Abstract
Unlabelled
Whether or not cholangiocytes or their hepatic progenitors undergo an epithelial-to-mesenchymal transition (EMT) to become matrix-producing myofibroblasts during biliary fibrosis is a significant ongoing controversy. To assess whether EMT is active during biliary fibrosis, we used Alfp-Cre × Rosa26-YFP mice, in which the epithelial cells of the liver (hepatocytes, cholangiocytes, and their bipotential progenitors) are heritably labeled at high efficiency with yellow fluorescent protein (YFP). Primary cholangiocytes isolated from our reporter strain were able to undergo EMT in vitro when treated with transforming growth factor-β1 alone or in combination with tumor necrosis factor-α, as indicated by adoption of fibroblastoid morphology, intracellular relocalization of E-cadherin, and expression of α-smooth muscle actin (α-SMA). To determine whether EMT occurs in vivo, we induced liver fibrosis in Alfp-Cre × Rosa26-YFP mice using the bile duct ligation (BDL) (2, 4, and 8 weeks), carbon tetrachloride (CCl(4) ) (3 weeks), and 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC; 2 and 3 weeks) models. In no case did we find evidence of colocalization of YFP with the mesenchymal markers S100A4, vimentin, α-SMA, or procollagen 1α2, although these proteins were abundant in the peribiliary regions.Conclusion
Hepatocytes and cholangiocytes do not undergo EMT in murine models of hepatic fibrosis.Free full text

Lineage tracing demonstrates no evidence of cholangiocyte epithelial-to-mesenchymal transition in murine models of hepatic fibrosis
Abstract
Whether or not cholangiocytes or their hepatic progenitors undergo an epithelial-to-mesenchymal transition (EMT) to become matrix-producing myofibroblasts during biliary fibrosis is a significant ongoing controversy. To assess whether EMT is active during biliary fibrosis, we used Alfp-Cre x Rosa26-YFP mice, in which the epithelial cells of the liver (hepatocytes, cholangiocytes, and their bipotential progenitors) are heritably labeled at high efficiency with yellow fluorescent protein (YFP). Primary cholangiocytes isolated from our reporter strain were able to undergo EMT in vitro when treated with transforming growth factor-β1 alone or in combination with tumor necrosis factor-α, as indicated by adoption of fibroblastoid morphology, intracellular relocalization of E-cadherin, and expression of α-smooth muscle actin (α-SMA). To determine whether EMT occurs in vivo, we induced liver fibrosis in Alfp-Cre x Rosa26-YFP mice using the bile duct ligation (BDL; 2, 4, and 8 weeks), carbon tetrachloride (CCl4, 3 weeks), and 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC; 2 and 3 weeks) models. In no case did we find evidence of co-localization of YFP with the mesenchymal markers S100A4, vimentin, α-SMA, or pro-collagen 1α2, although these proteins were abundant in the peribiliary regions.
Conclusion
Hepatocytes and cholangiocytes do not undergo EMT in murine models of hepatic fibrosis.
Liver fibrosis results from the deposition of excessive, abnormal extracellular matrix (ECM) by cells that differentiate into fibrogenic myofibroblasts during chronic liver injury. The biliary fibrosis subtype, which arises from persistent cholangiocyte injury, develops primarily in the portal/periportal regions and is characterized by a ductular reaction (a duct-like proliferation of bipotential progenitor cells, also called oval cells). Unlike other forms of fibrosis in which hepatic stellate cells are the dominant source of myofibroblasts, portal fibroblasts are a major myofibroblast precursor population in biliary fibrosis.1,2
A significant ongoing controversy is whether hepatic epithelial cells that undergo an epithelial-to-mesenchymal transition (EMT) represent another candidate myofibroblast precursor pool.3–10 EMT describes the phenomenon whereby epithelial cells adopt the structural and functional characteristics of mesenchymal cells with the acquisition of motility, loss of cell-cell contacts, development of a flat, spindle-like shape, downregulation of epithelial markers such as E-cadherin and keratins, and gain of mesenchymal markers such as vimentin and fibronectin. Substantial experimental evidence supports the occurrence of EMT in embryonic development and tumor metastasis, processes in which the motility phenotype of the transitioned cells is essential.11–13 For tissue fibrosis, however, there are conflicting data on whether or not EMT occurs.
Evidence favoring hepatocyte EMT primarily comes from cell culture studies, although an in vivo lineage tracing study also suggested that hepatocytes in mouse models of fibrosis express the putative EMT marker S100A4 (fibroblast-specific protein 1 [FSP1]).14 Evidence favoring biliary EMT, in contrast, comes largely from immunohistochemical studies of fibrotic human and rodent livers that identified cholangiocytes co-expressing epithelial markers (especially the cholangiocyte marker keratin 19 [K19]) and mesenchymal markers (i.e. S100A4, vimentin, and heat shock protein 47 [HSP47]).3–7,14 Notably, few of these studies reported co-expression of cholangiocyte markers with the definitive myofibroblast marker α-smooth muscle actin (α-SMA), and none demonstrated collagen deposition by cholangiocytes or their derivatives.
