48 Consistent with this notion, forced expression of LIGHT within tumor were shown to recruit APCs and naïve T cells into the tumor, intratumoral activation of naive T cells, leading to the eradication of established tumors as well as prevention of spontaneous metastases arising following surgical removal of the tumor.35,49 The efficacy of both proteins-engineered TC-1 cells was further confirmed in a therapeutic setting where a single or two injection of the vaccine resulted in prolonged survival in all mice with > 55 % tumor-free survival. However, vaccine efficacy required the display of higher levels of both proteins on the surface of TC-1 cells under the tested conditions. This observation is consistent with a recent study demonstrating a dose-depend efficacy in a therapeutic tumor model using an Ig.4-1BBL fusion protein for immunomodulation.50
The immune efficacy of the TC-1 cells engineered to codisplay both SA-LIGHT and SA-4-1BBL proteins on their surface as compared with cells engineered to display the individual proteins is consistent with several published studies using tumor cells genetically modified to coexpress two or more costimulatory molecules as vaccine. DNA vaccines encoding rat Her-2/neu as TAA given in combination with both 4-1BBL and either CD80 or CD86 costimulatory molecules generated cellular and humoral immune responses with antitumor efficacy.51 Vaccination with neuroblastoma cells transiently transfected to simultaneously express the co-stimulatory molecules CD54, CD80, CD86, and CD137L was effective in generating antitumor immunity that had efficacy in preventing tumor growth in a prophylactic setting in mice.37 Immunomodulation using the combination of 4-1BBL and LIGHT costimulatory molecules is particularly attractive because the expression patterns of these two proteins are distinct and connected in a spatiotemporal manner. LIGHT can initiate robust innate and primary T cells responses against the tumor by interacting with its LTβR and HVEM receptor constitutively expressed on various immune cells, such as DCs, monocytes, NK cells as well as T cells. This initial activation of immune cells by LIGHT can then set the stage for 4-1BBL activity by upregulating the expression of 4-1BB receptor. The engagement of 4-1BBL with 4-1BB on activated immune cells may augment LIGHT-generated immune responses with particular effect on selective expansion of T effector cells and establishment of long-term memory required for immunosurveillance and prevention of recurrences. In addition, 4-1BB signaling may also overcome various tumor-induced immune evasion mechanisms, such as clonal anergy/ignorance14,26 and CD4+CD25+FoxP3+ T regulatory cells.14
Mice that underwent successful immunotherapy generated potent E7-specific in vivo killing responses and in vitro E7-specific proliferation responses when boosted 60 days post-vaccination as compared with naïve primed mice, providing evidence for long-term T cell memory. Consistent with this notion, long-term mice with successful immunotherapy had significantly higher percentages of peripheral CD4+ and CD8+ T cells with memory phenotype as compared with primed naïve mice. T cells from these animals also generated higher IFN-γ response and had significantly higher levels of anti-E7 antibody titer in their sera. The enhanced T cell memory, CTL, and IFN-γ responses are consistent with the expected functions of LIGHT and 4-1BBL on the immune system and their demonstrated efficacies in generating these responses in various tumor settings.14,29 For example, exogenous soluble LIGHT was shown to promote monocyte-derived DC maturation in vitro by the up-regulation of CD86, CD80, CD83, and HLA-DR antigen expression, resulting in increased antigen presentation and T-cell activation in patients with myelodysplastic syndromes.52 The forced expression of LIGHT in the tumor environment induces a massive infiltration of naive T lymphocytes that correlates with an upregulation of both chemokine production and expression of adhesion molecules.29 Activation of these infiltrating T cells, possibly through HVEM, leads to the rejection of established, highly progressive tumors at local and distal sites.29 Furthermore, we have recently shown that a soluble from of 4-1BBL as the immunomodulatory component of a vaccine based on the HPV E7 protein was effective in eradicating TC-1 tumors. The therapeutic efficacy of the vaccine was associated with the generation of potent primary and memory CD8+ T cell responses, enhanced infiltration of these cells into the tumor, and their secretion of IFN-γ.14 Mice that failed immunotherapy and developed tumor had significantly higher levels of tumor infiltrating CD8+ T cells as compared with unvaccinated mice or mice vaccinated with SA-engineered TC-1 cells as control. Although these data provide evidence for the efficacy of our vaccine regimen to facilitate the infiltration of CD8+ T cells into the tumor, the molecular basis of inability of such cells to eradicate the tumor is not known. One possibility is that these CD8+ T cells are not specific for tumor antigens or functionally compromised or actively regulated by tumor microenvironment. Further studies will be required to address these issues.
