Abstract
Free full text

Adaptation of HIV-1 to Cells Expressing Rhesus Monkey TRIM5α
Abstract
The cross-species transmission of retroviruses is limited by host restriction factors that exhibit inter-species diversity. For example, the TRIM5α proteins of Old World monkeys block the early, post-entry steps in human immunodeficiency virus (HIV-1) infection. We adapted an HIV-1 isolate to replicate in cells expressing TRIM5αrh from rhesus monkeys, an Old World species. A single amino acid change in the cyclophilin-binding loop of the HIV-1 capsid protein allowed virus replication in cells expressing TRIM5αrh. The capsid of the escape virus exhibited a reduced affinity for TRIM5αrh, but retained the ability to bind cyclophilin A efficiently. Thus, a preferred HIV-1 escape pathway involves decreased binding to TRIM5α, a capsid-destabilizing factor, and retention of binding to cyclophilin A, a capsid-stabilizing factor.
Introduction
Human immunodeficiency virus (HIV-1), the major cause of acquired immunodeficiency syndrome (AIDS) in humans, arose as a result of cross-species transmission of related viruses in chimpanzees and gorillas (Hahn et al., 2000; Keele et al., 2006; Sharp et al., 2005; Van Heuverswyn et al., 2006; Van Heuverswyn and Peeters, 2007). These simian immunodeficiency viruses (SIVs) in apes are thought to have been acquired from African monkeys, many species of which are endemically infected by particular SIVs (Heeney et al., 2006; Van Heuverswyn and Peeters, 2007). In establishing infections in new species, the primate immunodeficiency viruses have encountered and adapted to a variety of host restriction factors that exhibit lineage-related differences in viral specificity. Appreciation of these restriction factors is relevant not only to natural cross-species transmission of viruses, but also to attempts to establish animal models of HIV-1 infection in humans.
Current animal models for HIV-1 infection are based on the infection of macaques with SIV or simian-immunodeficiency virus (SHIV) chimeras (Ambrose et al., 2007; Li et al., 1995). In the cells of Old World monkeys, HIV-1 encounters three main blocks: TRIM5α, APOBEC3 and BST2 (Mariani et al., 2003; McNatt et al., 2009; Stremlau et al., 2004). After HIV-1 entry, the cytoplasmic factor TRIM5α recognizes the incoming capsid and prematurely accelerates the uncoating of the retroviral capsid, compromising virus infectivity (Stremlau et al., 2006a). APOBEC3G and APOBEC3F are cellular cytidine deaminases that can be incorporated into newly produced virions and block virus replication by various mechanisms, including hypermutation of viral cDNA (Bishop et al., 2008; Lecossier et al., 2003; Mbisa et al., 2007). The HIV-1 and SIV Vif proteins can inhibit virion incorporation of APOBEC3G/F from some species by promoting proteasome-mediated degradation (Yu et al., 2003). BST2 tethers budding viral particles to the infected cell surface, blocking their release (Neil et al., 2008; Van Damme et al., 2008). The HIV-1 Vpu protein counters the impact of BST2 on virus release (Neil et al., 2008; Van Damme et al., 2008). Overcoming these blocks may allow the development of macaque models of infection with HIV-1-like viruses. Recently, two groups have generated HIV-1/SIV chimeras composed of about 90% HIV-1 sequences that efficiently infect macaque cells (Hatziioannou et al., 2006; Kamada et al., 2006). These monkey-tropic HIV-1 derivatives encode the SIVmac239 Vif protein and either a short 7-amino acid segment of the SIV capsid corresponding to the HIV-1 cyclophilin A-binding loop or the whole capsid of SIVmac.
In the present work, we adapted an HIV-1 isolate to replicate in HeLa-CD4 cells expressing TRIM5αrh.
Results
Adaptation of HIV-1 to cells expressing rhesus monkey TRIM5α
To generate an HIV-1 variant resistant to rhesus monkey TRIM5α, we employed an approach that was previously used to adapt HIV-1 to replicate in CD4-negative, CCR5-expressing cells (Kolchinsky et al., 1999) or in cells expressing CD4 and CXCR4 from common marmosets (Pacheco et al., 2008). The parental virus, HIV-1NL4-3, was generated by transfection of an infectious proviral molecular clone into 293T cells, and the resulting virus was passaged on HeLa cells expressing CD4. HeLa cells naturally express CXCR4, the coreceptor utilized by the NL4-3 envelope glycoproteins (Feng et al., 1996). This viral stock was used to infect a culture of HeLa-CD4 cells in which 50% of the cells expressed TRIM5αrh. Viral replication was determined by measuring reverse transcriptase (RT) activity in the supernatant of the cultures every 2-3 days. Viral supernatants derived from this culture at the peak of RT activity were used to infect cultures of HeLa-CD4 cells with 75% of cells expressing TRIM5αrh. This process was repeated with cultures consisting of 90% and then 100% HeLa-CD4 cells expressing TRIM5αrh. In this way, viruses that demonstrated the ability to replicate in cultures of 100% HeLa-CD4-TRIM5αrh cells were obtained.
The gag and pol sequences of the adapted viruses were examined by PCR amplification of the genomic DNA of infected cells extracted on the day when the supernatant RT activity reached a maximum. The Gag, Pro, RT and RNaseH amino acid changes observed in the adapted HIV-1NL4-3 variants are summarized in Table 1. After the first passage of the virus in cultures of 100% HeLa-CD4-TRIM5αrh cells, all of the sequenced proviral clones exhibited a mutation that converted valine 86 in the capsid protein to methionine. This change (V86M) was also present in all the proviral clones derived after additional passages of virus in cultures of 100% HeLa-CD4-TRIM5αrh cells (see Table 2). The V86M change has not been observed in natural HIV-1 strains (Kuiken et al., 2008), suggesting that its appearance may result from specific selective pressure associated with these experiments. A number of clones contained additional changes that affected HIV-1 proteins other than the capsid; however, most of these changes were found only in single clones and none were maintained between the first and third passages of virus in the 100% HeLa-CD4-TRIM5αrh cultures. Therefore, we focused on assessing the contribution of the V86M change in the capsid protein to virus adaptation to TRIM5αrh.
Table 1
Amino acid changes in the HIV-1NL4-3 gag and pol protein products following adaptation to TRIM5αrh-expressing cells.a
| Region | Predicted amino acid change | Presence of amino acid change in 1st passage | ||||
|---|---|---|---|---|---|---|
| Clone 1 | Clone 2 | Clone 3 | Clone 4 | Clone 5 | ||
| MA | R20W | X | X | |||
| CA | V86M | X | X | X | X | X |
| Protease | V11I | X | X | |||
| Protease | M46I | X | ||||
| RT | G196R | X | ||||
| RT | E296K | X | ||||
| RNase | V119I | X | ||||
| Region | Predicted amino acid change | Presence of amino acid change in 3rd passage | ||||
|---|---|---|---|---|---|---|
| Clone 1 | Clone 2 | Clone 3 | Clone 4 | Clone 5 | ||
| MA | S126R | X | ||||
| CA | V86M | X | X | X | X | X |
| p6 | F17I | X | ||||
| RT | R206G | X | ||||
| RT | A26T | X | ||||
| RNase | D48N | X | ||||
| Integrase | G70E | X | ||||
Table 2
Interaction of ligands with HIV-1 capsid variants
| Capsid variant | Relative cyclophilin A incorporationa | Relative TRIM5αrh bindingb | Relative TRIMCyp bindingb |
|---|---|---|---|
| wt | 1.00 | 1.00 | 1.00 |
| V86M | 0.84 ± 0.39 | 0.05 ± 0.05 | 0.88 ± 0.32 |
| H87Q | 0.75 ± 0.61 | 0.18 ± 0.18 | 0.46 ± 0.14 |
| YQ | 1.02 ± 0.57 | 1.77 ± 0.90 | 0.74 ±0.32 |
| G89A | 0.08 ± 0.04 | 0.84 ± 0.30 | 0.03 ± 0.008 |
| P90A | 0.09 ± 0.07 | 0.87 ± 0.18 | 0.06 ± 0.10 |
| SCA | 0.06 ± 0.07 | ND | ND |
Characterization of the sensitivity of the HIV-1 V86M capsid mutant to TRIM5αrh
Valine 86 is located in the cyclophilin A-binding loop of the HIV-1 capsid protein (Figure 1, A and B). Changes in the cyclophilin-binding loop of the capsid have been previously shown to reduce HIV-1 sensitivity to early-acting restriction factors in Old World monkey and owl monkey cells (Chatterji et al., 2005; Gatanaga et al., 2006; Hatziioannou et al., 2004; Ikeda et al., 2004; Kootstra et al., 2003, 2007; Nagao et al., 2009; Owens et al., 2004). Of note, some of these changes involve the histidine 87 residue, which is adjacent to valine 86. Other changes involving glycine 89 and proline 90 in the capsid cyclophilin-binding loop disrupt the binding of cyclophilin A, which in some cases appears to potentiate TRIM5αrh restriction of HIV-1 (Berthoux et al., 2005; Stremlau et al., 2006b; Towers et al., 2003). We compared the effect of some of these capsid changes with that of the V86M change on HIV-1 infection of HeLa-CD4 cells expressing TRIM5αrh. The effect of the capsid changes on the replication of the HIV-1NL4-KB9 SEMQ virus was examined; the SEMQ alteration in Vif is reported to overcome partially the replication block conferred by rhesus monkey APOBEC3G (Schrofelbauer et al., 2006). The replication of the HIV-1NL4-KB9 SEMQ virus with the wild-type and mutant capsids in HeLa-CD4-TRIM5αrh cells is shown in Figure 2A. HIV-1 viruses containing the V86M or H87Q changes replicated efficiently in these cells, reaching a peak of RT activity around days 15 and 19, respectively. Beginning at approximately 19 days after infection, RT activity was detected in the culture infected by the P90 mutant; the RT activity in this culture reached a peak around day 33 post-infection. Reverse transcriptase remained at background levels in cultures inoculated with HIV-1NL4-KB9 SEMQ bearing the wild-type capsids, even after 40 days in culture. In parallel, as a control, we incubated this panel of viruses with Cf2Th cells stably expressing human CD4 and CXCR4, but not TRIM5αrh. All the viruses replicated efficiently in this cell line (Figure 2B); the delay in replication of the P90A mutant is consistent with the consequences of poor cyclophilin A binding. These results demonstrate that the V86M capsid change associated with virus passage in TRIM5αrh-expressing cells increases the resistance of HIV-1 to the inhibitory effects of rhesus monkey TRIM5α.