Some studies have proposed that EMT leads to myofibroblast accumulation through a two-stage process. In the first stage, epithelial cells adopt a mesenchymal phenotype, while in the second stage, these mesenchymal cells further transition to myofibroblasts as part of what has been termed an epithelial-to-myofibroblast transition (EMyT).15–17 Although not stated as such in the literature, the debate in liver fibrosis has focused largely on whether epithelial cells undergo EMyT, thereby contributing to the population of fibrogenic myofibroblasts.8–10,15,18
Definitive evaluation of EMT requires the use of lineage tracing technology, whereby epithelial cells are irreversibly tagged with a heritable label that persists regardless of the presence or absence of epithelial markers. (For clarity, the term EMT will be used throughout to refer collectively to both EMT and EMyT). A recent lineage tracing study in which β-galactosidase was expressed under the control of the hepatocyte marker albumin in transgenic mice also expressing a collagen marker provided strong evidence against hepatocyte EMT in the carbon tetrachloride (CCl4) model of fibrosis.8 A similar study carried out with K19-CreERT x Rosa26-YFP (yellow fluorescent protein) mice found no evidence in the CCl4 or bile duct ligation (BDL) models that cholangiocytes ever expressed α-SMA or collagen.9 Although this work demonstrated that labeled K19-positive cells did not become myofibroblasts, the possibility remained that K19-positive cells undergoing EMT were not labeled or that K19-negative cholangiocyte precursors underwent EMT.19–27 Therefore, we undertook lineage tracing studies using Alfp-Cre x Rosa26-YFP mice, enabling us to track the behavior of virtually all bipotential epithelial progenitors and their progeny in liver injury.28,29
Methods
Mice and injury models
Mice were maintained in a pathogen-free environment. Alfp-Cre mice were crossed with Rosa26-YFP reporter mice to generate mice for lineage tracing (Suppl. Fig. 1A).28,30 Labeling efficiency was determined by calculating the percentage of cells stained with antibodies against K19, A6, or HNF4α also expressing YFP, as shown in Fig. 1. For all models, livers were harvested; rinsed in 1x PBS; fixed in methanol-free 4% formaldehyde/1x PBS; progressively cryoprotected with 10%, 20%, and 30% sucrose/1x PBS at 4°C; and freeze-embedded in Optical Cutting Temperature (Sakura Finetek, Torrance, CA). BDL was carried out according to standard methods.3 Animals were anesthetized with isoflurane. Following midline laparotomy, the common bile duct was ligated twice with 4-0 silk suture. Sham-operated animals served as controls. Mice were sacrificed at 2, 4, and 8 weeks after BDL. For the CCl4 model, CCl4 was mixed 1:1 with mineral oil and injected at a dose of 0.2 mL/100 grams body weight intraperitoneally twice weekly for 3 weeks before sacrifice. Mineral oil alone was administered to controls. For the 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) model, mice were fed a diet containing 0.1% (wt/wt) DDC (Sigma-Aldrich, St. Louis, MO) in #5015, PMI Mouse diet (Harlan Teklad, Madison, WI) for 2 and 3 weeks before sacrifice. Controls received normal chow. All animal studies were approved by the University of Pennsylvania Institutional Animal Care and Use Committee.
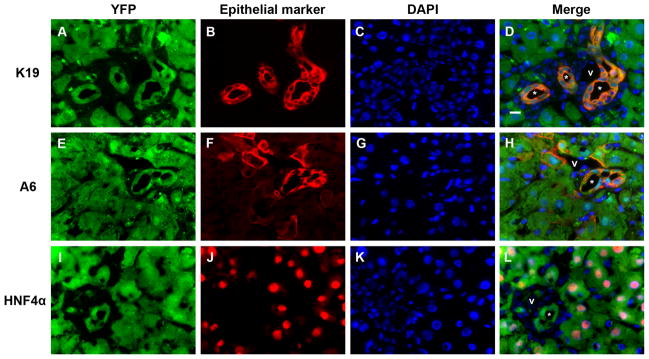
Cre recombination efficiency in Alfp-Cre x Rosa26-YFP mouse livers is high. Representative split color and merged panels from immunostaining of liver sections following 3 weeks of DDC treatment. (A-D) YFP (Cy5, green) nearly completely co-localizes with K19 staining (Cy3, red). (YFP+K19)/K19 ratio = 1041 cells/1044 cells (99.7%). (E-H) YFP (Cy5, green) and A6 (Cy3, red). (YFP+A6)/A6 ratio = 903 cells/916 cells (98.6%). (I-L) YFP (Cy5, green) and HNF4α (Cy3, red). (YFP+HNF4α)/HNF4α ratio = 899 cells/905 cells (99.3%). Similar co-localization between YFP and either K19 or HNF4α was observed for BDL and CCl4 models (not shown). Blue = DAPI; * = representative bile ducts; v = portal vein. Scale bar = 10 μm.
