Abstract
Free full text

Redox Regulation of the Mitochondrial KATP Channel in Cardioprotection
Associated Data
Abstract
The mitochondrial ATP-sensitive potassium channel (mKATP) is important in the protective mechanism of ischemic preconditioning (IPC). The channel is reportedly sensitive to reactive oxygen and nitrogen species, and the aim of this study was to compare such species in parallel, to build a more comprehensive picture of mKATP regulation. mKATP activity was measured by both osmotic swelling and Tl+ flux assays, in isolated rat heart mitochondria. An isolated adult rat cardiomyocyte model of ischemia-reperfusion (IR) injury was also used to determine the role of mKATP in cardioprotection by nitroxyl. Key findings were as follows: (i) mKATP was activated by O2•- and H2O2 but not other peroxides. (ii) mKATP was inhibited by NADPH. (iii) mKATP was activated by S-nitrosothiols, nitroxyl, and nitrolinoleate. The latter two species also inhibited mitochondrial complex II. (iv) Nitroxyl protected cardiomyocytes against IR injury in a mKATP-dependent manner. Overall, these results suggest that the mKATP channel is activated by specific reactive oxygen and nitrogen species, and inhibited by NADPH. The redox modulation of mKATP may be an underlying mechanism for its regulation in the context of IPC.
1. INTRODUCTION
The past 25 years has witnessed much investigation into the phenomenon of ischemic preconditioning (IPC), in which short non-lethal periods of ischemia and reperfusion (IR) can elicit protection against prolonged ischemia-reperfusion (IR) injury [1]. Despite this effort, the mechanism by which IPC protects organs such as the heart and brain from IR injury is still debated.
One proposed mechanism of IPC-induced cardioprotection is the opening of a mitochondrial ATP-sensitive potassium channel (mKATP), which elicits mild swelling of the mitochondrial matrix. This in turn is thought to impact on mitochondrial Ca2+ loading, reactive oxygen species (ROS) generation, metabolic efficiency, and assembly of the permeability transition pore [2], and these downstream events bring about protection via unclear mechanisms. Although the molecular identity of the mKATP channel remains unknown, several pharmacologic mKATP modulators mimic IPC, and many IPC signaling pathways are thought to converge on mKATP as an end effector [3].
Mitochondria are a quantitatively significant source of ROS, which contribute to tissue damage during ischemia, but are also mediators of IPC signaling [4]. Accumulating evidence suggests that redox signaling pathways play an important role in IPC [5-9], and can promote mKATP activation [10-15]. The primary ROS generated by mitochondria is superoxide (O2•-) [16,17], while hydrogen peroxide (H2O2) or lipid peroxides can be formed secondarily [17]. Both O2•- and H2O2 are thought to activate mKATP [13,18-20], although conflicting reports exist regarding O2•- [18,20]. The effect of other peroxides on mKATP is not known. Furthermore, it is apparent that some but not all types of antioxidants can inhibit IPC and mKATP activity [6,13], warranting further investigation. Table 1 summarizes the disparate results to date regarding redox regulation of the mKATP channel.
Table 1
Previous Studies on the Effects of Oxidants, Reactive Nitrogen Species, Antioxidants and Reducing Agents on mKATP Channel Activity.
| Reagent | Conc. | Effects | Experimental conditions | References |
|---|---|---|---|---|
| Ascorbate | 1 mM | No effect | Isolated mitochondria | [12] |
| DTE | 10 mM | Inhibits glyburide binding | Submitochondrial particles | [46] |
| DTNB | 500 μM | Inhibits glyburide binding | Submitochondrial particles | [46] |
| DTT | 100 μM | Inhibits activation by DZX | Isolated mitochondria | [12,13] |
| 1 mM | Activates rundown channels Loss in selectivity | Reconstituted channels | [66] | |
| 10 mM | Inhibits glyburide binding | Submitochondrial particles | [46] | |
| Mersalyl | 100 μM | Inhibits glyburide binding | Submitochondrial particles | [46] |
| MPG | 200 μM | Inhibits activation by DZX | Isolated mitochondria | [12,13,31] |
| NAC | 4 mM | Inhibits activation by DZX | Isolated mitochondria | [12,13] |
| NEM | 2 mM | Inhibits activation by O2.- | Reconstituted channels | [18] |
| Inhibits glyburide binding | Submitochondrial particles | [46] | ||
| 60 nmol/mg | Decreases selectivity | Isolated mitochondria | [67] | |
| Thimerosal | 500 μM | Inhibits glyburide binding | Submitochondrial particles | [46] |
| X/XO | 0.038 U/mL | Activates | Reconstituted channels | [18] |
| 6 mU/mL | No Effect | Isolated Mitochondria | [20] | |
| H2O2 | 1 μM | Activates | Isolated Mitochondria | [13] |
| 1 μM | Activates | Isolated Mitochondria | [19] | |
| 6 mU/mL X/XO + 30 U SOD | Activates | Isolated Mitochondria | [20] | |
| SNAP | 10 mM | Activates | Isolated Mitochondria | [20] |
Nitric oxide (NO•) is also implicated in IPC, and elicits a large variety of cardioprotective effects [21]. NO• has been detected in isolated mitochondrial preparations [22], and can secondarily generate many reactive nitrogen species (RNS) [23-25], which can serve either damaging or beneficial signaling roles [17,21,25,26]. mKATP is a potential target for such RNS, and while high doses (10 mM) of an S-nitrosothiol have been shown to activate the channel in intact mitochondria [20], evidence for more subtle physiologically relevant effects of NO• has mostly relied on indirect measures of channel activity [27] or study of the channel removed from its mitochondrial environment [28]. Thus, it is not clear whether the levels of NO• that would be experienced inside mitochondria are capable of modulating mKATP activity.