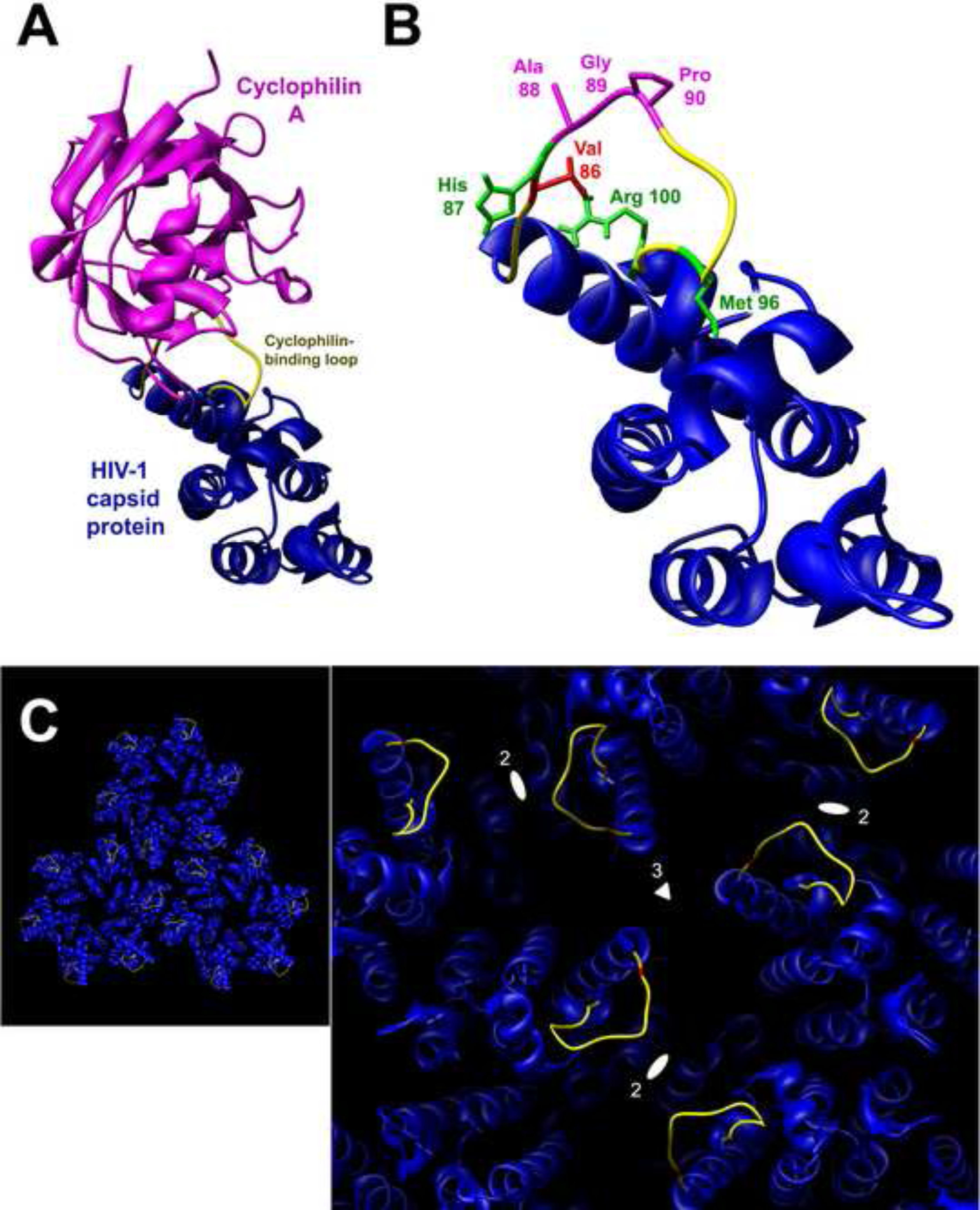
The cyclophilin A-binding loop of the HIV-1 capsid protein. (A) The ribbon structure of a cyclophilin A-HIV-1 p24 capsid protein complex is shown (Gamble et al., 1996). The cyclophilin A-binding loop is colored yellow. The complex is oriented so that, in an assembled capsid, the exposed surface of the capsid protein faces the viewer. (B) The HIV-1 capsid protein is oriented as in (A), with the side chains of the amino acid residues relevant to this study colored according to the following key: valine 86 (red), other residues in the cyclophilin A-binding loop that have been shown to influence HIV-1 sensitivity to rhesus monkey restriction (green) (Owens et al., 2004), and residues that directly contact cyclophilin A (magenta). (C) Three HIV-1 capsid hexamers are shown as they are related in a crystal lattice, approximating their relationship in an assembled capsid (Pornillos et al., 2009). The surface of the assembled capsid faces the viewer, and the cyclophilin A-binding loops (yellow) and valine 86 residues (red) are highlighted. The right panel provides a close-up of the region at the intersection of the three hexamers in the left panel. The local 3-fold (triangle) and 2-fold (ellipses) axes of symmetry are shown. The cyclophilin A-binding loops at the distal end of each hexamer spoke are located near the depressions on the surface of the assembled capsid that track along the 2- and 3-fold axes of pseudosymmetry.
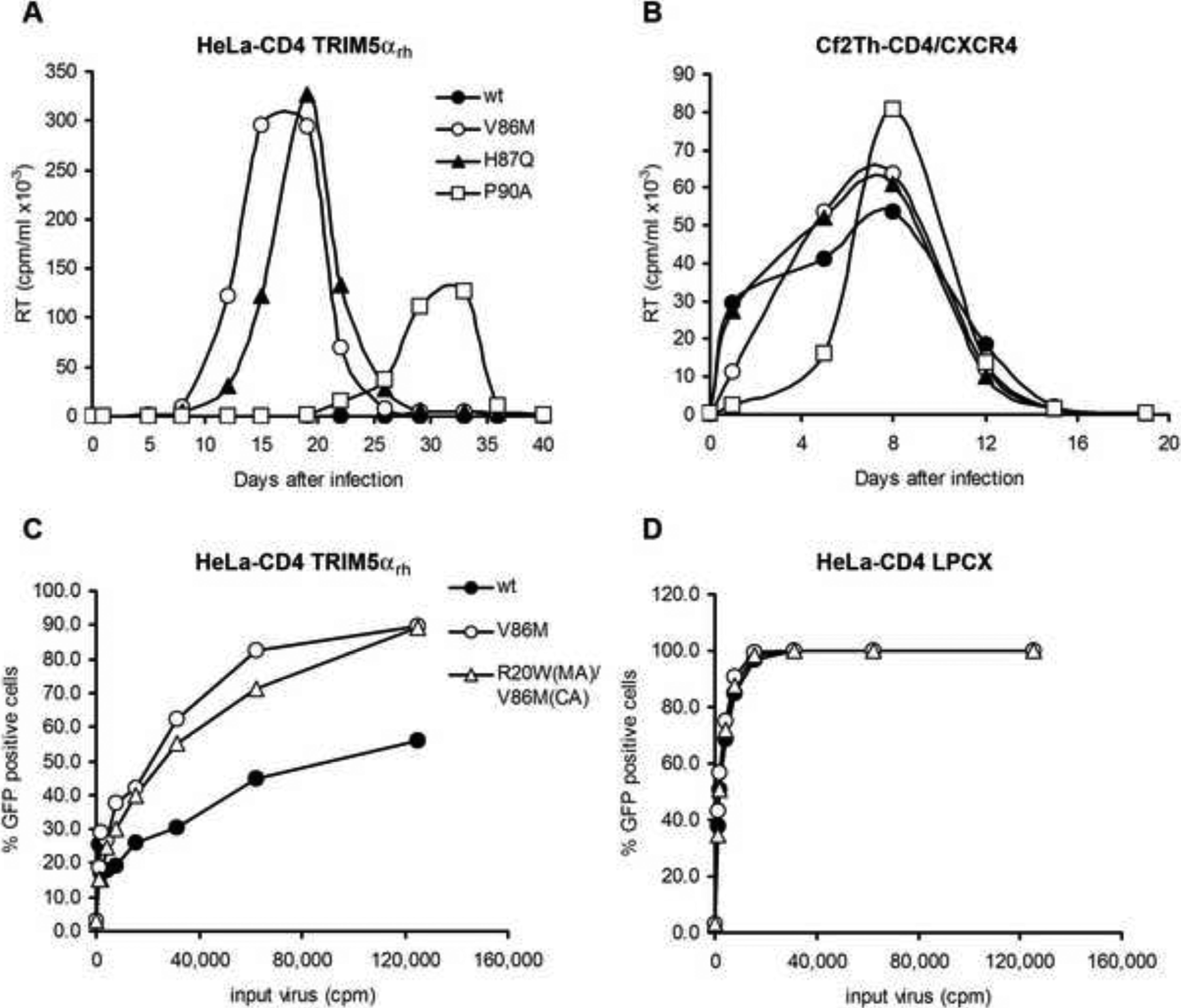
Replication of HIV-1 capsid mutants in HeLa-CD4 cells stably expressing TRIM5αrh. (A,B) HeLa-CD4 cells expressing TRIM5αrh (A) or control Cf2Th-CD4/CXCR4 cells (B) were incubated with 30,000 RT units of the indicated HIV-1NL4-KB9 SEMQ variants for 14 hours and then washed once with PBS. Every 3 or 4 days, cell supernatants were removed and used for RT assays. Cells were then trypsinized, diluted 1/10 in fresh medium and replated. (C,D) Recombinant, single-round HIV-1 expressing GFP were incubated with HeLa-CD4 cells expressing TRIM5αrh (C) or control HeLa-CD4 cells transduced with the empty LPCX vector (D). GFP expression was measured 48 hours later.