Immunofluorescence staining and imaging of mouse tissue
Six-micrometer sections were briefly post-fixed to slides using methanol-free 4% formaldehyde/1x PBS and rinsed with 1x PBS. Antigen retrieval suitable for cryostat sections was performed as described.31 Sections were blocked with 1% bovine serum albumin in 0.1% Triton X-100/1x PBS and incubated with primary antibodies (Suppl. Table 1) at 4°C overnight. Slides were incubated with the appropriate Cy3- (1:600) or Cy5-conjugated (1:400) secondary antibodies (Jackson ImmunoResearch, West Grove, PA) for 2 hours at room temperature then counterstained with DAPI. YFP was detected using a cross-reacting antibody against green fluorescent protein (GFP). Images were captured using a Nikon E600 microscope (Nikon, Melville, NY) equipped with a QICAM CCD camera (QImaging, Burnaby, BC, Canada) and processed using iVision software (BioVision Technologies, Exton, PA). We assessed at least 10 portal tracts from each animal and confirmed results by repeating stains in non-sequential sections using representatives from each treatment group.
See Supplemental Methods for Sirius Red staining, primary cholangiocyte isolation and culture, soluble factor treatment, immunocytochemistry, cell imaging, and real-time polymerase chain reaction.
Results
Labeling efficiency is high in Alfp-Cre x Rosa26-YFP mice
To carry out in vivo lineage tracing, we generated Alfp-Cre x Rosa26-YFP mice, in which YFP is constitutively expressed in all cells derived from precursors that express the hybrid albumin promoter and alpha-fetoprotein (AFP) enhancer (hepatocytes, cholangiocytes, and their bipotential progenitors; Suppl. Fig. 1A). Efficient recombination occurred in embryonic progenitors, resulting in YFP marking of greater than 98 percent of K19-, A6-, and HNF4α-positive cells in postnatal livers (Figs. 1, ,2B;2B; Suppl. Fig. 1B).32
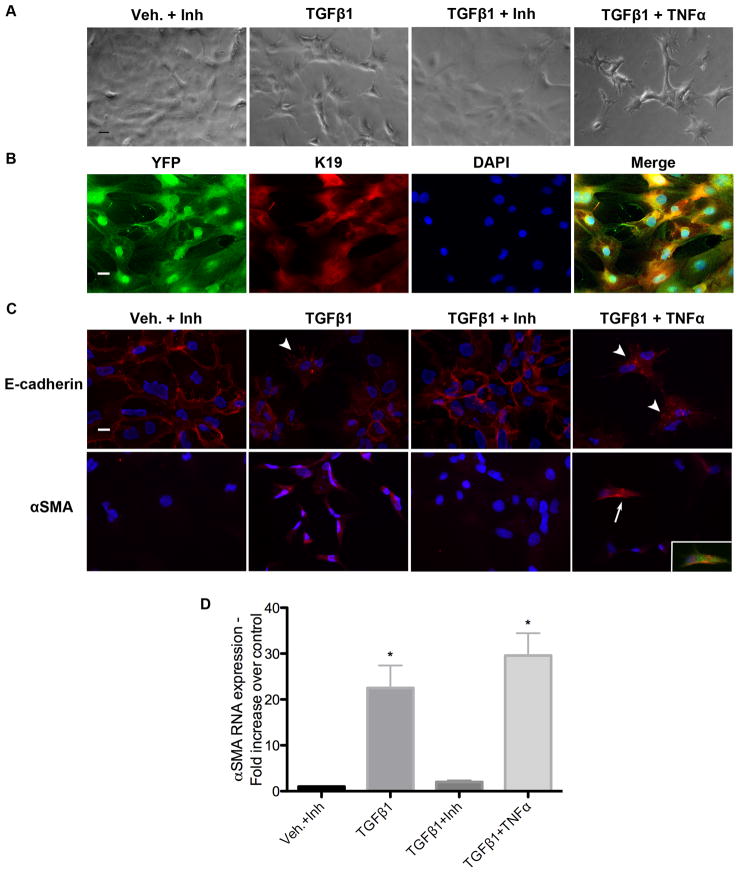
Cultured primary cholangiocytes can undergo EMT. Primary cholangiocytes were isolated from Alfp-Cre x Rosa26-YFP mice and cultured on type I collagen. (A) Phase contrast microscopy demonstrating fibroblast-like morphologic changes in cells treated with TGF-β1 alone and in combination with TNF-α for 72 hours compared with vehicle plus TGF-β receptor kinase-1 inhibitor (Inh) and TGF-β1 plus inhibitor. Scale bar = 50 μm. (B) K19 immunostaining (Cy3, red) of primary cholangiocytes completely co-localizes with YFP staining (Cy5, green). Blue = DAPI; scale bar = 20 μm. (C) Treatment with TGF-β1 alone and in combination with TNF-α resulted in intracellular relocalization of E-cadherin (arrowheads) and upregulation of α-SMA. Following combined treatment, rare cells with α-SMA stress fibers can be identified (arrow). These cells also express YFP, confirming their epithelial origin (inset). Scale bar = 20 μm. (D) Relative quantification of α-SMA mRNA expression by qRT-PCR. Graph represents average of 3 independent experiments each carried out in triplicate using cells isolated from the same animal. *P < 0.05 compared with vehicle controls.
