Abstract
Free full text

Placental Apoptosis in Health and Disease
Abstract
Apoptosis, programmed cell death, is an essential feature of normal placental development but is exaggerated in association with placental disease. Placental development relies upon effective implantation and invasion of the maternal decidua by the placental trophoblast. In normal pregnancy, trophoblast apoptosis increases with placental growth and advancing gestation. However, apoptosis is notably exaggerated in the pregnancy complications, hydatidiform mole, pre-eclampsia, and intra-uterine growth restriction (IUGR). Placental apoptosis may be initiated by a variety of stimuli, including hypoxia and oxidative stress. In common with other cell-types, trophoblast apoptosis follows the extrinsic or intrinsic pathways culminating in the activation of caspases. In contrast, the formation of apoptotic bodies is less clearly identified, but postulated by some to involve the clustering of apoptotic nuclei and liberation of this material into the maternal circulation. In addition to promoting a favorable maternal immune response, the release of this placental-derived material is thought to provoke the endothelial dysfunction of pre-eclampsia. Widespread apoptosis of the syncytiotrophoblast may also impair trophoblast function leading to the reduction in nutrient transport seen in IUGR. A clearer understanding of placental apoptosis and its regulation may provide new insights into placental pathologies, potentially suggesting therapeutic targets.
Introduction
Pre-eclampsia and intra-uterine growth restriction (IUGR) are major pregnancy complications, resulting in significant perinatal mortality and morbidity. While their precise etiology is unknown, it is hypothesized that placental dysfunction is central to their development. A common feature of the placenta in pre-eclampsia, IUGR and molar pregnancies, i.e. hydatidiform mole (trophoblast hyperplasia), is exaggerated placental apoptosis. As a result of these observations, apoptosis is suggested to be a key mechanism in placental dysfunction. This review describes the evidence for the presence of apoptosis in normal placental development, its alteration in placental dysfunction and development of placental diseases, such as pre-eclampsia.
Apoptosis
Apoptosis is evident in both physiological and pathological circumstances and was first described by Kerr and Wylie in 1972.1 Apoptosis (Greek: apo – from, ptosis – falling) is a form of programmed cell death characterized by the condensation of cell cytoplasm and organelles into membrane covered dense apoptotic bodies. During nuclear condensation, the nuclear lamina is dissembled, allowing the cleavage of DNA into 200 base pair fragments.2,3 Moreover, the cell membrane undergoes extensive alterations with loss of asymmetry, externalizing phosphatidylserine, promoting phagocytosis.4 In contrast to necrotic cell death, apoptosis represents a series of energy-dependent events, removing unwanted cell material while avoiding an immune response and damage to surrounding tissues. Apoptosis is initiated via the extrinsic or intrinsic pathway. Both pathways rely upon a cascade of protein interactions orchestrated by a family of 14 cysteine proteases, caspases, which are able to cleave structural proteins producing the morphological appearances typical of apoptosis. In addition, active caspases potentiate the apoptotic signal by activating a variety of pro-apoptotic proteins.