To examine whether the combination of the V86M or the H87Q capsid changes with the VifSEMQ changes were sufficient to allow HIV-1 to replicate in rhesus macaque cells, we infected the 221-89 lymphoid cell line (Alexander et al., 1997) and rhesus monkey PBMCs with HIV-1NL4-KB9 viruses containing these changes. Virus replication was not detected in this rhesus T cell line nor in rhesus macaque PBMCs (data not shown). This result suggests that additional changes in HIV-1 are needed to allow efficient replication in rhesus monkey cells. This result was not unexpected, given the existence of rhesus monkey restriction factors such as BST2 (tetherin) that operate during the late phase of HIV-1 replication (Neil et al., 2008; Van Damme et al., 2008). Rhesus monkey BST2 is not effectively countered by the HIV-1 Vpu protein (Jia et al., 2009; McNatt et al., 2009). Moreover, the SEMQ changes in the Vif protein may be insufficient to allow HIV-1 to overcome APOBEC3G restriction and to replicate in the cells of Old World monkeys (Hatcho et al., 2008; Kamada et al., 2009).
To examine the ability of V86M and other capsid variants to escape specifically from the early restriction imposed by TRIM5αrh, we infected cells with recombinant HIV-1 vectors containing the capsid changes. These viruses were pseudotyped with the vesicular stomatitis virus (VSV) G glycoprotein, which allows entry into a wide range of vertebrate cells. The recombinant viruses express either green fluorescent protein (GFP) or luciferase. The V86M change in the capsid protein allowed more efficient single-round infection of HeLa-CD4-TRIM5αrh cells than was observed for the wild-type recombinant HIV-1 vector (Figure 2C). Because the R20W change in the matrix protein appeared in two clones during an early passage of the virus in HeLa-CD4-TRIM5αrh cells, we also tested the contribution of this change. An HIV-1 vector containing the R20W change in the matrix protein in addition to the V86M change in capsid infected the HeLa-CD4-TRIM5αrh cells similarly to the virus with only the V86M change. All three recombinant viruses infected the control HeLa-CD4 cells efficiently (Figure 2D). We conclude that the V86M change in the capsid protein confers an advantage during the infection of HeLa cells expressing TRIM5αrh.
HeLa cells express human TRIM5α, which only minimally inhibits HIV-1 infection (Stremlau et al., 2004); the presence of human TRIM5α might influence the potency with which TRIM5αrh restricts HIV-1 infection. Therefore, we used single-round, luciferase-expressing HIV-1 to examine the contribution of the V86M capsid change to the early phase of infection of another cell type expressing TRIM5αrh. We compared the infectivity of recombinant HIV-1 with the V86M change, or with different changes in the capsid previously reported to affect sensitivity to TRIM5α restriction or cyclophilin A binding (Chatterji et al., 2005; Gatanaga et al., 2006; Hatziioannou et al., 2004; Ikeda et al., 2004; Kootstra et al., 2004, 2007; Owens et al., 2004; Towers et al., 2003). Canine Cf2Th cells, which do not express an endogenous TRIM5α protein (Sawyer et al., 2007), were used as target cells; in addition to the control cells transduced with the empty LPCX vector, we used Cf2Th cells expressing TRIM5αrh and Cf2Th cells expressing the owl monkey TRIMCyp protein. The latter protein is expressed in the New World owl monkey lineage as a result of a retrotransposition that fuses the amino-terminal part of TRIM5 with the cyclophilin A protein (Nisole et al., 2004; Sayah et al., 2004). In control canine Cf2Th cells transduced with an empty LPCX vector, all of the HIV-1 capsid mutants, with the exception of H87Q, infected less efficiently than the wild-type HIV-1 (Figure 3A). An HIV-1 vector (SCA) containing the SIVmac capsid (Owens et al., 2003) also infected these control cells less efficiently than wild-type HIV-1. In Cf2Th cells expressing TRIM5αrh, the SCA virus exhibited a higher level of infectivity than wild-type HIV-1. The V86M and H87Q HIV-1 variants also infected these cells slightly more efficiently than wild-type HIV-1 (Figure 3B). The G89A and P90A HIV-1 capsid mutants, which are defective for cyclophilin A binding (Braaten et al., 1996; Franke et al., 1994), infected the TRIM5αrh-expressing cells poorly. By contrast, these HIV-1 variants, as well as SCA, infected Cf2Th cells expressing owl monkey TRIMCyp much more efficiently than the other viruses, including wild-type HIV-1 (Figure 3C). This was expected, as neither the SIVmac nor the G89A or P90A HIV-1 capsids bind TRIMCyp (Hatziioannou et al., 2004; Sayah et al., 2004; Towers et al., 2003). These results indicate that the V86M and H87Q changes in the HIV-1 capsid are specifically advantageous during the infection of Cf2Th-TRIM5αrh cells.
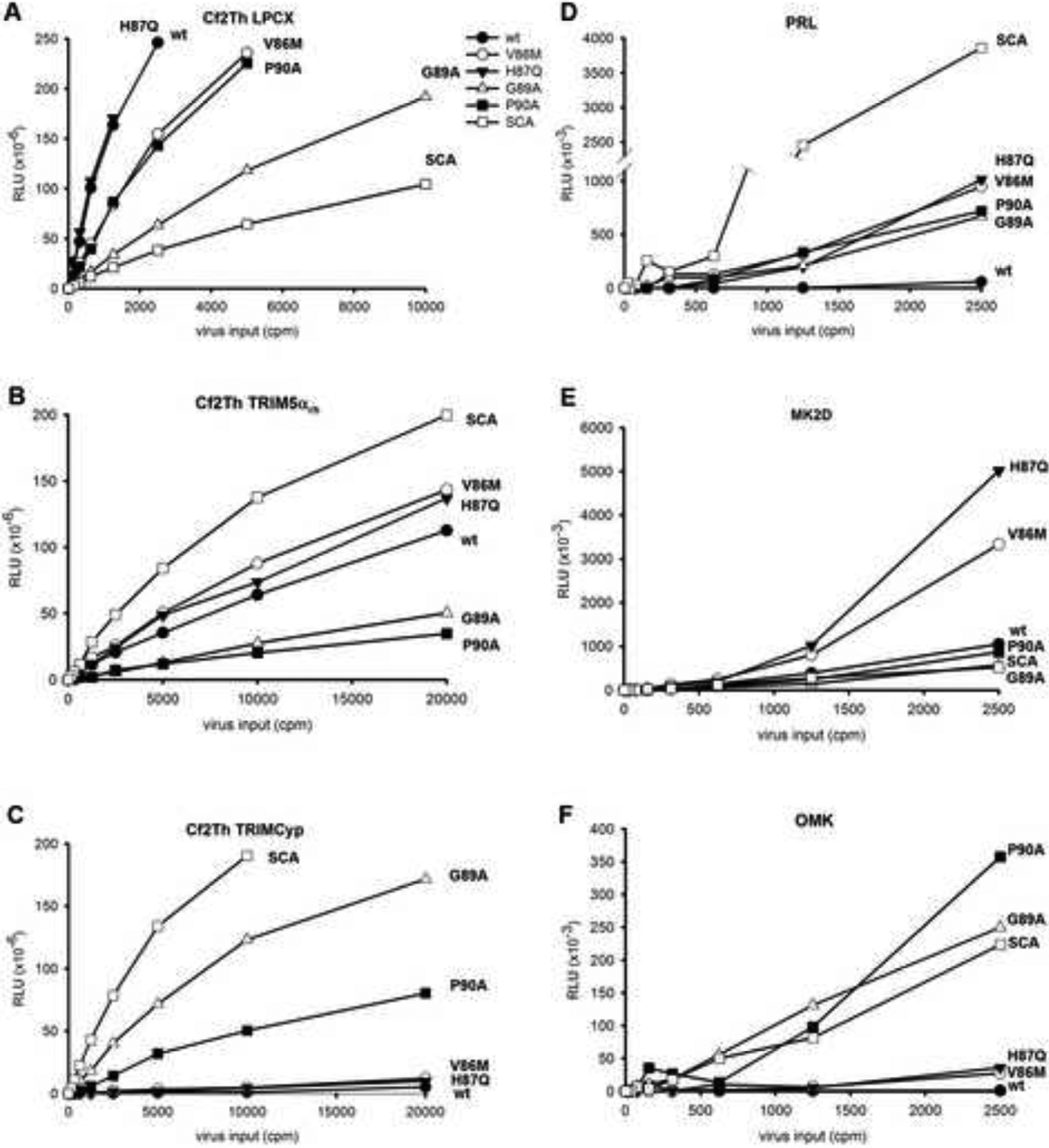
Infectivity of HIV-1 capsid mutants in different cell lines. Target cells were infected with single-round recombinant reporter viruses expressing firefly luciferase. Forty-eight hours after infection, cells were lysed and the luciferase activity was measured using an EG&G Berthold LB 96V microplate luminometer. The target cells were: Cf2Th cells stably transduced with the empty LPCX vector (A), Cf2Th cells stably expressing TRIM5αrh (B); Cf2Th cells stably expressing owl monkey TRIMCyp (C); primary rhesus monkey lung (PRL) fibroblasts (D), rhesus monkey kidney (MK2D) cells (E); and owl monkey kidney (OMK) cells (F). RLU, relative luciferase units.