Cultured primary cholangiocytes can undergo EMT
To assess whether primary cholangiocytes from our reporter strain are able to undergo EMT in vitro, we treated them with TGF-β1 either alone or in combination with TNF-α, which has been reported to drive EMT via stabilization of Snail.33 Cells treated with TGF-β1 for 72 hours appeared to lose cell-cell contacts and developed a fibroblast-like morphology (Fig. 2A). A TGF-β receptor inhibitor abrogated this effect, whereas combined TGF-β1/TNF-α treatment enhanced the phenotype. TGF-β1 treatment, alone and combined with TNF-α, also resulted in intracellular relocalization of E-cadherin from cell membranes and increased expression of α-SMA (Figs. 2C, D). At 72 hours, combined TGF-β1/TNF-α treatment yielded rare cells possessing α-SMA stress fibers (Fig. 2C), a characteristic of activated myofibroblasts. These were not contaminating cells, as they expressed YFP (Fig. 2C inset). Therefore, primary cholangiocytes from the double-transgenic mice are capable of adopting a myofibroblast phenotype in vitro, as has been reported for mouse and human cholangiocytes by others.7,34,35
Cholangiocytes do not undergo EMT in the mouse BDL and CCl4 models
To determine whether EMT occurs in vivo, we induced liver fibrosis in Alfp-Cre x Rosa26-YFP mice by BDL. Sirius red staining demonstrated progressively increasing hepatic fibrosis at 2, 4, and 8 weeks post-BDL (Fig. 3; Suppl. Fig. 2). At these time points (Figs. 4, ,5;5; Suppl. Fig. 3), there was no evidence of YFP co-localization with the mesenchymal markers S100A4, vimentin, α-SMA, or pro-collagen 1α2, despite significant peribiliary staining for these markers compared with untreated controls (Suppl. Figs. 1C and 5). Occasional YFP-negative, S100A4-positive cells appeared to infiltrate bile ducts (Fig. 5D); these may be lymphocytes or monocytes, which are known to express S100A4.36–38 Despite significant hepatic stellate cell accumulation by 8 weeks post-BDL, no YFP co-localization with the HSC marker desmin was noted (Fig. 7). The data indicate that EMT does not occur at these time points in the mouse BDL model.
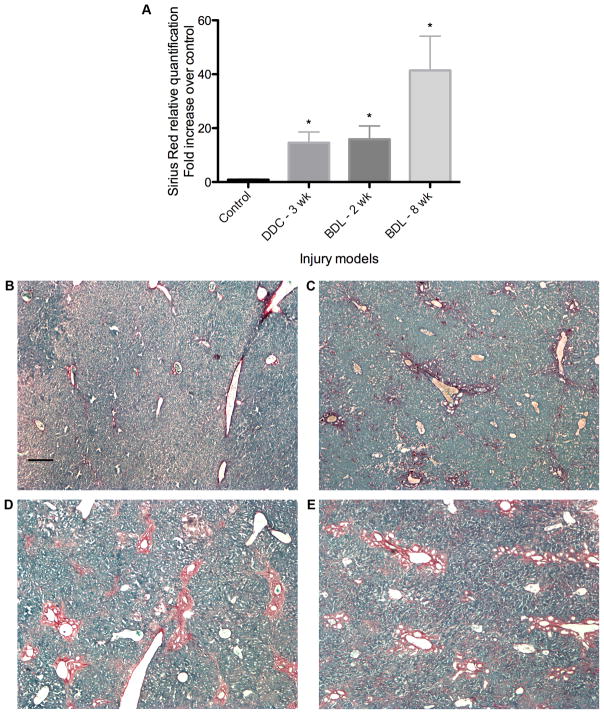
Mouse livers demonstrate significant Sirius red staining in fibrosis models. (A) Relative quantification of Sirius red staining, expressed as fold-increase over control. *P < 0.05 compared with controls. (B) Sirius red staining of representative control section with Fast Green counterstain. (C-E) Representative sections from stained livers (C) after 3 weeks of DDC treatment, (D) 2 weeks post-BDL, and (E) 8 weeks post-BDL. Scale bar = 200 μm.