The one electron reduction product of NO•, nitroxyl (HNO) may also modulate the mKATP channel. Nitroxyl is protective in IR injury [29], and while it shares some signaling pathways with NO•, it also possesses distinct biochemistry from NO•, such as a direct interaction with thiols. In this regard, the nitroxyl donor Angeli's salt (AS) inhibits mitochondrial complex II in a manner sensitive to glutathione and independent of S-nitrosation [30]. mKATP activity is exquisitely sensitive to complex II modulation [31,32], and herein we explored the concept that nitroxyl may regulate mKATP via effects on complex II.
Despite a collection of studies to date examining the effect of single redox agents on mKATP, often at high doses, a comparative study across a wide range of doses is lacking. In addition, unique chemical properties of certain NO• derived species have precluded their use to date in studying mKATP. The current study aimed to address such issues, and the collective results suggest mitochondrial redox state is an important regulator of mKATP channel activity, mitochondrial function, and cardioprotection in the context of IPC.
2. MATERIALS AND METHODS
Full experimental details are in the online supplement.
2.1 Animals, chemicals and supplies
Male Sprague-Dawley rats, 200–300 g, were purchased from Harlan (Indianapolis, IN) or bred at the Biotério do Conjunto das Químicas (Universidade de São Paulo) housed on a 12 hr. light/dark cycle with food and water available ad libitum. All procedures were performed in accordance with the US National Institutes of Health “Guide for the care and use of laboratory animals” and the Colégio Brasileiro de Experimentação Animal, and were approved by the appropriate university animal ethics committees. Linoleic peroxide was a kind gift from Sayuri Miyamoto (São Paulo) and stored under argon in methanol [33]. Nitro-linoleate was synthesized and analyzed as previously described [34]. All other reagents used were analytical grade or higher, obtained from Sigma (St. Louis MO) or EMD (Gibbstown NJ).
2.2 Mitochondrial isolation, Cx-II, and mKATP assays
Heart mitochondria were rapidly isolated as previously described [31,35]. Cx-II activity was measured as previously described [31,32]. mKATP activity was measured by osmotic swelling as previously described [32,36]. All channel modulating agents (reactive oxygen and nitrogen species, antioxidants etc.) were present in the assay buffer prior to mitochondrial addition. The nature of the mKATP osmotic swelling assay, requiring mitochondrial addition last of all, precludes its use to study highly reactive species such as HNO [37]. In such cases a fluorescence-based Tl+ flux assay for mKATP activity [38] was used, permitting incubation of mitochondria prior to assay initiation by Tl+ addition.
2.3 Cardiomyocyte model of IR injury
Adult rat ventricular myocytes were isolated, and a model of simulated IR (SIR) injury was as previously described [32]. Cells were incubated in anoxic glucose-free Krebs Henseleit (KH) buffer at pH 6.5 for 30 min., followed by reoxygenation in glucose-replete KH at pH 7.4. Where indicated, compounds were present 20 min. prior to the onset of simulated ischemia. At the end of all protocols, viability was determined by Trypan blue exclusion.
2.4 Statistics
All experiments were performed on at least 3 independent mitochondrial or cell preparations, and results are presented as mean ± SEM. Statistical significance between groups was determined by ANOVA.