We also examined the ability of these single-round recombinant viruses to infect primary rhesus monkey lung (PRL) fibroblasts, MK2D rhesus macaque cells and the OMK owl monkey kidney cell line. The PRL and MK2D cells express TRIM5αrh, whereas the OMK cells express TRIMCyp from owl monkeys, a New World monkey species (Sayah et al., 2004; Stremlau et al., 2004; Wilson et al., 2008). Wild-type HIV-1 infection of PRL cells was nearly undetectable, whereas SCA infected these cells very efficiently (Figure 3D). The V86M and H87Q HIV-1 mutants and, to a slightly lesser extent, the G89A and P90A mutants, infected the PRL cells more efficiently than wild-type HIV-1. In the MK2D rhesus monkey cells, infection by wild-type HIV-1 and the G89A and P90A mutants was much less efficient than infection mediated by the V86M and H87Q mutants. In the OMK cells from the owl monkey, only SCA and the G89A and P90A HIV-1 mutants infected efficiently. Thus, as was observed in the Cf2Th cells above, the V86M and H87Q changes in the HIV-1 capsid confer a specific advantage during the early phase of infection of cells expressing rhesus monkey TRIM5α.
Competition of wild-type and mutant virus-like particles for restriction factors
Previous studies have shown that the restriction of HIV-1 in Old World monkey cells can be saturated by incubation of the cells with enveloped virus-like particles (VLPs) (Cowan et al., 2002; Hatziioannou et al., 2003; Munk et al., 2002). To test whether the HIV-1 capsid mutants were able to compete for restriction factors in primary rhesus monkey cells, we incubated PRL cells with the wild-type HIV-1 reporter virus encoding firefly luciferase along with wild-type or mutant VLPs. The presence of wild-type HIV-1 VLPs increased the infectivity of the HIV-1 reporter virus (Figure 4), suggesting that these VLPs are able to compete for the binding to restriction factors. The V86M, H87Q, G89A and P90A mutant VLPs exhibited reduced ability to compete for restriction factors in the PRL cells, compared with the wild-type HIV-1 VLPs. The YQ VLPs, which contain two changes in the HIV-1 capsid that reduce susceptibility to Old World monkey TRIM5α without affecting restriction factor binding (Owens et al. 2004), competed efficiently for restricting activity. As expected, HIV-1 VLPs without envelope glycoproteins or the SCA VLPs, which have an SIVmac capsid in an HIV-1 background (Owens et al., 2003), did not compete for the PRL restriction factor(s). The results suggest that all of the HIV-1 VLPs with changes in the cyclophilin-binding loop interact less efficiently with restriction factors following entry into PRL cells.
Competition assay with HIV-1 VLPs. Target PRL cells were infected with 2,500 RT units of the wild-type recombinant HIV-1 expressing luciferase in the presence of 100,000 RT units of virus-like particles (VLPs) lacking a reporter gene. The capsid proteins of the VLPs were either wild-type HIV-1 (wt) or contained the indicated changes. All VLPs were pseudotyped with the VSV G glycoprotein, except control VLPs with wild-type HIV-1 capsids but with no added envelope glycoproteins (no Env). Forty-eight hours after infection, cells were lysed and the luciferase activity was measured.
Capsid binding to cyclophilin A, TRIM5αrh and TRIMCyp
To determine the effects of the HIV-1 capsid changes on the binding of cyclophilin A, we examined cyclophilin A incorporation into HIV-1 VLPs that were produced in cyclophilin A-expressing cells. Although the major effects of cyclophilin A incorporation are manifested during the early phase of HIV-1 infection (Hatziioannou et al., 2005), the level of cyclophilin A in the virions is a surrogate for capsid-binding affinity. We transfected 293T cells with plasmids expressing the wild-type or mutant HIV-1 Gag-Pol proteins, the HA-tagged human cyclophilin A and the HIV-1 Rev protein. As one control, we used a chimeric HIV-1/SIVmac provirus (SCA) encoding the SIVmac capsid (Owens et al., 2003). As a second control, a wild-type HIV-1 VLP was produced in the absence of HA-tagged cyclophilin A. The VLPs were pelleted, lysed and Western blotted with anti-HA and anti-capsid antibodies (Figure 5A and Table 2). The V86M, H87Q and YQ mutant VLPs efficiently incorporated cyclophilin A, whereas the G89A and P90A mutant VLPs incorporated only trace amounts of cyclophilin A. The HIV-1/SIVmac chimera (SCA) with the SIVmac capsid did not detectably incorporate cyclophilin A into VLPs. We conclude that the V86M capsid mutant retains the ability to bind cyclophilin A efficiently.
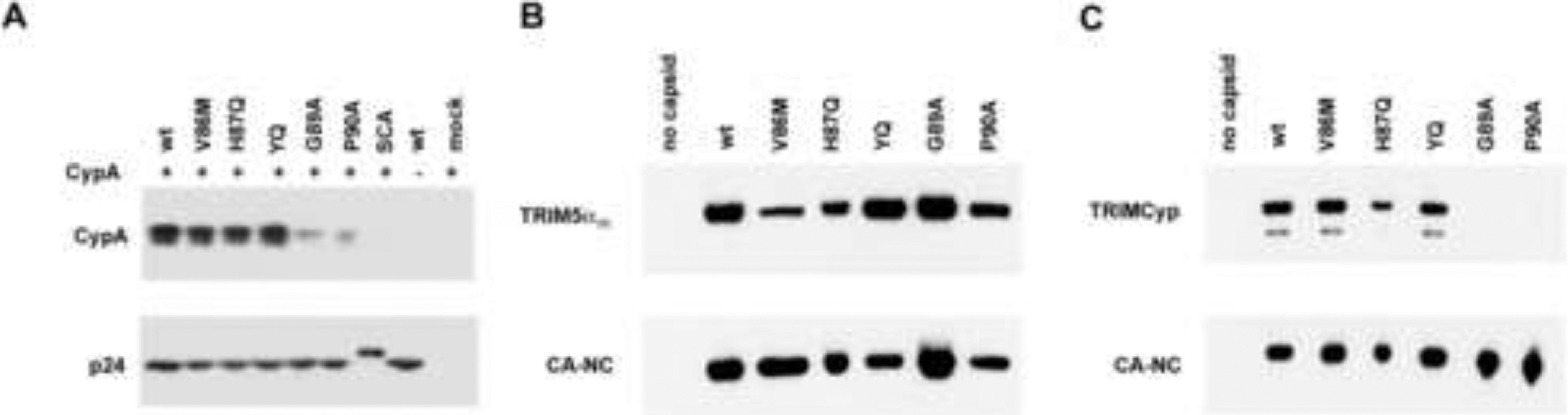
Interaction of ligands with the HIV-1 capsid variants. (A) Cyclophilin A incorporation into HIV-1 VLPs was measured. VLPs produced in 239T cells in the presence or absence of coexpressed FLAG-tagged cyclophilin A (CypA) were pelleted through a 20% sucrose cushion prepared in PBS. The pellets were analyzed by Western blotting with anti-p24 capsid and anti-FLAG antibodies. The incorporation of cyclophilin A into mutant VLPs, relative to that of the wild-type HIV-1 VLP, is reported in Table 2. The Western blot of one representative experiment is shown. (B,C) The binding of TRIM5αrh or owl monkey TRIMCyp to HIV-1 CA-NC complexes is shown. Binding to the HIV-1 CA-NC complexes was measured as previously described (Stremlau et al., 2006a). The samples were analyzed by Western blotting with an anti-p24 antibody (4F6) conjugated to horseradish peroxidase (Immunodiagnostics, Inc.) and an anti-HA antibody (3F10) conjugated to horseradish peroxidase (Roche). The binding of TRIM5α and TRIMCyp to each mutant CA-NC complex, relative to the binding to wt HIV-1 CA-NC complexes, is reported in Table 2. The results of a typical experiment are shown. The YQ mutant has the Q50Y/T54Q changes in the HIV-1 capsid protein (Owens et al., 2004).
TRIM5α specifically recognizes assembled retroviral capsids rather than individual capsid proteins (Dodding et al., 2005; Sebastian and Luban, 2005). To investigate the ability of TRIM5αrh to recognize the mutant HIV-1 capsids, we analyzed the binding of HA-tagged TRIM5αrh to wild-type and mutant capsid-nucleocapsid (CANC) complexes that had been assembled in vitro, as previously described (Stremlau et al., 2006a). TRIM5αrh bound efficiently to the wild-type, YQ, G89A and P90A CA-NC complexes (Figure 5B and Table 2). In fact, the binding of TRIM5αrh to the YQ CA-NC complexes was more efficient than to the wild-type CA-NC complexes. By contrast, TRIM5αrh binding to the V86M and H87Q CA-NC complexes was significantly reduced compared to the binding observed for the wild-type CA-NC complexes (Table 2). Thus, diminished binding of TRIM5αrh by the V86M and H87Q capsids provides a natural explanation for the ability of these mutants to escape TRIM5αrh restriction.