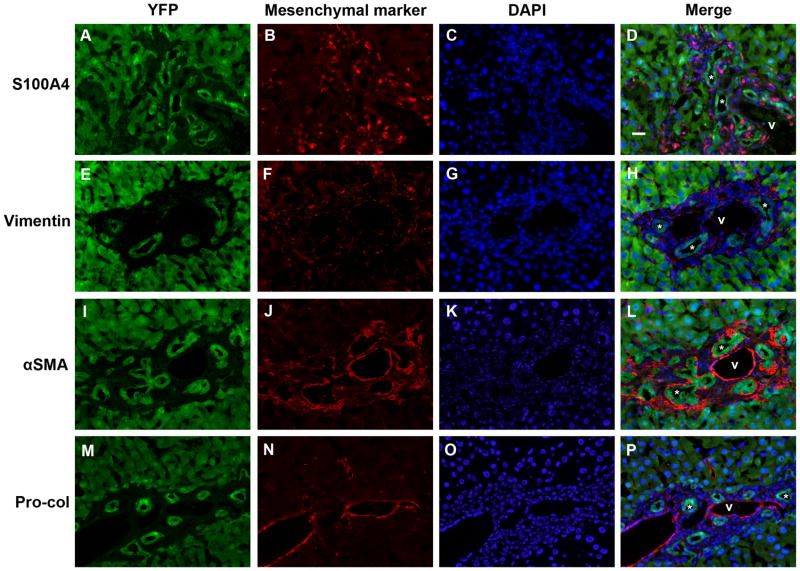
No evidence of epithelial-mesenchymal marker co-expression in mouse livers 2 weeks post-BDL. Split color and merged panels from representative mouse liver sections from 3 animals, stained with antibodies against (A-D) S100A4; (E-H) vimentin; (I-L) α-SMA; and (M-P) pro-collagen 1α2 (Pro-Col). Green = YFP (Cy5), red = mesenchymal marker (Cy3), blue = DAPI; * = representative bile ducts; v = portal vein. Scale bar = 20 μm.
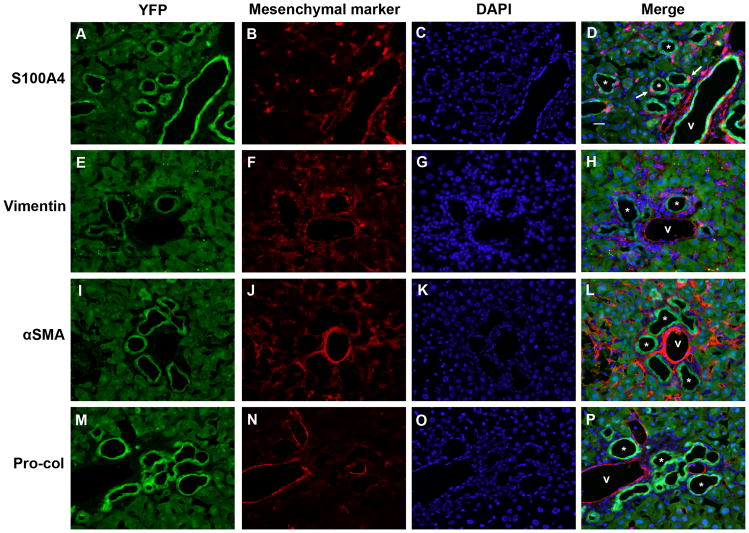
No evidence of epithelial-mesenchymal marker co-expression in mouse livers 8 weeks post-BDL. Split color and merged panels from representative mouse liver sections from 2 animals, stained with antibodies against (A-D) S100A4; (E-H) vimentin; (I-L) α-SMA; and (M-P) pro-collagen 1α2 (Pro-Col). Green = YFP (Cy5), red = mesenchymal marker (Cy3), blue = DAPI. Scattered YFP-negative, S100A4-positive cells were seen in bile ducts (arrows), likely representing infiltrating lymphocytes. * = representative bile ducts; v = portal vein. Scale bar = 20 μm.
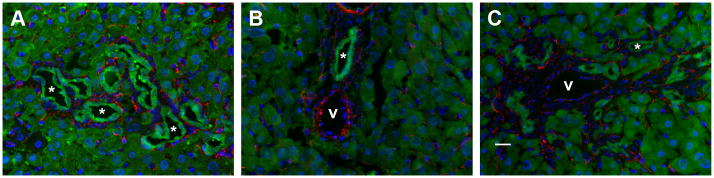
No evidence of YFP co-expression with the hepatic stellate marker desmin in murine models of biliary injury. Representative merged images of liver sections from mice (A) 8 weeks post-BDL, (B) after 3 weeks of CCl4 treatment, and (C) after 3 weeks of DDC treatment. Note the distinct staining of long, thin cells in the sinusoids. Green = YFP (Cy5), red = desmin (Cy3), blue = DAPI. * = representative bile ducts; v = portal vein. Scale bar = 20 μm.