This report demonstrates the feasibility of engineering tumor cells in an effective manner to display on their surface more than one immunomodulatory molecules at desired levels in a rapid and effective manner. Cells engineered with two costimulatory molecules as vaccine had immune efficacy against tumor than cells engineered with individual proteins. The efficacy of this vaccine approach is not limited to the TC-1 tumor model expressing the xenogeneic E7 oncogene since a vaccine based on A20 tumor cells, which do not express xenogeneic TAAs, engineered with the CD80 costimulatory molecule had efficacy in an autologous setting.18 The rapid, flexible, and effective engineering of tumor cells under clinically applicable conditions presents a novel and new means of cell-based cancer immunotherapy. This approach is particularly suited for autologous cell based vaccines where primary tumor cells can be resected from patients, engineered to display on their surface immunological molecules of interest, and used as vaccine within the same day; therefore, obviating time, labor, and safety issues associated with genetic manipulation of primary tumor cells for vaccine purposes.
Acknowledgments
We thank O. Grimany for his technical assistance in proteins production and purification.
Grant support: This work was funded in parts by grants from the NIH (R43 AI071618, R41 CA121665, R44 AI071618, and R43AI074176), Kentucky Lung Cancer Research Program, W.M. Keck Foundation, and the Commonwealth of Kentucky Research Challenge Trust Fund.
Footnotes
Disclosure of Potential Conflict of Interest: The SA-4-1BBL described in this manuscript is licensed from UofL by ApoImmune, Inc., Louisville, KY, for which Haval Shirwan serves as CSO and Haval Shirwan and Esma S. Yolcu have significant equity interest in the Company. The other authors disclosed no potential conflict of interest.
References
Full text links
Read article at publisher's site: https://doi.org/10.1038/cgt.2010.29
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc2941532?pdf=render
Citations & impact
Impact metrics
Article citations
A novel agonist of 4-1BB costimulatory receptor shows therapeutic efficacy against a tobacco carcinogen-induced lung cancer.
Cancer Immunol Immunother, 72(11):3567-3579, 22 Aug 2023
Cited by: 1 article | PMID: 37605009 | PMCID: PMC10991934
Engineering pancreatic islets with a novel form of thrombomodulin protein to overcome early graft loss triggered by instant blood-mediated inflammatory reaction.
Am J Transplant, 23(5):619-628, 01 Mar 2023
Cited by: 3 articles | PMID: 36863480 | PMCID: PMC10318623
Targeting hydrogen sulphide signaling in breast cancer.
J Adv Res, 27:177-190, 16 Jul 2020
Cited by: 33 articles | PMID: 33318876 | PMCID: PMC7728592
Review Free full text in Europe PMC
Localized Immunomodulation with PD-L1 Results in Sustained Survival and Function of Allogeneic Islets without Chronic Immunosuppression.
J Immunol, 204(10):2840-2851, 06 Apr 2020
Cited by: 18 articles | PMID: 32253240 | PMCID: PMC7334868
Pancreatic islets engineered with a FasL protein induce systemic tolerance at the induction phase that evolves into long-term graft-localized immune privilege.
Am J Transplant, 20(5):1285-1295, 05 Jan 2020
Cited by: 13 articles | PMID: 31850658 | PMCID: PMC7299172
Go to all (16) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
SA-4-1BBL as the immunomodulatory component of a HPV-16 E7 protein based vaccine shows robust therapeutic efficacy in a mouse cervical cancer model.
Vaccine, 28(36):5794-5802, 04 Jul 2010
Cited by: 19 articles | PMID: 20603135 | PMCID: PMC2921468
4-1BB ligand as an effective multifunctional immunomodulator and antigen delivery vehicle for the development of therapeutic cancer vaccines.
Cancer Res, 70(10):3945-3954, 20 Apr 2010
Cited by: 40 articles | PMID: 20406989 | PMCID: PMC2872136
Provision of 4-1BB ligand enhances effector and memory CTL responses generated by immunization with dendritic cells expressing a human tumor-associated antigen.
J Immunol, 170(6):2912-2922, 01 Mar 2003
Cited by: 40 articles | PMID: 12626542
SA-4-1BBL as a novel adjuvant for the development of therapeutic cancer vaccines.
Expert Rev Vaccines, 13(3):387-398, 01 Mar 2014
Cited by: 8 articles | PMID: 24521311 | PMCID: PMC4721633
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NCI NIH HHS (2)
Grant ID: R41 CA121665
Grant ID: R41 CA121665-01A2
NIAID NIH HHS (7)
Grant ID: R43 AI074176
Grant ID: R43AI074176
Grant ID: R44 AI071618
Grant ID: R43 AI071618
Grant ID: R43 AI074176-01A2
Grant ID: R43 AI071618-01A1
Grant ID: R44 AI071618-02