3. RESULTS & DISCUSSION
3.1 mKATP is activated by some but not all peroxides
While the ability of ROS to modulate mKATP activity has been reported [13,18-20], the differential action of various classes of ROS is less well understood. In particular, ROS such as H2O2 may initiate chemistry that generates secondary peroxides such as lipid hydroperoxides (LOOH) [17]. We therefore hypothesized that peroxides may modulate mKATP activity. In Figure 1A, the effects of H2O2, t-butyl hydroperoxide (t-BuOOH), and linoleic hydroperoxide (LOOH) on mKATP were tested. Interestingly, while H2O2 robustly opened the channel at 1 μM, higher concentrations of t-BuOOH and LOOH did not. The differential hydrophobicity of t-BuOOH, LOOH, and H2O2 suggests that the peroxide sensor of mKATP may be in a hydrophilic region of the molecule. Alternatively, it has been suggested that H2O2 activation of the mKATP may occur via a PKCε dependent mechanism [20], although the location of the kinase in this particular mitochondrial preparation is unknown.
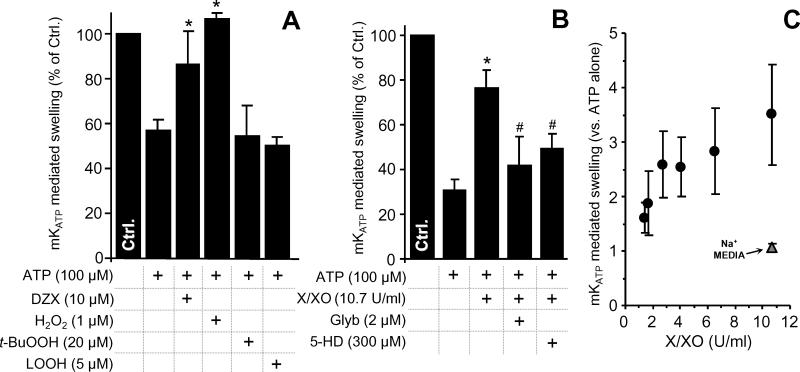
mKATP activity was measured by osmotic swelling as detailed in the methods. Controls (Na+ based media) are in Figure S1. (A): mKATP is activated by H2O2 but not by t-BuOOH or LOOH. Diazoxide (DZX) opening of mKATP was used as a positive control. (B): mKATP activation by O2•- generated by the X/XO system. Glyburide (Glyb) and 5-hydroxydecanoate (5-HD) are mKATP antagonists. (C): Dose response of mKATP activation by X/XO in K+ (black circles) or Na+ (gray triangles) based media. *p<0.05 vs. ATP alone. #p<0.05 vs. ATP + H2O2 or X/XO. Experimental conditions are listed below the X axis.
3.2 mKATP is activated by supra-physiological levels of superoxide
Activation of mKATP by O2•- has been reported previously [18], but at relatively high doses. It is unclear whether the amounts of O2•- made by mitochondria are capable of modulating mKATP activity. To investigate this, a xanthine/xanthine oxidase (X/XO) system was used to modulate ROS flux. Figures 1B and 1C show that XO levels as low as 1.4 U/ml (approximating to a O2•- flux of 1.2 μM/min) activated the channel. Reported maximal rates of O2•- generation by heart mitochondria range from 30 nM/min to 1 μM/min [39,40]. Thus, it is unlikely that mitochondrial O2•- generation under normal conditions approaches levels required for mKATP channel activation. This finding suggests that the channel would only be activated in situ with ROS originated from non-mitochondrial sources or conditions that increase ROS production such as pre- and post-conditioning. Also under conditions of reverse electron flow, O2•- in the mitochondrial microenvironment may reach levels capable of activating the channel [13]. No effect of H2O2 or X/XO on mitochondrial swelling was observed in Na+ based media (supplemental Figure 1).
3.3 mKATP is inhibited by some but not all reductants/antioxidants
While IPC is inhibited by both thiol antioxidants and catalase (suggesting a role for H2O2 [12]), cardioprotection by mKATP agonists such as diazoxide is prevented only by thiol antioxidants [13], suggesting that antioxidant-sensitive proteins distinct from mKATP may be important in IPC (e.g. SERCA, [41]). Thus, a detailed understanding of the selective regulation of mKATP by reductants is a key step toward understanding its role in IPC.
The effect of several reducing agents on mKATP activity was tested under baseline conditions and conditions of maximal channel opening (presence of ATP and diazoxide). Figure 2 shows that while most reducing agents had a mild inhibitory effect on mKATP activity, NADPH was a strong inhibitor, almost completely preventing channel activity. Compiling these data with those from our previous study [13], supplemental Figure 2 shows the ability of reductants to inhibit mKATP channel activity correlated with redox potential (r2 = 0.73). A notable outlier to this correlation was NADPH (inclusion of NADPH in the linear regression fit lowers r2 to 0.43). The underlying mechanism for a difference in the effect of NADH vs. NADPH, despite their identical redox potentials, is elusive at this stage. Interestingly, while both surface KATP channels [42-45] and the mKATP [12,13,18,46,47] do have redox active thiols, NADPH does not directly reduce thiols, suggesting its direct redox activity is not the mechanism of channel regulation.