To corroborate these findings, we exposed mice to CCl4, a hepatotoxin that induces hepatic (though non-biliary-specific) fibrosis. While progenitor cell proliferation has been described in this model, the phenotype depends mostly on hyperplasia of mature cholangiocytes.39,40 After 3 weeks of treatment, the increase in fibrosis compared to controls was modest but statistically significant (Suppl. Fig. 2). There was no evidence of YFP co-staining with mesenchymal markers noted in any of the animals despite significant peribiliary staining (Fig. 7; Suppl. Figs. 4 and 5). Neither the CCl4 model nor the BDL model produced evidence of marker co-localization in YFP-labeled hepatocytes, consistent with a recent study suggesting that hepatocyte EMT does not occur.8
Cholangiocytes do not undergo EMT in the setting of fibrosis with an oval cell response
BDL is the most widely used rodent model of biliary fibrosis, although it is an imperfect model for many human diseases because the phenotype results from marked proliferation of mature cholangiocytes (primarily from the large bile ducts) rather than a ductular reaction, which is proposed to involve activation of bipotential progenitor cell populations (oval cells).39,41,42 The ductular reaction is a dominant feature of several fibrosing biliary diseases, including biliary atresia.43,44 To assess the possibility that EMT occurs in cholangiocyte precursors, before the expression of K19, we used the DDC dietary model, which results in an oval cell response with a marked ductular reaction and sclerosing cholangitis.45,46
Mice fed the DDC diet for 2 weeks developed moderate fibrosis (data not shown) compared to mice fed normal chow. At 3 weeks of treatment, fibrosis was more marked and roughly equivalent by Sirius red staining to mice 2 weeks post-BDL (Fig. 3). After 2 weeks, livers showed no co-localization of YFP with mesenchymal markers (data not shown). Three weeks of DDC treatment produced the same findings despite clear expression of these mesenchymal proteins by other cells in the surrounding stroma (Figs. 6 and and7).7). Therefore, we conclude that EMT does not occur in this model of biliary fibrosis characterized by bipotential progenitor cell proliferation.
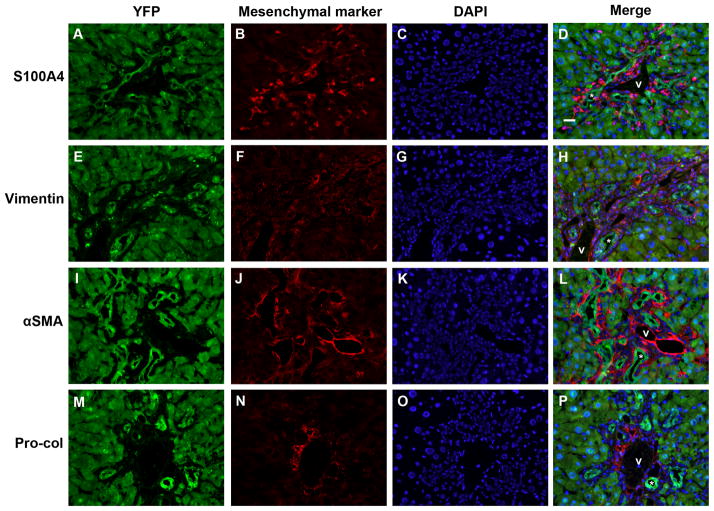
No evidence of epithelial-mesenchymal marker co-expression in mouse livers after 3 weeks of DDC treatment. Split color and merged panels from representative mouse liver sections from 2 animals, stained with antibodies against (A-D) S100A4; (E-H) vimentin; (I-L) α-SMA; and (M-P) pro-collagen 1α2 (Pro-Col). Green = YFP (Cy5), red = mesenchymal marker (Cy3), blue = DAPI. * = representative bile ducts; v = portal vein. Scale bar = 20 μm.
Discussion
We report here a broad-based approach to the study of EMT in liver fibrosis. Employing a heritable marker expressed in nearly all bipotential progenitor cells, cholangiocytes, and hepatocytes, we found no evidence in three different fibrosis models that epithelial cells in the adult liver undergo transition to fibrogenic myofibroblasts or other mesenchymal cells during hepatic injury.