The extrinsic pathway is controlled by members of the tumour necrosis factor (TNF) death receptor family. There are eight members of this family with Fas (CD95/APO-1), TNF-R1 (CD120a), and TNF-related apoptosis inducing ligand (TRAIL), being the most studied.5 Binding of an external ligand to the death receptor allows protein–protein interactions between the receptor and a cytoplasmic death domain, such as Fas-associated death domain (FADD) or TNF-R-associated death domain.6 Binding of FADD to the death receptor recruits procaspase-8 or procaspase-10 via death effector domains.7–9 The combination of these proteins forms the death-inducing signaling complex which cleaves procaspase-8 and procaspase-10 to their active forms, initiating the caspase cascade.10 Sometimes this signal is further amplified by cleavage of Bid by caspase 8, which also activates the intrinsic pathway.11
The intrinsic pathway is initiated by cellular stress; such as DNA damage, reactive oxygen species, the unfolded protein response, or removal of growth factor support. Activation of the intrinsic pathway leads to alteration in mitochondrial membrane permeability because of an imbalance in the relationship of pro- and anti-apoptotic Bcl-2 proteins.12 Increased mitochondrial permeability causes membrane pore formation and leakage of cytochrome c into the cytosol.13 In the cytosol, cytochrome c is bound by apoptosis protease activating factor-1 (APAF-1) forming the apoptosome.14 The apoptosome cleaves procaspase-9 activating the terminal pathway of apoptosis. During apoptosis, other mitochondrial contents such as smac/Diablo are also released from the mitochondria, antagonizing anti-apoptotic inhibitor of apoptosis proteins.15,16 Both pathways culminate in a terminal pathway involving the cleavage and activation of caspase-3, 6, and 7 initiating cell destruction by activating DNAses and cleaving DNA repair enzymes such as PARP.17,18
Apoptotic cells can be identified in a variety of ways. The initial description of cell morphology was based upon observations of ultrastructure by electron microscopy including increased nuclear cytoplasmic ratio, cytoplasmic condensation, and deposition of euchromatin around the nuclear periphery. Subsequently, some of these changes in cell morphology particularly increased nuclear/cytoplasmic ratio have been observed by light microscopy. 19 Biochemical measures have also been utilized to confirm the presence of cleaved DNA into 200 bp fragments by the presence of DNA ladders (Fig. 1).20 More recently, combined histological and biochemical approaches have led to the development of staining techniques that recognize cleaved DNA by terminal deoxy-uridine nick-end labeling (TUNEL), 21 by externalization of phosphatidylserine or by cytoskeletal cleavage products indicative of caspase activity e.g. cytokeratin M30 neoepitope22 (Fig. 2). Often studies will combine multiple measures to ensure apoptotic specificity.
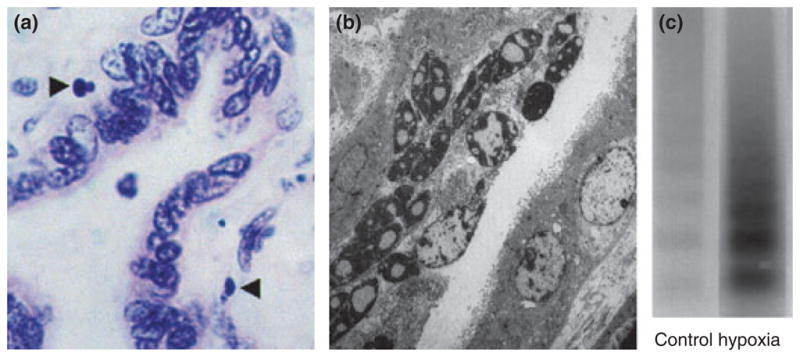
Apoptosis in the villous placenta. (a) Light micrograph (oil immersion, H & E stained) of 4 μm section of term villous placenta. Apoptotic nuclei of trophoblast highlighted (arrows). Magnification × 1000. (b) Electron micrograph showing pyknotic and apoptotic nuclei within the syncytiotrophoblast (original magnification × 4250). Smith et al. 1997, reproduced with permission. (c) Increased DNA fragmentation (laddering) in term trophoblast under hypoxic conditions. Reproduced from Levy et al. 2000 with permission.