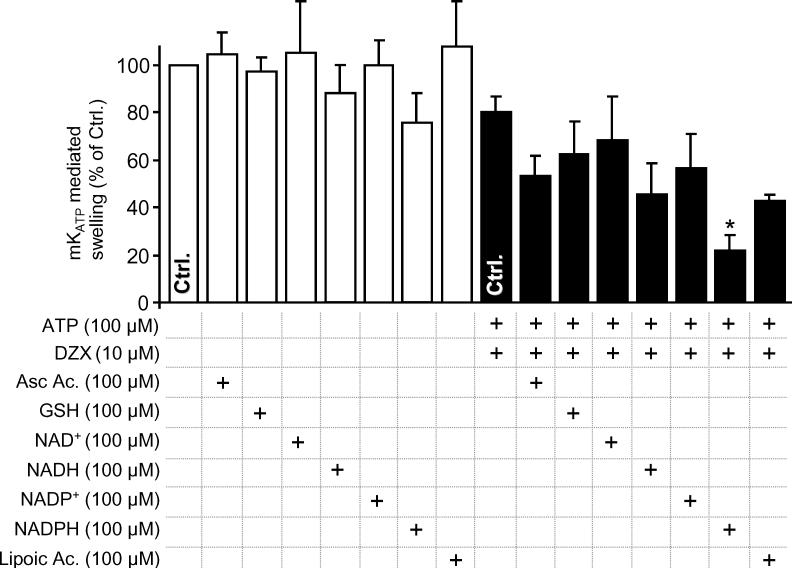
mKATP activity was measured by osmotic swelling as detailed in the methods. Data are shown for the baseline condition (open bars) or maximal swelling in the presence of both ATP and DZX (filled bars). Reductants were present in the media before mitochondrial addition, at the concentrations indicated. *p<0.05 vs. the appropriate control (bar marked Ctrl.) in the absence of reductant.
Nevertheless, NADPH does play an important role in overall mitochondrial redox status; transhydrogenases reduce mitochondrial NADP+ using electrons from NADH and the electrochemical proton gradient as an energy source [48]. The resulting NADPH is used as an electron source for thiol peroxidase removal systems, including glutathione and thioredoxin peroxidase/reductase [17]. Thus, the finding that NADPH can regulate mKATP activity suggests this channel may play a role in sensing both energy metabolism and redox status [13,49].
Notably, surface KATP channel activity has been shown to be sensitive to pyridine nucleotides [50], possibly at the same site as adenine nucleotides modulate channel activity. Furthermore, insulin secretion in pancreatic β cells, which occurs secondarily to KATP closure, correlates with NADPH/NADP+ ratios [51]. Thus, a direct modulation of the mKATP channel by NADPH binding may occur. Another possibility might be that NADPH competes with DZX for a binding site on the mKATP channel. However, we consider this unlikely since NADPH was also able to inhibit channel opening by the structurally unrelated opener atpenin A5 [32] (supplemental Figure 3).
3.4 mKATP is activation by S-nitrosothiols and L-arginine
In addition to ROS, much recent interest has focused on mKATP as a possible target for NO• or its redox congeners [20]. While NO• effects on mKATP activity in intact cells are thought to be mediated via cGMP signaling [52,53], direct effects of NO• on the purified channel have been measured in planar bilayer studies [28]. In addition NO• was shown to activate mKATP in mitochondria by using flavoprotein fluorescence as a read-out [27]. However, the dose response of mKATP to NO• in intact mitochondria is unknown. Figures 3A and 3B show that S-nitrosoacetylpenacillamine (SNAP) dose-dependently activated mKATP, in a glybenclamide and 5-HD sensitive manner. Notably, >10 μM SNAP led to mKATP inhibition, presumably due to NO• inhibition of cytochrome c oxidase [54] leading to mitochondrial deenergization, removing the driving force for K+ uptake. SNAP did not activate swelling in Na+ based buffers (supplemental Figure 4). Notably, the optimal SNAP concentration for mKATP channel opening in this study (10 μM) is 3 orders of magnitude lower than previously reported (Table 1). We are unsure as to the origin of this very large discrepancy [20].