Our labeling strategy enabled us to bypass potential problems associated with the use of the cholangiocyte marker K19 for fate mapping. Several studies have suggested that K19 is expressed in only a subset of biliary epithelial cells.47 Tan et al. demonstrated in diseased human livers that K19 is absent in some keratin 7-positive biliary cells27, and additionally, studies with human and rodent livers affected by biliary pathology found that a significant number of putative hepatic progenitor cells are K19-negative, even in the ducts.48,49 Unlike K19, AFP is expressed in mice beginning E8.25–8.5. In the Alfp-Cre strain, Cre recombinase activity is detectible by E9.5, and its efficient recombination results in labeling of more than 98% of cholangiocytes and hepatocytes (Figs. 1, ,2B;2B; Suppl. Fig. 1B).28.29,32 A significant advantage of this approach is the marking of K19-negative cholangiocyte progenitors. These potentially include hepatocytes, which stop expressing K19 after maturation but may transdifferentiate into cholangiocytes19–23, and AFP-positive/K19-negative progenitor cells.24–26,50
An important part of our study was the use of the DDC model, which permits a more complete assessment of bipotential progenitor cell fate during the ductular reaction than is possible with the CCl4 and BDL models. Bipotential progenitor cell activation is central to the “ductular reaction” that characterizes biliary fibrosis.43,51 Recent studies have demonstrated a direct association between the degree of the ductular reaction and the severity of fibrosis in diseases including biliary atresia and hepatitis C.52 We have previously shown that co-staining of epithelial and mesenchymal markers primarily occurs in diseases with a marked ductular reaction. Notably, in livers from patients with primary biliary cirrhosis, marker co-staining was limited to epithelial cells of the ductular reaction and was not seen in mature ducts.4 Using the DDC model, in which bipotential progenitor cell expansion is associated with fibrosis, we observed no evidence of EMT. Although lineage-tracing methodology cannot absolutely rule out EMT in a minor subgroup of cells, and we cannot prove that all bipotential epithelial progenitors were labeled, our work clearly demonstrates that neither hepatocyte nor cholangiocyte EMT contributes to fibrosis in these three mouse models. The fate of putative multipotent, mixed epithelial-mesenchymal precursors is not addressed in our study, except to say that such cells are not marked by the Alfp-Cre transgene.53
Many studies of EMT in fibrosis have failed to define EMT rigorously or to differentiate between the transition to a mesenchymal (EMT) versus a myofibroblast (EMyT) phenotype. Type I collagen expression is the most direct measure of fibrogenesis, and the literature suggests that α-SMA-positive cells are the primary effectors of fibrogenesis.15,18,54,55 Nevertheless, surrogate fibroblast markers have often been used to identify EMT, most notably S100A4, despite recent data suggesting that it is non-specific.10,18,38 In our study, we examined the expression of four different mesenchymal markers, including S100A4, vimentin, α-SMA and procollagen I. Their lack of co-localization with YFP in the setting of fibrosis supports the conclusion that in these models, EMT does not contribute to fibrosis. The lack of vimentin co-localization is particularly interesting. Although often said to be a non-specific indicator of cholangiocyte damage, it is in fact a highly specific marker of the mesenchymal state and has been used as a marker of EMT in non-fibrosis contexts (e.g. embryonic development and cancer).56 The complete absence of its co-localization with YFP in our study suggests that liver epithelial cells do not transition to either mesenchymal cells or myofibroblasts in the mouse models examined. Furthermore, our data contradict the recently proposed hypothesis that stellate cells are derived from epithelial progenitors.57 Rather, they are consistent with studies that have shown that stellate cells originate in the hepatic submesothelium.58–60
Although our data stand in contrast to another study that supported the concept of hepatocyte EMT(14), they complement those of Taura et al. and Scholten et al., which provide strong, direct evidence that EMT does not occur in rodent models of fibrosis.8,9 As definitive as these lineage-tracing data are, however, it is difficult to reconcile them with the co-staining data, primarily from human tissue, which originally led to the concept of cholangiocyte EMT.3–7,14 Although most of these studies demonstrated little or no co-expression of cholangiocyte markers with α-SMA, there was significant cholangiocyte expression of other mesenchymal markers, including vimentin and the collagen chaperone HSP47. It may be that in human livers, EMT occurs in cirrhosis, a state not well modeled in rodents, and may require a florid ductular reaction, which is also poorly mimicked by rodent models. Alternatively, this discrepancy may reflect the limitations of immunohistochemistry-based lineage-tracing methodology, although this is mitigated in our study by the high efficiency of Cre recombination.
In summary, we show, using robust lineage-tracing methods, that EMT does not occur in common rodent models of liver fibrosis. Future work should focus on better understanding the direct contribution of dysfunctional epithelial cells to liver fibrosis, as well as determining the mechanistic relationships between fibrogenesis and the progenitor cell activation characteristic of the ductular reaction. This will ultimately require the development of animal models of biliary fibrosis that better reflect human disease.