We also tested the binding of owl monkey TRIMCyp to the wild-type and mutant CA-NC complexes (Figure 5C and Table 2). TRIMCyp bound efficiently to the wild-type, V86M and YQ CA-NC complexes, and with an intermediate level of efficiency to the H87Q variant. In agreement with their reduced affinity for cyclophilin A, the G89A and P90A CA-NC complexes did not detectably bind TRIMCyp.
Discussion
Selection of an HIV-1 variant for replication in cells expressing TRIM5αrh allowed an assessment of the preferred viral solution to the challenge of circumventing a potent restriction factor. A single amino acid change, V86M, in the cyclophilin A-binding loop of the capsid protein was sufficient to allow efficient HIV-1 replication in HeLa-CD4 cells expressing TRIM5αrh. Of note, changes in the cyclophilin A-binding loop of capsid, including one change (V86Q) in valine 86, were previously shown to confer improved HIV-1 infectivity in Old World monkey target cells (Hatziioannou et al., 2004; Ikeda et al., 2004; Kootstra et al., 2003, 2007; Nagao et al., 2009; Owens et al., 2004). These changes involved valine 86, histidine 87, methionine 96 and arginine 100, residues that are located near the point of departure of the cyclophilin A-binding loop from the main body of the capsid protein (Figure 1B). Because of this location, changes in these residues have the potential to alter the conformation/orientation of the distal cyclophilin A-binding loop. The impact of such alterations on the structure of the capsid surface could be amplified by the location of the cyclophilin A-binding loop at the end of the hexamer spoke, proximal to 2-fold and three-fold axes of pseudosymmetry (Figure 1C).
Our results suggest that the mechanism of escape of the V86M mutant is a reduced interaction of the capsid with TRIM5αrh. Both the V86M and the H87Q VLPs competed for restriction factors in PRL cells less efficiently than wild-type HIV-1 VLPs. The TRIM5αrh protein in cell lysates bound less to the V86M and H87Q capsidnucleocapsid complexes than to wild-type HIV-1 CA-NC complexes. Previous studies have suggested that TRIM5α recognizes assembled capsid structures rather than individual capsid proteins (Dodding et al., 2005; Sebastian and Luban, 2005). Moreover, TRIM5α B-box 2 mutants that can dimerize but not form higher-order oligomers exhibit reductions in capsid-binding ability and virus restriction (Diaz-Griffero et al., 2009; Li and Sodroski, 2008). Thus, retrovirus restriction apparently is mediated by an array of TRIM5α dimers that must first assemble on the surface of the viral capsid. In light of this requirement, the potential of valine 86 and histidine 87 changes to affect the conformation of the cyclophilin A-binding loop and thereby to alter the boundaries of the two- or three-fold symmetric pockets on the capsid surface is noteworthy. To gain avidity for the capsid, TRIM5α dimers are expected to use both B30.2(SPRY) domains to contact the capsid, perhaps via a 2-fold symmetric mode of binding. The V86M and H87Q changes could potentially disrupt this initial TRIM5αrh binding. Although the precise higher-order assembly of TRIM5α is not understood, the formation of TRIM5α hexamers in solution has been reported (Nepveu-Traversy et al., 2009). It is possible that alterations of the conformation of the cyclophilin A-binding loop resulting from the V86M or H87Q changes also disrupt one or more aspects of the higher-order assembly of TRIM5α dimers.
The binding of cyclophilin A to the capsid contributes to the potency of HIV-1 restriction by some TRIM5α proteins, including TRIM5αrh (Berthoux et al., 2005; Stremlau et al., 2006b; Towers et al., 2003). Decreased binding of the V86M variant to cyclophilin A, however, does not explain the observed decrease in susceptibility to TRIM5αrh restriction. First, the P90A mutant, which is markedly defective in the ability to bind cyclophilin A, replicated less efficiently in TRIM5αrh-expressing cells than the V86M or H87Q mutants; this was true even in HeLa cells where cyclophilin A binding is not required by HIV-1 for efficient infection (Li et al., 2009). Second, although valine 86 and histidine 87 are close to the cyclophilin A-binding site on the HIV-1 capsid, they do not appear to contribute significantly to the affinity of the cyclophilin-capsid interaction. Valine 86 does not contact cyclophilin A, being just beyond a van der Waals contact with alanine 103 of cyclophilin A (Gamble et al., 1996; Gatanaga et al., 2006). The methionine substitution at capsid residue 86 might even be expected to increase opportunities for such contacts with cyclophilin A. Histidine 87 makes no van der Waals contacts with cyclophilin A; at best, the Nδ1 of histidine 87 is predicted to form only a non-ideal hydrogen bond with the backbone carbonyl oxygen of asparagine 71 of cyclophilin A (Gamble et al., 1996; Gatanaga et al., 2006). Consistent with these structural observations, the V86M change in the CA-NC complexes resulted in only very slight decreases in the ability to interact with cyclophilin A and TRIMCyp, relative to the wild-type CA-NC complexes. Slightly greater decreases in cyclophilin A and TRIMCyp binding were observed for the H87Q CA-NC complexes. Apparently, cyclophilin A and TRIM5αrh bind proximal but distinct structures on the HIV-1 capsid, the former present on the monomer and the latter formed by quaternary interactions (Dodding et al., 2005; Sebastian and Luban, 2005). In adapting to the TRIM5αrh-expressing cell, HIV-1 apparently seeks a path that decreases TRIM5α binding while preserving the greater part of cyclophilin A binding. Both the binding of a restricting TRIM5α protein and failure to bind cyclophilin A have been shown to lead to a decrease in the stability of the HIV-1 capsid in the cytosol of infected cells and a consequent decrease in infectivity (Li et al., 2009; Stremlau et al., 2006a).
Our results provide some insight into the mechanism whereby cyclophilin A interaction with the HIV-1 capsid in an infected cell potentiates TRIM5αrh restriction. Both the G89A and P90A VLPs, which are deficient in cyclophilin A binding, were inefficient in competing for restriction factors in PRL cells, compared with the wild-type HIV-1 VLPs. The deduced decrease in TRIM5αrh binding in these circumstances may be secondary to the decreased stability, in most cell types, of HIV-1 capsids that do not bind cyclophilin A (Li et al., 2009). Changes in the stability of the HIV-1 capsid have been shown to affect the ability of VLPs to saturate TRIM5α restriction (Shi and Aiken, 2006). Of note, the G89A and P90A changes exert only minimal effects on TRIM5αrh binding to CA-NC complexes in vitro; this is consistent with previous observations that the P90A change only minimally decreases the in vitro stability of capsid assemblies (Li et al., 2009). Any slight direct effect of the G89A and P90A changes on TRIM5αrh binding to the HIV-1 capsid may be amplified by the decreased ability of cyclophilin A-binding-deficient capsids to present a higher-order quaternary structure to TRIM5αrh in the cytosol.
Future studies will investigate in greater detail the mechanisms by which HIV-1 crosses species barriers and explore opportunities to use this information in creating animal models for HIV-1 infection.
Materials and Methods
Cell lines
OMK, MK2D, 293T and Cf2Th cells were obtained from the American Type Culture Collection and maintained in DMEM supplemented with 10% fetal bovine serum (DMEM-10). HeLa-CD4 cells expressing TRIM5αrh have been previously described (Stremlau et al., 2004) and were maintained in DMEM-10. Primary rhesus lung (PRL) fibroblasts have been previously described (Hofmann et al., 1999) and were maintained in DMEM-10. Rhesus monkey peripheral blood mononuclear cells (PBMC) were isolated from rhesus monkey blood provided by the New England Primate Research Center (NEPRC). After isolation with Ficoll-Paque Plus, PBMCs were activated for 3 days with 1 μg of PHA-P and cultured in complete lymphocyte medium (RPMI-1640 supplemented with 20% FBS, 10% IL-2, 25 mM HEPES, 2 mM glutamine and antibiotics). The rhesus monkey T-cell line 221-98 (Alexander et al., 1997), kindly provided by Dr. Ronald Desrosiers (NEPRC), was maintained in complete lymphocyte medium.
Cf2Th cell lines expressing TRIM5αrh from rhesus macaques or TRIMCyp from owl monkeys were prepared by transducing Cf2Th cells with murine leukemia virus (MLV) vectors harboring TRIM5α or TRIMCyp cDNA. Recombinant TRIM-expressing MLV viruses pseudotyped with VSV-G were generated by cotransfecting 293T cells with 4 μg of the pVSV-G plasmid, 4 μg of the pLPCX-TRIM vector plasmid and 12 μg of the pcPack-B-MLV plasmid using the calcium phosphate method (Invitrogen). The resulting viral particles were used to transduce canine Cf2Th cells. Transduced Cf2Th cells were selected with 5 μg/ml of puromycin.
Virus replication
Replication-competent HIV-1 viruses were generated by transfecting 20 μg of the pNL4-3 plasmid, which contains an infectious HIV-1NL4-3 provirus (Adachi et al., 1986), into 2×106 293T cells using the calcium phosphate transfection method (Invitrogen). Forty-eight hours after transfection, supernatants containing viruses were harvested and cleared by low-speed centrifugation. The level of virus in the supernatant was determined by measuring reverse transcriptase (RT), as described previously (Rho et al., 1981).