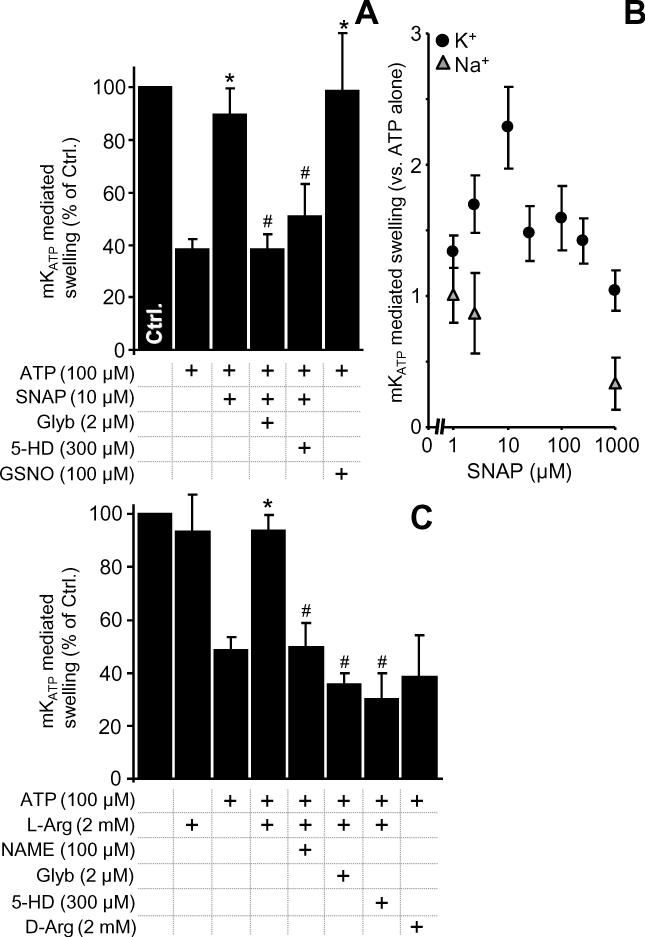
mKATP activity was measured by osmotic swelling as detailed in the methods. Controls (Na+ based media) are in Figure S2. (A): mKATP was activated by S-nitrosoacetylpenacillamine (SNAP) and S-nitrosoglutathione (GSNO), at the indicated doses. (B): Dose-response of mKATP activation by SNAP in K+ (black circles) or Na+ (gray triangles) based media. *p<0.05 vs. ATP alone. #p<0.05 vs. ATP plus SNAP. (C): mKATP activation by NOS modulators. L- or D-Arginine, L-nitroarginine methyl ester (NAME), Glyb and 5-HD were present at the concentrations indicated. *p<0.05 vs. *p<0.05 vs. ATP alone. #p<0.05 vs. ATP plus L-arginine.
There has been much interest in the possibility that mitochondria may contain a nitric oxide synthase, termed “mtNOS”. Despite recent developments including retraction of some work [55-57], NOS is a common contaminant of isolated mitochondrial preparations [58]. This may be particularly applicable to mKATP studies, since a rapid and crude mitochondrial isolation is required to effectively measure channel activity (full experimental details are in the online supplement) [32,36]. The data in Figure 3C show that the NOS substrate L-arginine stimulates mKATP opening, in a manner sensitive to the NOS inhibitor L-NAME. No effect was seen with D-arginine, suggesting the origin of this effect resides at the level of a mitochondrially associated NOS or L-NAME-sensitive enzyme. Unfortunately, further purification of mitochondria that would be required to assert a mitochondria-resident NOS in mediating these effects, also results in loss of mKATP channel activity, in a manner mechanistically related to classical channel “run-down” [38].
3.5 Certain RNS can activate mKATP, via a mechanism involving mitochondrial complex-II
In addition to “classical” RNS such as NO•, RNS such as nitro-lipids and nitroxyl may regulate mKATP. Nitro-lipids are an emerging class of anti-inflammatory signaling lipids which can mediate NO• signaling, [59,60] and are known to be generated during IPC [34,61]. One example of a nitro-lipid, nitrolinoleate (LA-NO2), can elicit cardioprotection in a cGMP-independent manner [34,62]. Figure 4A shows that, low doses (0.5 μM) of LA-NO2 opened the mKATP channel in a 5-HD- and glyburide-sensitive manner, while native linoleic acid (LA) did not. Complex II of the mitochondrial respiratory chain has been proposed as an important regulator of mKATP activity [31,32,63], and in this regard Figure 4B shows that LA-NO2, but not LA, inhibited complex-II in a dose-dependent manner. Notably however, the amount of LA-NO2 required to open the channel was significantly lower than that required to inhibit complex II (see below for discussion).