Apoptosis and implantation
Normal placental development relies upon the invasion of the maternal decidua by extravillous trophoblast, and the subsequent remodeling of maternal spiral arteries to provide stability to the placenta and efficient utero-placental blood flow. Extravillous trophoblast invades the maternal decidua as far as the proximal third of the myometrium, with a greater depth observed centrally.23 A subgroup of extravillous trophoblast invades the maternal spiral arteries replacing the endothelium and remodeling the maternal vasculature. Initially this process is trophoblast independent,24 although eventually requiring trophoblast for completion.25 Trophoblast disrupts the endothelium causing the loss of underlying smooth muscle allowing further trophoblast invasion of the decidua.26 Recently, it has been suggested that vascular remodeling may be indirectly controlled by intravascular trophoblast that stimulates endothelial cells to secrete chemokines. These chemokines attract decidual leukocytes, particularly uterine natural killer cells and macrophages, leading to vascular smooth muscle cell apoptosis.27 A suggested mechanism for endothelial cell destruction is via the Fas/FasL system, which is present on endothelial and vascular smooth muscle cells of the uterine spiral arteries.25
The remodeling of maternal spiral arteries is again not uniform throughout the decidua, with a higher incidence in the center region compared to the periphery.23,28 In addition to remodeling, trophoblast has been suggested to block maternal vessels allowing the fetus to develop initially in a hypoxic environment, protected from reactive oxygen species until around 10 weeks gestation, when placental blood flow is restored.29
In pre-eclampsia and IUGR, there may be a reduction in the number of trophoblast cells within the spiral arteries, which has been associated with increased apoptosis and a reduced luminal size.30,31 Others have found a reduction in the extent of trophoblast invasion in severe pre-eclampsia, both in the spiral arteries and the myometrium.32 Poor trophoblast invasion and remodeling of uterine spiral arteries have been suggested to lead to the development of a high pressure placental blood supply, which may in turn damage the developing villous tree causing a change in placental structure.33,34 It has been suggested that this damage leads to hypoxia and impaired blood flow, as determined by aberrant Doppler ultrasound waveforms in IUGR and severe pre-eclampsia.35
Apoptosis in placental villi
After 10 weeks gestation, the human villous placenta develops within a lake of maternal blood, with the tree-like structure becoming progressively more branched and convoluted to form the terminal villi. These terminal villi consist of stroma, containing fetal capillaries, beneath a layer of progenitor cytotrophoblast cells, which are in turn covered by a continuous multinucleated syncytium called the syncytiotrophoblast. The syncytiotrophoblast forms a barrier between the fetal and maternal circulations and is essential for the normal immunological, endocrine, and nutritional functions of the placenta. Fusion between the cytotrophoblasts and the syncytiotrophoblast has been suggested as a means of replenishing the syncytiotrophoblast, although the exact physiological function is unknown. Fusion of the villous cytotrophoblast with the syncytiotrophoblast is associated with the presence of GCM-1,36 Syncytin-1 & -237 and caspase-8.38
The amount of apoptosis in placental villi changes throughout normal pregnancy, being lowest in the first trimester, increasing in the third, and markedly accelerating beyond 40 weeks gestation.39,40 Interest has been stimulated by the observation that increased levels of villous trophoblast apoptosis have been identified in placental pathologies (Fig. 3), including early pregnancy loss,41,42 pre-eclampsia,43–46 IUGR,45,47–49 and gestational trophoblastic disease, including partial and complete hydatidiform mole and choriocarcinoma.50,51 Systemic disease can also impact upon the human placenta, as maternal diabetes is similarly associated with increased placental apoptosis.52 Moreover, the finding of increased apoptotic maternal is not confined to the placenta. In pre-eclampsia, increased levels of syncytial derived cytokeratin M30 neoepitope can be detected in maternal serum.53
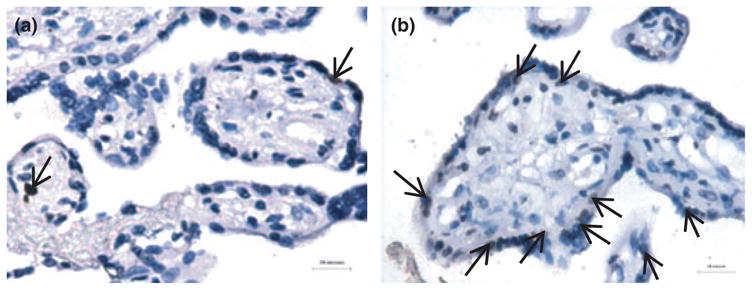
Exaggerated trophoblast apoptosis in pre-eclampsia. (a) occasional TUNEL positive nuclei (arrows) in the syncytiotrophoblast in normal pregnancy. (b) increased apoptotic events in pre-eclampsia (brown stained nuclei). Reproduced from Heazell et al. 2008 with permission.