Supplementary Material
Supp Figure S1
Supp Figure S2
Supp Figure S3
Supp Figure S4
Supp Figure S5
Supplementary Data
Acknowledgments
We are grateful to Archanna Panikkar for assistance with mouse husbandry and to Carlo Spirli (Yale University) for training in cholangiocyte isolation. We acknowledge the assistance of Gary Swain and the Morphology Core of the University of Pennsylvania NIDDK Center for the Study of Digestive and Liver Diseases (Philadelphia, PA; P30 DK50306) in immunostaining and imaging. The TROMA-III antibody developed by Rolf Kemler was obtained from the Developmental Studies Hybridoma Bank, which is supported by the NICHD and maintained by The University of Iowa (Iowa City, IA).
This work was supported by National Institutes of Health R01 grant DK-058123 (to R.G.W.), R01 grant DK-083355 (to B.Z.S.), and by a grant from the Fred and Suzanne Biesecker Pediatric Liver Center (to R.G.W.). A.C. is a Fellow of the Pediatric Scientist Development Program and is supported by award K12-HD000850 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, by a Childhood Liver Disease Research and Education Network training grant, and by the American Liver Foundation Alexander M. White, III Postdoctoral Research Fellowship Award. R.D. was supported by the National Institutes of Health institutional training grant T32 DK07066.
Abbreviations
- ECM
- extracellular matrix
- EMT
- epithelial-to-mesenchymal transition
- K19
- keratin 19
- HSP47
- heat shock protein 47
- α-SMA
- α-smooth muscle actin
- EMyT
- epithelial-to-myofibroblast transition
- YFP
- yellow fluorescent protein
- CCl4
- carbon tetrachloride
- BDL
- bile duct ligation
- DDC
- 3,5-diethoxycarbonyl-1,4-dihydrocollidine
- GFP
- green fluorescent protein
- AFP
- alpha-fetoprotein
- TGF-β1
- transforming growth factor-β1
- HNF4α
- hepatocyte nuclear factor-4α
- TNFα
- tumor necrosis factor-α
References
Full text links
Read article at publisher's site: https://doi.org/10.1002/hep.24206
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3082729?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1002/hep.24206
Article citations
Systematic review of the mechanism and assessment of liver fibrosis in biliary atresia.
Pediatr Surg Int, 40(1):205, 20 Jul 2024
Cited by: 0 articles | PMID: 39033225
Review
Lack of basic rationale in epithelial-mesenchymal transition and its related concepts.
Cell Biosci, 14(1):104, 20 Aug 2024
Cited by: 0 articles | PMID: 39164745 | PMCID: PMC11334496
Review Free full text in Europe PMC
The possible pathogenesis of liver fibrosis: therapeutic potential of natural polyphenols.
Pharmacol Rep, 76(5):944-961, 20 Aug 2024
Cited by: 0 articles | PMID: 39162986
Review
Liver Fibrosis: From Basic Science towards Clinical Progress, Focusing on the Central Role of Hepatic Stellate Cells.
Int J Mol Sci, 25(14):7873, 18 Jul 2024
Cited by: 7 articles | PMID: 39063116 | PMCID: PMC11277292
Review Free full text in Europe PMC
Epithelial-mesenchymal transition in tissue repair and degeneration.
Nat Rev Mol Cell Biol, 25(9):720-739, 29 Apr 2024
Cited by: 3 articles | PMID: 38684869
Review
Go to all (140) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Genetic labeling does not detect epithelial-to-mesenchymal transition of cholangiocytes in liver fibrosis in mice.
Gastroenterology, 139(3):987-998, 20 Jun 2010
Cited by: 160 articles | PMID: 20546735 | PMCID: PMC2930026
Hepatocytes do not undergo epithelial-mesenchymal transition in liver fibrosis in mice.
Hepatology, 51(3):1027-1036, 01 Mar 2010
Cited by: 230 articles | PMID: 20052656 | PMCID: PMC2906231
Mesodermal mesenchymal cells give rise to myofibroblasts, but not epithelial cells, in mouse liver injury.
Hepatology, 60(1):311-322, 28 Apr 2014
Cited by: 35 articles | PMID: 24488807 | PMCID: PMC4077971
Cholangiocyte proliferation and liver fibrosis.
Expert Rev Mol Med, 11:e7, 25 Feb 2009
Cited by: 115 articles | PMID: 19239726 | PMCID: PMC2675635
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NICHD NIH HHS (2)
Grant ID: K12-HD000850
Grant ID: K12 HD000850
NIDDK NIH HHS (8)
Grant ID: T32 DK007066
Grant ID: R01 DK083355
Grant ID: DK-058123
Grant ID: DK-083355
Grant ID: R01 DK058123-10
Grant ID: R01 DK058123
Grant ID: T32 DK07066
Grant ID: P30 DK050306