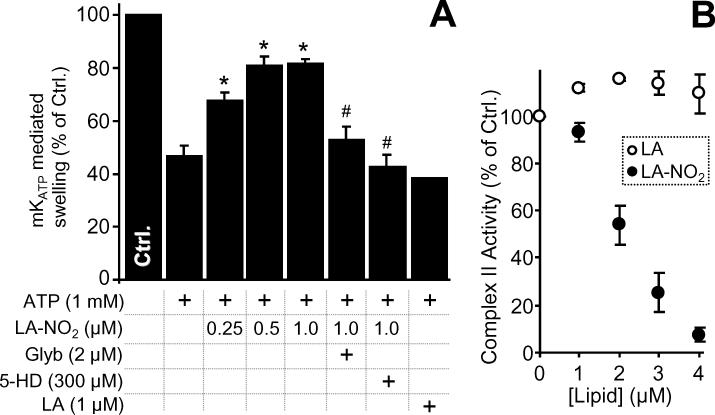
(A): mKATP activity was measured by osmotic swelling as detailed in the methods. LA-NO2, native linoleate (LA), Glyb, and 5-HD were present at the indicated concentrations. *p<0.05 vs. ATP alone. #p<0.05 vs. ATP plus LA-NO2. (B): Complex II activity in the presence of LA-NO2 was determined as detailed in the methods. Values are expressed as percentage of control complex II rate (128 ± 26 nmols DCPIP . min-1 . mg protein-1).
The disparate effects of LA-NO2 (stimulation mKATP activity), and lipid hydroperoxide (no effect on mKATP, see section 3.1) highlight the different chemical properties of these species. While both are hydrophobic reactive lipids, only LA-NO2 possesses an electrophilic moiety that can adduct thiols by Michael addition [61]. Thus it is suggested that the mechanism of LA-NO2 mediated mKATP opening may involve modification of thiols on the channel (c.f. section 3.3).
The levels of LA-NO2 generated inside mitochondria during IPC may reach 1 μM [34], raising the possibility that LA-NO2 is an important endogenous mKATP regulator. However, we previously showed that cardioprotection induced by exogenously added LA-NO2 was insensitive to mKATP blockers [34], suggesting that other mechanisms of LA-NO2 action (e.g. mild uncoupling) may account for its cardioprotective effects.
Similar to nitro-lipids, the importance of nitroxyl in cardiovascular signaling has also been the subject of recent attention [29,37]. In agreement with previous findings, we showed that the nitroxyl donor Angeli's salt (AS) dose-dependently inhibited complex II in rat heart mitochondria (Figure 5A). Consistent with an interaction between complex II and the mKATP channel, we also found that Angeli's salt opened mKATP in a manner sensitive to 5-HD and glyburide (Figure 5B). Furthermore, in agreement with previous studies [29,64] Angeli's salt was protective in a cardiomyocyte model of IR injury. This protection was sensitive to 5-HD and glyburide, but insensitive to the guanylate cyclase inhibitor ODQ or the protein kinase G inhibitor KT-5823 (Figure 5C). The role of other known components of the preconditioning signaling pathway (e.g. PKC, ERK, PI3 kinase) in mediating the effects of Angeli's salt is currently unknown, although none of these signals has previously been linked to nitroxyl. Together, these data suggest that Angeli's salt mediated cardioprotection proceeds via non-PKG mediated activation of mKATP, possibly involving an inhibition of complex II.
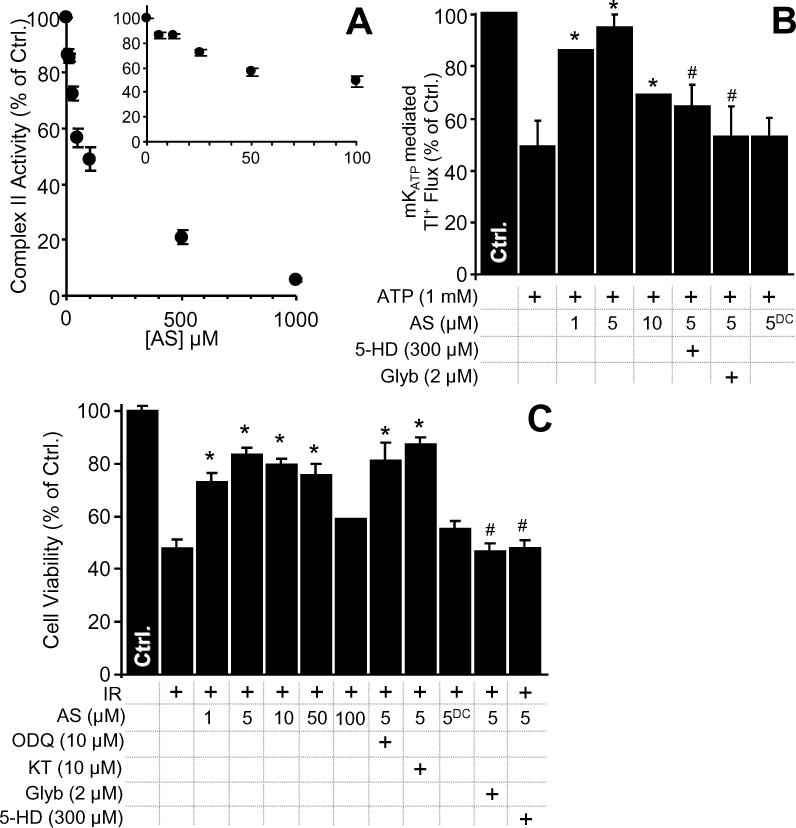
(A): Complex II activity was measured, following nitroxyl exposure of mitochondria, as described in the methods. Values are expressed as percentage of control complex II rate (108 ± 8 nmols DCPIP . min-1 . mg protein-1). (B): Nitroxyl activation of mKATP was monitored using a novel Tl+ flux assay as described in the methods. Data show the magnitude of change in intra-mitochondrial Tl+ based fluorescence following Tl+ addition, relative to control. 5-HD, Glyb, the nitroxyl donor Angeli's salt (AS) and decomposed AS (ASDC) were present at the indicated concentrations. *p<0.05 vs. ATP alone. #p<0.05 vs. ATP plus 5 μM AS. (C): Nitroxyl protects against cardiomyocyte IR injury. Cell viability was measured via Trypan blue exclusion at the end of reoxygenation, as described in the methods, and expressed as percentage of control (normoxic) cell viability. 5-HD, Glyb, Angeli's salt (AS), decomposed AS (ASDC), the PKG inhibitor KT-5823 (KT) or the soluble guanylate cyclase inhibitor ODQ were present at the indicated concentrations. *p<0.05 vs. IR alone. #p<0.05 vs. IR plus 5 μM AS.