Apoptosis within the villus is predominantly localized to the syncytiotrophoblast with virtually no events within term cytotrophoblast and a very low incidence in first trimester cytotrophoblast.19,54–56 In support, the syncytiotrophoblast demonstrates features consistent with apoptosis, such as externalization of phosphatidylserine, caspase-8 and caspase-9 activation, cytokeratin-18 cleavage, and DNAse activity.38,56–58 Importantly, apoptotic pathways may be involved in the maintenance of the syncytiotrophoblast. This stems from two observations, first that caspase 8 is involved in the fusion of cytotrophoblast with syncytiotrophoblast, and second that some syncytiotrophoblast nuclei exhibit morphological features of apoptosis with peripheral chromatin condensation and gradual pyknosis.
Within the syncytiotrophoblast, syncytial knots are aggregations of pyknotic nuclei which accumulate at the syncytial surface before being lost into the maternal circulation as membrane bound entities. The presence of such material has been identified within the maternal venous circulation prior to destruction by pulmonary macrophages.59,60 It is the release of this apoptotic material into the maternal circulation that has been suggested as a mechanism for maternal endothelial disruption in pre-eclampsia.61 However, the theory that syncytial knots consist of truly apoptotic nuclei has recently been challenged.62 Nevertheless, apoptosis occurs in discrete areas within the syncytiotrophoblast, particularly those associated with damage e.g. fibrin deposition. This is unexpected as apoptosis in other organs and tissues is confined to individual cells,63 and an apoptotic signal would be expected to spread throughout the syncytiotrophoblast.
The origin of exaggerated apoptosis in pregnancy complications is not clear. Although the extrinsic pathway is active in trophoblast,64,65 the in vivo association with abnormal conversion of the uterine spiral arteries, more readily implicates hypoxia and/or oxidative stress. In support, exaggerated apoptosis can be reproduced in trophoblast in vitro by exposure to hypoxia66 and reactive oxygen species.67 Interestingly, villous trophoblast from placentas of pregnancies complicated by IUGR or pre-eclampsia demonstrate increased susceptibility to apoptosis, an inherent change rendering these cells more vulnerable to oxidative damage.43–45,47,48 In molar pregnancies, increased apoptosis is thought to reflect the cell fate of some trophoblast resulting from uncontrolled hyperplasia. This is confirmed by greater levels of apoptosis in more invasive and proliferative disease.50
The interplay of pro and anti-apoptotic regulators is crucial for the control of apoptosis and the expression of these in villous trophoblast has been the subject of significant study (reviewed in detail by Heazell and Crocker, 2008).68 It is well recognized that both cytotrophoblast and syncytiotrophoblast express TNF receptors, Fas and Fas ligand, and TRAIL69,70 and its death and decoy receptors, each may play an important role, not only in apoptosis but also in immune regulation (comprehensively reviewed by Straszewski-Chavez et al., 2005). Other important regulators of cell fate include proteins such as the transcription factor p53 and members of the Bcl-2 family. p53 has been identified in cytotrophoblasts but is only rarely observed in the syncytiotrophoblast.71,72 Placental p53 is present in a non-mutated, wild-type form in both normal tissue and gestational trophoblast disease.73,74 By contrast, the natural inhibitor of p53, Mdm2, is expressed within both the cytotrophoblast and syncytiotrophoblast in the first trimester and at reduced levels in third-trimester tissue.74–76 A variety of p53 target proteins are also located in the villous placenta including the cell cycle regulator p21, which is strongly expressed in first trimester cytotrophoblasts and more weakly expressed in the syncytiotrophoblast and third trimester tissue.71,75,77
The regulation of apoptosis in placental disease
In general, more dramatic morphological and developmental changes occur in the villous placenta in cases of IUGR and early-onset pre-eclampsia, i.e. before 32 weeks gestation. These changes predominantly relate to impoverished villus development and reduced capillary growth.78 In pre-eclampsia, apoptosis has been associated with a reduction in syncytiotrophoblast, a response not seen in idiopathic IUGR,79 and an observation supportive of irregular syncytiotrophoblast formation or excessive syncytial loss.