Cells were infected with 30,000 RT units of HIV-1NL4-KB9 variants for 14 hours and then washed once with PBS. Every 3 or 4 days, cell supernatants were removed and used for RT assays. Cells were trypsinized, diluted 1/10 in fresh medium and replated.
Selected gag mutations were introduced into the HIV-1NL4-KB9 provirus encoding the SEMQ Vif variant (Schrofelbauer et al., 2006). Infectious viruses were generated and tested for replicative ability as described above.
Analysis of Gag and Pol sequences
The gag and pol genes of the adapted viruses were amplified by polymerase chain reaction (PCR) of genomic DNA isolated from infected cells with the QIAamp® DNA Blood Mini Kit (QIAGEN). A 4.5-kb fragment containing the full gag-pol sequence was generated by PCR with PfuUltra™ High-Fidelity DNA polymerase (Stratagene) and the primers gag-pol-forward: 5’-CTCTCGACGCAGGACTCG-3’ and gag-pol-reverse: 5’-AAACCAGTCCTTAGCTTTCCTTG -3’. The 4.5 kb fragment was cloned into the pCR®4blunt-TOPO plasmid (Invitrogen). The inserts from five individual clones were sequenced to obtain the consensus sequence in the gag and pol region of the adapted virus.
Site-directed mutagenesis
DNA sequence changes specifying the V86M alteration in capsid, which was associated with viral adaptation to TRIM5αrh, were introduced by site-directed mutagenesis using the QuikChange II XL Site-Directed Mutagenesis protocol (Stratagene) into the pCMVΔP1ΔenvpA plasmid containing the gag, pol and vif regions of NL4-3 HIV-1. DNA sequences specifying the previously described capsid variants H87Q, Q50Y/T54Y, G89A and P90A (Bukovsky et al., 1997; Franke et al., 1994; Owens et al., 2004) were also introduced by site-directed mutagenesis into this plasmid for comparison purposes. The presence of the desired mutations was verified by automated DNA sequencing.
Single-round infection assay
The efficiency of a single round of HIV-1 infection was measured by using recombinant reporter viruses expressing firefly luciferase in place of Nef and pseudotyped with the vesicular stomatitis virus G glycoprotein (VSV-G). Recombinant luciferase-expressing HIV-1 viruses (Helseth et al., 1990) were generated by transfecting 293T cells using the calcium phosphate transfection method (Invitrogen) with 2 μg of the pHCMV-G plasmid expressing the VSV envelope glycoprotein G, 2 μg of a Rev-expressing plasmid, 4 μg of the pCMVΔP1ΔenvpA packaging plasmid expressing the corresponding CA mutant, and 12 μg of an HIV-1 vector plasmid. The HIV-1 vector plasmid expresses an RNA that can be packaged into virions, reverse transcribed, and integrated into a target cell, where it encodes firefly luciferase. Forty-eight hours after transfection, supernatants containing reporter viruses were harvested and cleared by low-speed centrifugation. The amounts of virus in the supernatants were quantified by measuring RT activity.
Target cells were seeded at a density of 6,000 cells/well in 96-well luminometer-compatible tissue culture plates. Twenty-four hours later, medium was changed and different amount of viruses were added to the cells. After 14 h of incubation, the supernatant was removed and fresh medium was added. Forty-eight hours later, the medium was removed and cells were lysed with 30 μl of passive lysis buffer (Promega). Luciferase activity was measured using an EG&G Berthold LB 96V microplate luminometer in accordance with the luciferase assay system technical bulletin (Promega).
Competition for restriction factors with virus-like particles (VLPs)
For the competition assay with VLPs, the PRL cells were infected with 2,500 RT units of wild-type luciferase reporter virus in the presence of 100,000 RT units of virus-like particles lacking a reporter. These VLPs were generated by co-transfecting 293T cells with 4 μg of the pHCMV-G plasmid expressing the VSV envelope glycoprotein G, 4 μg of a Rev-expressing plasmid (pRev), and 12 μg of the pCMVΔP1ΔenvpA packaging plasmid expressing the corresponding CA mutant. VLPs were concentrated by pelleting through a 20% sucrose cushion.
Cyclophilin A incorporation into virions
Eight million 293T cells were transfected with 3 μg of pcDNA-CypA-FLAG, 1 μg of pRev, and 8 μg of pCMVΔP1ΔenvpA harboring different capsid changes. Forty-eight hours after transfection, supernatants containing the viral particles were collected and spun at low speed to remove cell debris. Cleared supernatants were pelleted through a 20% sucrose cushion prepared in PBS for 2 h at 30,000 rpm in an SW41 rotor at 4°C. Pelleted VLPs were resuspended in RT suspension buffer. Similar amount of VLPs, normalized for RT activity, were analyzed by SDS-PAGE and Western blotting with anti-p24 and anti-FLAG antibodies. Western blots were exposed to film for time periods that allowed detection of signals but avoided saturation of the film.
TRIM5αrh and TRIMCyp binding to HIV-1 CA-NC complexes
Purification of recombinantly expressed HIV-1 CA-NC protein from Escherichia coli was carried out as described previously (Ganser et al., 1999). High-molecular-weight HIV-1–capsid complexes were assembled using 0.3 mM CA-NC protein and 60 μM (TG)50 DNA oligonucleotide in a volume of 50 μl of 50 mM Tris·HCl, pH 8.0 and 500 mM NaCl. The reaction was allowed to proceed overnight at 4°C, and the assembled CA–NC complexes were stored at 4°C until needed.
Binding of TRIM5α and TRIMCyp to HIV-1 CA-NC complexes was measured as previously described (Stremlau et al., 2006a). For a source of TRIM5 protein, about 4 × 107 Cf2Th cells stably expressing TRIM5αrh or TRIMCyp were lysed in 1 ml of hypotonic lysis buffer (10 mM Tris·HCl pH 8.0, 10 mM KCl, 1 mM EDTA) and placed on ice for 15 min. The cells were frozen/thawed once and the cell debris were removed by centrifugation at 4°C for 10 min at maximum speed (14,000 × g) in an Eppendorf microfuge. The concentration of NaCl was adjusted to 150 mM. One hundred microliters of the cleared cell lysate was combined with 5 μl of HIV-1 CA–NC complexes from the assembly reaction. The mixture was incubated for 1 h at room temperature with gentle mixing. After incubation, the mixture was layered onto a 4.5 ml 70% sucrose cushion (prepared in PBS) and centrifuged at 110,000 × g for 2 h at 4°C in a Beckman SW55Ti rotor. The pellet was resuspended in 1× SDS sample buffer and subjected to SDS/PAGE and Western blotting with an anti-p24 antibody (4F6) conjugated to horseradish peroxidase (Immunodiagnostics, Inc.) and an anti-HA antibody (3F10) conjugated to HRP (Roche). Western blots were exposed to film for time periods that allowed detection of signals but avoided saturation of the film.