Both nitroxyl and LA-NO2 inhibit mitochondrial complex II, activate mKATP, and are known to react with complex II thiols [30,34]. This suggests that modification of complex II thiols may underlie the mechanism of mKATP activation. However, the concentrations of nitroxyl and LA-NO2 which activated mKATP did not significantly inhibit complex II enzymatic activity (Figures 4 and and5).5). In this regard, nitroxyl and LA-NO2 are similar to other species which activate the mKATP at doses far below those at which they inhibit complex II, including atpenin A5 [32], malonate [31] and diazoxide [65]. Thus, the regulation of mKATP activity appears to be mechanistically divorced from the bulk enzymatic activity of complex II itself. The fact that 5 unrelated compounds which inhibit complex II by distinct mechanisms all activate mKATP at lower concentrations, collectively suggests that a small sub-population of complex II may play an important role in regulating mKATP activity, while not impacting total complex II enzymatic activity.
4. CONCLUSIONS
In summary, a variety of redox active species, including ROS, RNS, antioxidants, and reductants, all act on the mKATP. While a broad conclusion of this work can be summarized as “oxidants activate, reductants inhibit”, it is apparent that many species do not conform to this simple model. Key examples include NADPH, which may regulates the channel via direct binding, and LA-NO2 and nitroxyl, which are thought to mediate their effects via complex II. A summary of the potential mechanisms of mKATP channel modulation by various species is shown in Figure 6. Clearly, further work in this area, including the molecular identification of the mKATP itself, and the redox-sensitive residues within it, will facilitate a better understanding of the role that channel regulation by redox plays in events such as IPC.
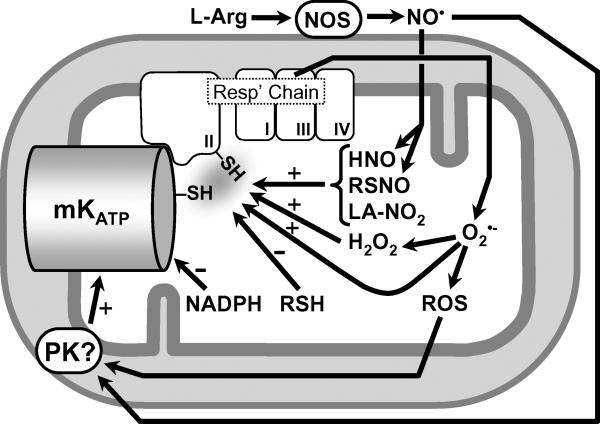
Nitroxyl (HNO), RSNO, and LA-NO2 activate the channel, possibly via thiols on the channel itself or on complex II of the respiratory chain. The ability of low molecular weight thiols (RSH) to inhibit the channel may also be mediated via thiols on the channel or on complex II. In contrast, the effects of NADPH are likely no mediated via thiols. The ability of NO• to activate the channel may be mediated via the generation of secondary RNS (e.g. RSNO, LA-NO2, HNO), which can activate the channel via PKG-independent mechanisms, or via classical NO• protein kinase signaling. ROS (in particular H2O2) can also activate the channel, via mechanisms that may include thiol modification or protein kinase signaling. The nature of the interaction between complex II and the subunits of the mKATP channel itself remains to be elucidated.