Alterations in a variety of pro- and anti-apoptotic proteins have been observed in pre-eclampsia and IUGR (Table 1). For example, p5380 and the pro-apoptotic isoform of Mcl-181 are increased in pre-eclampsia and Bcl-245 and syncytin decreased.81 Others have found p53 to be increased in severe pre-eclampsia with HELLP syndrome.82 In IUGR, the pattern is similar with increased staining observed for p53,47 caspase-3,48 and p21.83 However, not all proteins are affected equally with most observers finding no effect upon Bax43,47 and conflicting results reported for Bcl-2.45,47
Table I
Selected Studies of Apoptosis in Spiral Artery Remodeling and in the Placental Pathologies, Pre-eclampsia, and Intrauterine Growth Restriction (IUGR)
| Author | Year | Tissue | Method | Main findings |
|---|---|---|---|---|
| IUGR | ||||
 Smith et al. Smith et al. | 1997 | Villous TB | IHC, EM | TUNEL staining demonstrates an increase in apoptosis in IUGR |
 Ishihara et al. Ishihara et al. | 2002 | Villous TB | IHC, EM | Increased TUNEL-positive nuclei in pre-eclampsia in both ST and CT. BcL-2 reduced in severe pre-eclampsia and IUGR. No difference in Fas |
 Levy et al. Levy et al. | 2002 | Villous TB | IHC | Increased TUNEL-positive nuclei in IUGR. Increased p53 in CT of IUGR but no difference in BcL-2 family proteins |
 Crocker et al. Crocker et al. | 2003 | Villous TB | IHC | Enhanced apoptosis in IUGR and pre-eclampsia after treatment with 3%O2 or TNFa |
 Crocker et al. Crocker et al. | 2004 | Villous TB | IHC | Increased apoptosis in placentas from women with pre-eclampsia or IUGR exposed to TNFa or 3%O2 |
 Daayana et al. Daayana et al. | 2004 | Villous TB | Microscopy | Reduced syncytial area in IUGR. Reduction in syncytial/villous area ratio in pre-eclampsia but not in IUGR |
 Endo et al. Endo et al. | 2005 | Villous TB | IHC, EM | TUNEL staining and activated caspase-3 showed increased apoptosis in IUGR vs normal. No difference in p53 or Bax |
 Kadyrov et al. Kadyrov et al. | 2006 | EVT | IHC | Severely impaired trophoblast invasion in pre-eclampsia and IUGR. Increased EVT apoptosis |
 Davy et al. Davy et al. | 2009 | Villous TB | Southern analysis | Increase in cell senescence regulators p21, p16, and EF-1 alpha in FGR placentas |
| Pre-eclampsia | ||||
 Difederico et al. Difederico et al. | 1999 | EVT | IHC | 15–50% EVT apoptosis in pre-eclampsia, virtually zero in control. Lack of Bcl-2 staining in preeclamptic EVT |
 Allaire et al. Allaire et al. | 2000 | Villous TB | IHC | Increased TUNEL-positive nuclei, increased Fas and reduced FasL in the villous TB of preeclamptic patients vs controls |
 Leung et al. Leung et al. | 2001 | Villous TB | EM, microscopy | Increased apoptosis in placentas from women with pre-eclampsia |
 Ishihara et al. Ishihara et al. | 2002 | Villous TB | IHC, EM | Increased TUNEL-positive nuclei in pre-eclampsia in both ST and CT. BcL-2 reduced in severe pre-eclampsia and IUGR. No difference in Fas |
 Crocker et al. Crocker et al. | 2003 | Villous TB | IHC | Enhanced apoptosis in IUGR and pre-eclampsia after treatment with 3%O2 or TNFa |
 Crocker et al. Crocker et al. | 2004 | Villous TB | IHC | Increased apoptosis in placentas from women with pre-eclampsia or IUGR exposed to TNFa or 3%O2 |
 Daayana et al. Daayana et al. | 2004 | Villous TB | Microscopy | Reduced syncytial area in IUGR. Reduction in syncytial/villous area ratio in pre-eclampsia but not in IUGR |
 Heazell et al. Heazell et al. | 2005 | Villous TB | IHC | Increased expression of p53 in ST nuclei and ST cytoplasm in placentas from women with pre-eclampsia. Reduction in Mdm2 in pre-eclampsia |
 Jeschke et al. Jeschke et al. | 2006 | Villous TB | IHC, IF | p53 and ki67 elevated in HELLP syndrome but not in pre-eclampsia. p53 reduced in CT from IUGR placentas, no effect upon proliferation |