Acknowledgments
We thank Yvette McLaughlin and Elizabeth Carpelan for manuscript preparation. We acknowledge Angela Carville and the New England Primate Research Center for providing the rhesus monkey blood. We acknowledge Dr. Ronald Desrosiers from the New England Primate Research Center for kindly providing the 221-89 rhesus cell line. We acknowledge ATCC for the 293T, Cf2Th, MK2D and OMK cell lines. This work was supported by grants from the National Institutes of Health (AI063987 and a Center for AIDS Research Award AI060354) and a gift from the late William F. McCarty-Cooper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adachi A, Gendelman HE, Koenig S, Folks T, Willey R, Rabson A, Martin MA. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986;59:284–291. [Europe PMC free article] [Abstract] [Google Scholar]
- Alexander L, Du Z, Rosenzweig M, Jung JU, Desrosiers RC. A role for natural simian immunodeficiency virus and human immunodeficiency virus type 1 nef alleles in lymphocyte activation. J Virol. 1997;71:6094–6099. [Europe PMC free article] [Abstract] [Google Scholar]
- Ambrose Z, KewalRamani VN, Bieniasz PD, Hatziioannou T. HIV/AIDS: in search of an animal model. Trends Biotechnol. 2007;25:333–337. [Abstract] [Google Scholar]
- Berthoux L, Sebastian S, Sokolskaja E, Luban J. Cyclophilin A is required for TRIM5{alpha}-mediated resistance to HIV-1 in Old World monkey cells. Proc Natl Acad Sci U S A. 2005;102:14849–14853. [Europe PMC free article] [Abstract] [Google Scholar]
- Bishop KN, Verma M, Kim EY, Wolinsky SM, Malim MH. APOBEC3G inhibits elongation of HIV-1 reverse transcripts. PLoS Pathog. 2008;4:e1000231. [Europe PMC free article] [Abstract] [Google Scholar]
- Braaten D, Franke EK, Luban J. Cyclophilin A is required for an early step in the life cycle of human immunodeficiency virus type 1 before the initiation of reverse transcription. J Virol. 1996;70:3551–3560. [Europe PMC free article] [Abstract] [Google Scholar]
- Bukovsky AA, Weimann A, Accola MA, Gottlinger HG. Transfer of the HIV-1 cyclophilin-binding site to simian immunodeficiency virus from Macaca mulatta can confer both cyclosporin sensitivity and cyclosporin dependence. Proc Natl Acad Sci U S A. 1997;94:10943–10948. [Europe PMC free article] [Abstract] [Google Scholar]
- Chatterji U, Bobardt MD, Stanfield R, Ptak RG, Pallansch LA, Ward PA, Jones MJ, Stoddart CA, Scalfaro P, Dumont JM, Besseghir K, Rosenwirth B, Gallay PA. Naturally occurring capsid substitutions render HIV-1 cyclophilin A independent in human cells and TRIM-cyclophilin-resistant in Owl monkey cells. J Biol Chem. 2005;280:40293–40300. [Abstract] [Google Scholar]
- Cowan S, Hatziioannou T, Cunningham T, Muesing MA, Gottlinger HG, Bieniasz PD. Cellular inhibitors with Fv1-like activity restrict human and simian immunodeficiency virus tropism. Proc Natl Acad Sci U S A. 2002;99:11914–11919. [Europe PMC free article] [Abstract] [Google Scholar]
- Diaz-Griffero F, Qin XR, Hayashi F, Kigawa T, Finzi A, Sarnak Z, Lienlaf M, Yokoyama S, Sodroski J. A B-box 2 surface patch important for TRIM5alpha self-association, capsid binding avidity, and retrovirus restriction. J Virol. 2009;83:10737–10751. [Europe PMC free article] [Abstract] [Google Scholar]
- Dodding MP, Bock M, Yap MW, Stoye JP. Capsid processing requirements for abrogation of Fv1 and Ref1 restriction. J Virol. 2005;79:10571–10577. [Europe PMC free article] [Abstract] [Google Scholar]
- Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. [Abstract] [Google Scholar]
- Franke EK, Yuan HE, Luban J. Specific incorporation of cyclophilin A into HIV-1 virions. Nature. 1994;372:359–362. [Abstract] [Google Scholar]
- Gamble TR, Vajdos FF, Yoo S, Worthylake DK, Houseweart M, Sundquist WI, Hill CP. Crystal structure of human cyclophilin A bound to the amino-terminal domain of HIV-1 capsid. Cell. 1996;87:1285–1294. [Abstract] [Google Scholar]
- Ganser BK, Li S, Klishko VY, Finch JT, Sundquist WI. Assembly and analysis of conical models for the HIV-1 core. Science. 1999;283:80–83. [Abstract] [Google Scholar]
- Gatanaga H, Das D, Suzuki Y, Yeh DD, Hussain KA, Ghosh AK, Mitsuya H. Altered HIV-1 gag protein interactions with cyclophilin A (CypA) on the acquisition of H219Q and H219P substitutions in the CypA binding loop. J Biol Chem. 2006;281:1241–1250. [Abstract] [Google Scholar]
- Hahn BH, Shaw GM, De Cock KM, Sharp PM. AIDS as a zoonosis: scientific and public health implications. Science. 2000;287:607–614. [Abstract] [Google Scholar]
- Hatcho K, Kamada K, Yamashita T, Adachi A, Nomaguchi M. Replication potentials of vif variant viruses generated from monkey cell-tropic HIV-1 derivative clones NL-DT5/NL-DT5R. Microbes Infect. 2008;10:1218–1222. [Abstract] [Google Scholar]
- Hatziioannou T, Cowan S, Goff SP, Bieniasz PD, Towers GJ. Restriction of multiple divergent retroviruses by Lv1 and Ref1. Embo J. 2003;22:385–394. [Europe PMC free article] [Abstract] [Google Scholar]
- Hatziioannou T, Cowan S, Von Schwedler UK, Sundquist WI, Bieniasz PD. Species-specific tropism determinants in the human immunodeficiency virus type 1 capsid. J Virol. 2004;78:6005–6012. [Europe PMC free article] [Abstract] [Google Scholar]
- Hatziioannou T, Perez-Caballero D, Cowan S, Bieniasz PD. Cyclophilin interactions with incoming human immunodeficiency virus type 1 capsids with opposing effects on infectivity in human cells. J Virol. 2005;79:176–183. [Europe PMC free article] [Abstract] [Google Scholar]
- Hatziioannou T, Princiotta M, Piatak M, Jr., Yuan F, Zhang F, Lifson JD, Bieniasz PD. Generation of simian-tropic HIV-1 by restriction factor evasion. Science. 2006;314:95. [Abstract] [Google Scholar]
- Heeney JL, Dalgleish AG, Weiss RA. Origins of HIV and the evolution of resistance to AIDS. Science. 2006;313:462–466. [Abstract] [Google Scholar]
- Helseth E, Kowalski M, Gabuzda D, Olshevsky U, Haseltine W, Sodroski J. Rapid complementation assays measuring replicative potential of human immunodeficiency virus type 1 envelope glycoprotein mutants. J Virol. 1990;64:2416–2420. [Europe PMC free article] [Abstract] [Google Scholar]
- Hofmann W, Schubert D, LaBonte J, Munson L, Gibson S, Scammell J, Ferrigno P, Sodroski J. Species-specific, postentry barriers to primate immunodeficiency virus infection. J Virol. 1999;73:10020–10028. [Europe PMC free article] [Abstract] [Google Scholar]
- Ikeda Y, Ylinen L, Kahar-Bador M, Towers GJ. The influence of gag on HIV-1 species specific tropism. J Virol. 2004;78:1816–11822. [Europe PMC free article] [Abstract] [Google Scholar]
- Jia B, Serra-Morena R, Neidermyer W, Rahmberg A, Mackey J, Fofana IB, Johnson WE, Westmoreland S, Evans DT. Species-specific activity of SIV Nef and HIV-1 Vpu in overcoming restriction by tetherin/BST2. PLoS Pathog. 2009;5:e1000429. [Europe PMC free article] [Abstract] [Google Scholar]
- Kamada K, Igarashi T, Martin MA, Khamsri B, Hatcho K, Yamashita T, Fujita M, Uchiyama T, Adachi A. Generation of HIV-1 derivatives that productively infect macaque monkey lymphoid cells. Proc Natl Acad Sci U S A. 2006;103:16959–16964. [Europe PMC free article] [Abstract] [Google Scholar]
- Kamada K, Yamashita T, Hatcho K, Adachi A, Nomaguchi M. Evasion from CypA- and APOBEC-mediated restrictions is insufficient for HIV-1 to efficiently grow in simian cells. Microbes Infect. 2009;11:164–171. [Abstract] [Google Scholar]
- Keele BF, Van Heuverswyn F, Li Y, Bailes E, Takehisa J, Santiago ML, Bibollet-Ruche F, Chen Y, Wain LV, Liegeois F, Loul S, Ngole EM, Bienvenue Y, Delaporte E, Brookfield JF, Sharp PM, Shaw GM, Peeters M, Hahn BH. Chimpanzee reservoirs of pandemic and nonpandemic HIV-1. Science. 2006;313:523–526. [Europe PMC free article] [Abstract] [Google Scholar]
- Kolchinsky P, Mirzabekov T, Farzan M, Kiprilov E, Cayabyab M, Mooney LJ, Choe H, Sodroski J. Adaptation of a CCR5-using, primary human immunodeficiency virus type 1 isolate for CD4-independent replication. J Virol. 1999;73:8120–8126. [Europe PMC free article] [Abstract] [Google Scholar]
- Kootstra NA, Munk C, Tonnu N, Landau NR, Verma IM. Abrogation of postentry restriction of HIV-1-based lentiviral vector transduction in simian cells. Proc Natl Acad Sci U S A. 2003;100:1298–1303. [Europe PMC free article] [Abstract] [Google Scholar]
- Kootstra NA, Navis M, Beugeling C, Van Dort KA, Schuitemaker H. The presence of the TRIM5alpha escape mutation H87Q in the capsid of late stage HIV-1 variants is preceded by a prolonged asymptomativ infection phase. AIDS. 2007;21:2015–2023. [Abstract] [Google Scholar]
- Kuiken C, Leitner T, Foley B, Hahn B, Marx P, McCutchan F, Wolinsky S, Korber B. HIV Sequence Compendium 2008. Los Alamos National Laboratory, Theoretical Biology and Biophysics; Los Alamos, New Mexico: 2008. [Google Scholar]
- Lecossier D, Bouchonnet F, Clavel F, Hance AJ. Hypermutation of HIV-1 DNA in the absence of the Vif protein. Science. 2003;300:1112. [Abstract] [Google Scholar]
- Li JT, Halloran M, Lord CI, Watson A, Ranchalis J, Fung M, Letvin NL, Sodroski JG. Persistent infection of macaques with simian-human immunodeficiency viruses. J Virol. 1995;69:7061–7067. [Europe PMC free article] [Abstract] [Google Scholar]