ACKNOWLEDGMENTS
We thank Camille C. Caldeira da Silva (São Paulo) and Emily Redman (Rochester) for technical support, and Sayuri Miyamoto (São Paulo) for providing linoleic hydroperoxide. This work was supported by the National Institutes of Health [HL-071158 and GM-087483 to PSB]; the John Simon Guggenheim Memorial Foundation [AJK]; the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) [AJK]; the Instituto Nacional de Ciência e Tecnologia de Processos Redox em Biomedicina (Redoxoma) [AJK]; the American Heart Association Founders Affiliate [0815770D to APW]; and FAPESP Predoctoral Fellowship [BBQ].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
RESEARCH HIGHLIGHTS, Queliconi et al.
Several redox active species have been proposed to modulate the activity of the mitochondrial ATP sensitive potassium channel (mKATP), but a comprehensive analysis is lacking. We show that:
Superoxide and hydrogen peroxide activate mKATP, but other reactive oxygen species do not.
Several reactive nitrogen species including S-nitrosothiols, nitroxyl, and nitrated lipids all activate the mKATP channel. Some of this activation is likely mediated via complex II of the mitochondrial respiratory chain.
Reducing agents show variable ability to inhibit the mKATP channel, but NADPH is particularly effective, via a mechanism unrelated to its redox potential.
LITERATURE CITED
Citations & impact
Impact metrics
Citations of article over time
Article citations
Redox Regulation of Mitochondrial Potassium Channels Activity.
Antioxidants (Basel), 13(4):434, 03 Apr 2024
Cited by: 1 article | PMID: 38671882 | PMCID: PMC11047711
Review Free full text in Europe PMC
Potassium ion channels as a molecular target to reduce virus infection and mortality of honey bee colonies.
Virol J, 20(1):134, 22 Jun 2023
Cited by: 2 articles | PMID: 37349817 | PMCID: PMC10286336
Influence of Short and Long Hyperglycemia on Cardioprotection by Remote Ischemic Preconditioning-A Translational Approach.
Int J Mol Sci, 23(23):14557, 22 Nov 2022
Cited by: 1 article | PMID: 36498885 | PMCID: PMC9738494
The effects of red LED light on pig sperm function rely upon mitochondrial electron chain activity rather than on a PKC-mediated mechanism.
Front Cell Dev Biol, 10:930855, 07 Oct 2022
Cited by: 2 articles | PMID: 36274839 | PMCID: PMC9585505
Mitochondrial trafficking and redox/phosphorylation signaling supporting cell migration phenotypes.
Front Mol Biosci, 9:925755, 22 Jul 2022
Cited by: 4 articles | PMID: 35936783 | PMCID: PMC9355248
Review Free full text in Europe PMC
Go to all (70) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
A novel mitochondrial K(ATP) channel assay.
Circ Res, 106(7):1190-1196, 25 Feb 2010
Cited by: 36 articles | PMID: 20185796 | PMCID: PMC2857559
The complex II inhibitor atpenin A5 protects against cardiac ischemia-reperfusion injury via activation of mitochondrial KATP channels.
Basic Res Cardiol, 104(2):121-129, 26 Feb 2009
Cited by: 76 articles | PMID: 19242645 | PMCID: PMC2776710
Mitochondrial reactive oxygen species: which ROS signals cardioprotection?
Am J Physiol Heart Circ Physiol, 305(7):H960-8, 02 Aug 2013
Cited by: 37 articles | PMID: 23913710 | PMCID: PMC3798754
Mitochondrial K(ATP) channels: role in cardioprotection.
Cardiovasc Res, 55(3):429-437, 01 Aug 2002
Cited by: 115 articles | PMID: 12160940
Review
Funding
Funders who supported this work.
American Heart Association Founders Affiliate (1)
Grant ID: 0815770D
FAPESP Predoctoral Fellowship
Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP)
Instituto Nacional de Ciência e Tecnologia de Processos Redox em Biomedicina (Redoxoma)
John Simon Guggenheim Memorial Foundation
NHLBI NIH HHS (3)
Grant ID: R01 HL071158
Grant ID: R01 HL071158-09
Grant ID: HL-071158
NIGMS NIH HHS (2)
Grant ID: R01 GM087483
Grant ID: GM-087483
National Institutes of Health (2)
Grant ID: GM-087483
Grant ID: HL-071158