 Kadyrov et al. Kadyrov et al. | 2006 | EVT | IHC | Severely impaired trophoblast invasion in pre-eclampsia and IUGR. Increased EVT apoptosis |
 De Falco et al. De Falco et al. | 2007 | Villous TB | IHC | p21 is expressed by CT and ST in pre-eclampsia |
 Cobellis et al. Cobellis et al. | 2007 | Villous TB | IHC | Increased Bax expression in miscarriage vs termination. Reduced Bax in Cesarean section vs normal birth. Also increased Bax in pre-eclampsia |
| Vascular remodelling | ||||
 Craven et al. Craven et al. | 1998 | EVT | IHC | Initial spiral artery changes, such as VCAM-1 expression and smooth muscle disruption are independent of trophoblast. |
 Difederico et al. Difederico et al. | 1999 | EVT | IHC | 15–50% EVT apoptosis in pre-eclampsia, virtually zero in control. Lack of Bcl-2 staining in preeclamptic EVT |
 Dunk et al. Dunk et al. | 2003 | EVT | IHC | EVTs penetrate the decidua and stimulate endothelial and smooth muscle disruption. Not seen in vessels cultured in the absence of EVT |
 Ashton et al. Ashton et al. | 2005 | EVT | IHC, WB | Endothelial cells and VSMC express Fas and FasL. Trophoblast induced apoptosis in cultured endothelial cells |
 Kadyrov et al. Kadyrov et al. | 2006 | EVT | IHC | Severely impaired trophoblast invasion in pre-eclampsia and IUGR. Increased EVT apoptosis |
 Smith et al. Smith et al. | 2009 | EVT | IHC | 4-stage model of trophoblast remodelling of spiral arteries. Transient role for uNK cells and macrophages in VSMC apoptosis |
ST, syncytiotrophoblast; CT, cytotrophoblast; EVT, extravillous trophoblast; FGR, fetal growth restricted; TB, trophoblast; VSMC, vascular smooth muscle cells; uNK, uterine natural killer.
Apoptosis and the maternal vasculature
Pre-eclampsia and IUGR are associated not only with apoptosis but also with excessive syncytial knot formation. With 10–30% of normal terminal villi containing knots compared to virtually all terminal villi in pre-eclampsia.62,84,85 Small microparticles of syncytiotrophoblast microvillous membrane (STBMs), are found in the maternal circulation from the second trimester and in increasing amounts with gestation and are thought to represent apoptotic material released as part of normal syncytiotrophoblast turnover. 60 Exaggerated levels of this material are associated with pre-eclampsia, but not IUGR.86 This reconciles with greater syncytiotrophoblast loss and damage.60,87,88 This finding is reproducible under hypoxic conditions in vitro, further supporting hypoxia and reactive oxygen species as the underlying pathophysiological trigger in the formation of syncytial debris.89,90 Other smaller fragments, including cell-free fetal DNA, are also released and may also be associated with severe pre-eclampsia.91
Syncytial debris and systemic inflammatory response
The presence of STBMs, and newly identified nano-particles 92 within the maternal circulation are associated with alterations in immunological response, specifically neutrophil activation93,94 and the release of superoxide radicals.95 Neutrophil activation may be further exaggerated in pre-eclampsia as shown by an increased incidence of DNA lattices (NETS) in placentae from these pregnancies.94 The culture of syncytiotrophoblast-derived particles with non-pregnant peripheral blood monocytes stimulates the release of the cytokines, TNF-alpha, IL-1beta, IL-6, IL-8, IL12p70, IL-18, with an additional increase in adhesion molecule CD54.96,97 These findings suggest a systemic inflammatory response in pregnancy, one further exaggerated in pre-eclampsia. In support, maternal levels of monocyte derived IL-1β, IL-6, and IL-8 are increased in pre-eclampsia.98 Furthermore, STBMs are able to disrupt endothelial cells in vitro, again promoting a link between placental apoptosis, syncytial microparticle liberation and the maternal vascular complications, characteristic of the pre-eclamptic syndrome.99,100