- Li X, Sodroski J. The TRIM5alpha B-box 2 domain promotes cooperative binding to the retroviral capsid by mediating higher-order self-association. J Virol. 2008;82:11495–11502. [Europe PMC free article] [Abstract] [Google Scholar]
- Li Y, Kar AK, Sodroski J. Target cell type-dependent modulation of human immunodeficiency virus type 1 capsid disassembly by cyclophilin A. J Virol. 2009;83:10951–10962. [Europe PMC free article] [Abstract] [Google Scholar]
- Mariani R, Chen D, Schrofelbauer B, Navarro F, Konig R, Bollman B, Munk C, Nymark-McMahon H, Landau NR. Species-specific exclusion of APOBEC3G from HIV-1 virions by Vif. Cell. 2003;114:21–31. [Abstract] [Google Scholar]
- Mbisa JL, Barr R, Thomas JA, Vandegraaff N, Dorweiler IJ, Svarovskaia ES, Brown WL, Mansky LM, Gorelick RJ, Harris RS, Engelman A, Pathak VK. Human immunodeficiency virus type 1 cDNAs produced in the presence of APOBEC3G exhibit defects in plus-strand DNA transfer and integration. J Virol. 2007;81:7099–7110. [Europe PMC free article] [Abstract] [Google Scholar]
- McNatt MW, Zang T, Hatziioannou T, Bartlett M, Fofana IB, Johnson WE, Neil SJ, Bieniasz PD. Species-specific activity of HIV-1 Vpu and positive selection of tetherin transmembrane domain variants. PLoS Pathog. 2009;5:e1000300. [Europe PMC free article] [Abstract] [Google Scholar]
- Munk C, Brandt SM, Lucero G, Landau NR. A dominant block to HIV-1 replication at reverse transcription in simian cells. Proc Natl Acad Sci U S A. 2002;99:13843–13848. [Europe PMC free article] [Abstract] [Google Scholar]
- Nagao T, Hatcho K, Doi N, Fugiwara S, Adachi A, Nomaguchi M. Amino acid alterations in Gag that confer the ability to grow in simian cells on HIV-1 are located at a narrow CA region. J Med Invest. 2009;56:21–25. [Abstract] [Google Scholar]
- Neil SJ, Zang T, Bieniasz PD. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008;451:425–430. [Abstract] [Google Scholar]
- Nepveu-Traversy ME, Berube J, Berthoux L. TRIM5alpha and TRIMCyp form apparent hexamers and their multimeric state is not affected by exposure to restriction-sensitive viruses or by treatment with pharmacological inhibitors. Retrovirology. 2009;6:100. [Europe PMC free article] [Abstract] [Google Scholar]
- Nisole S, Lynch C, Stoye JP, Yap MW. A Trim5-cyclophilin A fusion protein found in owl monkey kidney cells can restrict HIV-1. Proc Natl Acad Sci U S A. 2004;101:13324–13328. [Europe PMC free article] [Abstract] [Google Scholar]
- Owens CM, Song B, Perron MJ, Yang PC, Stremlau M, Sodroski J. Binding and susceptibility to postentry restriction factors in monkey cells are specified by distinct regions of the human immunodeficiency virus type 1 capsid. J Virol. 2004;78:5423–5437. [Europe PMC free article] [Abstract] [Google Scholar]
- Owens CM, Yang PC, Gottlinger H, Sodroski J. Human and simian immunodeficiency virus capsid proteins are major viral determinants of early, postentry replication blocks in simian cells. J Virol. 2003;77:726–731. [Europe PMC free article] [Abstract] [Google Scholar]
- Pacheco B, Basmaciogullari S, Labonte JA, Xiang SH, Sodroski J. Adaptation of the human immunodeficiency virus type 1 envelope glycoproteins to new world monkey receptors. J Virol. 2008;82:346–357. [Europe PMC free article] [Abstract] [Google Scholar]
- Pornillos O, Ganser-Pornillos BK, Kelly BN, Hua Y, Whitby FG, Stout CD, Sundquist WI, Hill CP, Yeager M. X-ray structures of the hexameric building block of the HIV capsid. Cell. 2009;137:1282–1292. [Europe PMC free article] [Abstract] [Google Scholar]
- Rho HM, Poiesz B, Ruscetti FW, Gallo RC. Characterization of the reverse transcriptase from a new retrovirus (HTLV) produced by a human cutaneous T-cell lymphoma cell line. Virology. 1981;112:355–360. [Abstract] [Google Scholar]
- Sawyer SL, Emerman M, Malik HS. Discordant evolution of the adjacent antiretroviral genes TRIM22 and TRIM5 in mammals. PLoS Pathog. 2007;3:e197. [Europe PMC free article] [Abstract] [Google Scholar]
- Sayah DM, Sokolskaja E, Berthoux L, Luban J. Cyclophilin A retrotransposition into TRIM5 explains owl monkey resistance to HIV-1. Nature. 2004;430:569–573. [Abstract] [Google Scholar]
- Schrofelbauer B, Senger T, Manning G, Landau NR. Mutational alteration of human immunodeficiency virus type 1 Vif allows for functional interaction with nonhuman primate APOBEC3G. J Virol. 2006;80:5984–5991. [Europe PMC free article] [Abstract] [Google Scholar]
- Sebastian S, Luban J. TRIM5alpha selectively binds a restriction-sensitive retroviral capsid. Retrovirology. 2005;2:40. [Europe PMC free article] [Abstract] [Google Scholar]
- Sharp PM, Shaw GM, Hahn BH. Simian immunodeficiency virus infection of chimpanzees. J Virol. 2005;79:3891–3902. [Europe PMC free article] [Abstract] [Google Scholar]
- Shi J, Aiken C. Saturation of TRIM5 alpha-mediated restriction of HIV-1 infection depends on the stability of the incoming viral capsid. Virology. 2006;350:493–500. [Abstract] [Google Scholar]
- Stremlau M, Owens CM, Perron MJ, Kiessling M, Autissier P, Sodroski J. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature. 2004;427:848–853. [Abstract] [Google Scholar]
- Stremlau M, Perron M, Lee M, Li Y, Song B, Javanbakht H, Diaz-Griffero F, Anderson DJ, Sundquist WI, Sodroski J. Specific recognition and accelerated uncoating of retroviral capsids by the TRIM5alpha restriction factor. Proc Natl Acad Sci U S A. 2006a;103:5514–5519. [Europe PMC free article] [Abstract] [Google Scholar]
- Stremlau M, Song B, Javanbakht H, Perron M, Sodroski J. Cyclophilin A: an auxiliary but not necessary cofactor for TRIM5alpha restriction of HIV-1. Virology. 2006b;351:112–120. [Abstract] [Google Scholar]
- Towers GJ, Hatziioannou T, Cowan S, Goff SP, Luban J, Bieniasz PD. Cyclophilin A modulates the sensitivity of HIV-1 to host restriction factors. Nat Med. 2003;9:1138–1143. [Abstract] [Google Scholar]
- Van Damme N, Goff D, Katsura C, Jorgenson RL, Mitchell R, Johnson MC, Stephens EB, Guatelli J. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe. 2008;3:245–252. [Europe PMC free article] [Abstract] [Google Scholar]
- Van Heuverswyn F, Li Y, Neel C, Bailes E, Keele BF, Liu W, Loul S, Butel C, Liegeois F, Bienvenue Y, Ngolle EM, Sharp PM, Shaw GM, Delaporte E, Hahn BH, Peeters M. Human immunodeficiency viruses: SIV infection in wild gorillas. Nature. 2006;444:164. [Abstract] [Google Scholar]
- Van Heuverswyn F, Peeters M. The origins of HIV and implications for the global epidemic. Curr Infect Dis Rep. 2007;9:338–346. [Abstract] [Google Scholar]
- Wilson SJ, Webb BL, Ylinen LM, Verschoor E, Heeney JL, Towers GJ. Independent evolution of an antiviral TRIMCyp in rhesus macaques. Proc Natl Acad Sci U S A. 2008;105:3557–3562. [Europe PMC free article] [Abstract] [Google Scholar]
- Yu X, Yu Y, Liu B, Luo K, Kong W, Mao P, Yu XF. Induction of APOBEC3G ubiquitination and degradation by an HIV-1 Vif-Cul5-SCF complex. Science. 2003;302:1056–1060. [Abstract] [Google Scholar]
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/116988503
Article citations
A nanobody interaction with SARS-COV-2 Spike allows the versatile targeting of lentivirus vectors.
J Virol, 98(9):e0079524, 29 Aug 2024
Cited by: 0 articles | PMID: 39207135
Generation of a bovine cell line for gene engineering using an HIV-1-based lentiviral vector.
Sci Rep, 12(1):16952, 18 Oct 2022
Cited by: 3 articles | PMID: 36258028 | PMCID: PMC9579131
HIV-1 capsid variability: viral exploitation and evasion of capsid-binding molecules.
Retrovirology, 18(1):32, 26 Oct 2021
Cited by: 14 articles | PMID: 34702294 | PMCID: PMC8549334
Review Free full text in Europe PMC
TRIM7 inhibits enterovirus replication and promotes emergence of a viral variant with increased pathogenicity.
Cell, 184(13):3410-3425.e17, 31 May 2021
Cited by: 36 articles | PMID: 34062120 | PMCID: PMC8276836
Restriction of HIV-1 and other retroviruses by TRIM5.
Nat Rev Microbiol, 17(9):546-556, 16 Jul 2019
Cited by: 77 articles | PMID: 31312031 | PMCID: PMC6858284
Review Free full text in Europe PMC
Go to all (26) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The Three-Fold Axis of the HIV-1 Capsid Lattice Is the Species-Specific Binding Interface for TRIM5α.
J Virol, 92(5):e01541-17, 12 Feb 2018
Cited by: 18 articles | PMID: 29237846 | PMCID: PMC5809731
Species-specific variation in the B30.2(SPRY) domain of TRIM5alpha determines the potency of human immunodeficiency virus restriction.
J Virol, 79(5):3139-3145, 01 Mar 2005
Cited by: 286 articles | PMID: 15709033 | PMCID: PMC548447
General Model for Retroviral Capsid Pattern Recognition by TRIM5 Proteins.
J Virol, 92(4):e01563-17, 30 Jan 2018
Cited by: 15 articles | PMID: 29187540 | PMCID: PMC5790955
Funding
Funders who supported this work.
Center for AIDS Research Award (1)
Grant ID: AI060354
NIAID NIH HHS (6)
Grant ID: AI060354
Grant ID: P30 AI060354-04
Grant ID: R01 AI063987-05
Grant ID: P30 AI060354
Grant ID: AI063987
Grant ID: R01 AI063987
National Institutes of Health (1)
Grant ID: AI063987