Conclusion
In conclusion, apoptosis is a feature of villous trophoblast throughout pregnancy and is an essential feature of placental invasion, cytotrophoblast fusion, and syncytiotrophoblast function as well as potentially playing a role in maternal immune tolerance. This process is not uncontrolled or haphazard in nature. In fact, the many key proteins and cytokines involved in presenting foreign material to the placenta and controlling the response of this tissue to external stimuli have only been partially explored. We do know that alterations to placental function by external factors such as hypoxia and reactive oxygen species can lead to significant increases in placental apoptosis, which may be the underlying cause in the pathophysiology of pre-eclampsia and IUGR. A growing body of evidence also suggests that abnormal placental apoptosis has effects beyond the placenta upon maternal vascular endothelial behavior and immune tolerance. Our understanding of the role of key protein pathways involved in regulating placental apoptosis is constantly expanding and it is at this mechanistic level the future therapeutic strategies may be derived.
Acknowledgments
Andrew Sharp is funded by a research training fellowship from The Wellcome Trust
References
Full text links
Read article at publisher's site: https://doi.org/10.1111/j.1600-0897.2010.00837.x
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3025811?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1111/j.1600-0897.2010.00837.x
Article citations
Impact of Ionizing Radiation Exposure on Placental Function and Implications for Fetal Programming.
Int J Mol Sci, 25(18):9862, 12 Sep 2024
Cited by: 0 articles | PMID: 39337351 | PMCID: PMC11432287
Review Free full text in Europe PMC
Antioxidant Defenses, Oxidative Stress Responses, and Apoptosis Modulation in Spontaneous Abortion: An Immunohistochemistry Analysis of First-Trimester Chorionic Villi.
Life (Basel), 14(9):1074, 28 Aug 2024
Cited by: 0 articles | PMID: 39337859 | PMCID: PMC11432807
Impact of the Immunomodulatory Factor Soluble B7-H4 in the Progress of Preeclampsia by Inhibiting Essential Functions of Extravillous Trophoblast Cells.
Cells, 13(16):1372, 17 Aug 2024
Cited by: 1 article | PMID: 39195262 | PMCID: PMC11352994
Studying the O-GlcNAcome of human placentas using banked tissue samples.
Glycobiology, 34(4):cwae005, 01 Apr 2024
Cited by: 0 articles | PMID: 38253038
Melatonin: the placental antioxidant and anti-inflammatory.
Front Immunol, 15:1339304, 01 Feb 2024
Cited by: 7 articles | PMID: 38361952 | PMCID: PMC10867115
Review Free full text in Europe PMC
Go to all (159) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Apoptotic and non-apoptotic roles of caspases in placenta physiology and pathology.
Placenta, 151:37-47, 30 Mar 2024
Cited by: 0 articles | PMID: 38703713
Review
An integrated model of preeclampsia: a multifaceted syndrome of the maternal cardiovascular-placental-fetal array.
Am J Obstet Gynecol, 226(2s):S963-S972, 09 Mar 2021
Cited by: 18 articles | PMID: 33712272
Review
Cell death mechanisms and their roles in pregnancy related disorders.
Adv Protein Chem Struct Biol, 126:195-225, 27 Feb 2021
Cited by: 15 articles | PMID: 34090615
Review
Apoptosis in the trophoblast--role of apoptosis in placental morphogenesis.
J Soc Gynecol Investig, 11(6):353-362, 01 Sep 2004
Cited by: 151 articles | PMID: 15350247
Review
Funding
Funders who supported this work.
NICHD NIH HHS (2)
Grant ID: P01 HD054713
Grant ID: P01 HD054713-03






