Abstract
Free full text

From the Cover
Prevention of maternal aging-associated oocyte aneuploidy and meiotic spindle defects in mice by dietary and genetic strategies
Abstract
Increased meiotic spindle abnormalities and aneuploidy in oocytes of women of advanced maternal ages lead to elevated rates of infertility, miscarriage, and trisomic conceptions. Despite the significance of the problem, strategies to sustain oocyte quality with age have remained elusive. Here we report that adult female mice maintained under 40% caloric restriction (CR) did not exhibit aging-related increases in oocyte aneuploidy, chromosomal misalignment on the metaphase plate, meiotic spindle abnormalities, or mitochondrial dysfunction (aggregation, impaired ATP production), all of which occurred in oocytes of age-matched ad libitum-fed controls. The effects of CR on oocyte quality in aging females were reproduced by deletion of the metabolic regulator, peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α). Thus, CR during adulthood or loss of PGC-1α function maintains female germline chromosomal stability and its proper segregation during meiosis, such that ovulated oocytes of aged female mice previously maintained on CR or lacking PGC-1α are comparable to those of young females during prime reproductive life.
Chromosomal and spindle abnormalities become much more prevalent in oocytes with age and are considered the major factors responsible for the increased incidence of infertility, fetal loss (miscarriage), and conceptions resulting in birth defects—most notably trisomy 21 or Down syndrome—in women over 35 y of age (1–4). This latter problem is compounded by modern fertility trends in that first birth rates for women 35–44 y of age in the United States have increased by more than eightfold in the past four decades (5, 6). Although the occurrence and consequences of aging-related aneuploidy in oocytes of humans and animal models have been extensively studied (1–4, 7–9), approaches to maintain fidelity of chromosome segregation during meiotic cell division with age have remained elusive. Management of fertility issues associated with advancing maternal age thus remains a challenge, even with the aid of modern assisted reproductive technologies (ARTs).
The only strategy identified thus far that can improve oocyte quality in aging females has been developed using mice as a model and involves chronic administration of pharmacologic doses of antioxidants during the juvenile period and throughout adult reproductive life (10). However, this approach has significant long-term negative effects on ovarian and uterine function, leading to higher fetal death and resorptions and decreased litter frequency and size in treated animals (11). Whereas clinical translation of chronic antioxidant therapy for maintaining oocyte quality is therefore impractical, these studies tie the free radical theory of aging (12) to reduced oocyte quality in females of advanced reproductive age (13). In further support of this, induced oxidative stress in isolated mouse oocytes reduces ATP levels and increases meiotic spindle abnormalities leading to chromosomal misalignment (14). Additionally, whereas meiotic maturation of human oocytes can proceed over a range of ATP levels, oocytes with higher ATP show a greater potential for successful embryogenesis, implantation, and development (15).
Restricted caloric intake without malnutrition extends lifespan and attenuates severity of aging-related health complications in many species (16–18). A common feature of the caloric restriction (CR) response appears to be an alteration of metabolic regulators that affect mitochondrial dynamics and accumulated oxidative stress in organs with age (19–21). For example, the growth hormone/insulin/insulin-like growth factor-1 axis, mammalian target of rapamycin, AMP-activated protein kinase, and sirtuins have all been implicated as mediators of CR (18, 22–24). Several of these pathways reportedly converge on peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α), a transcriptional regulator that is highly responsive to nutritional cues. Among its actions, PGC-1α promotes adaptation to energy deficiency by modulating expression of genes involved in mitochondrial respiration (18, 22–26). Surprisingly, deletion of PGC-1α in mice produces only subtle phenotypes, although several metabolic abnormalities manifest much more robustly upon a challenge such as acute fasting (27–29). To our knowledge, however, no studies have tested the functional relationship between PGC-1α and CR in any tissue with age by subjecting Pgc-1α–null mice to a reduced calorie diet. Herein we undertook a 4-y investigation to elucidate whether CR during adulthood without or with manipulation of PGC-1α influences oocyte quality in female mice on the verge of reproductive failure due to advancing maternal age.
Results
We first evaluated yield, maturational status, and postfertilization developmental competency of oocytes obtained from 12-mo-old (aged) female mice returned to an ad libitum (AL) diet for 1 mo following 7.5 mo of dietary CR (CR–AL fed) initiated in a stepwise fashion at 3.5 mo of age. This protocol was based on prior work showing that female mice maintained on CR during adulthood continue to breed and deliver offspring into advanced ages after their return to an AL diet (30). In control females allowed to AL feed during the entire study period, the total number of oocytes and number of fully mature oocytes (oocytes that reached meiotic metaphase II, MII) ovulated per female decreased significantly between 3 and 12 mo of age (Fig. 1A). However, the age-related decline in both total and mature oocyte yield was abrogated in 12-mo-old female mice maintained on CR (Fig. 1A). Following analysis of 284 (3-mo-old AL fed), 93 (12-mo-old AL fed), and 198 (12-mo-old CR–AL fed) oocytes, no differences were observed with respect to in vitro fertilization (IVF) or preimplantation embryonic development rates (Fig. S1). However, because CR improved the yield of MII oocytes per female after an induced ovulation cycle at 12 mo of age (Fig. 1A), the number of blastocysts obtained following IVF of oocytes obtained from each aged CR–AL-fed mouse was similar to that obtained using young mice and significantly higher than that using aged AL-fed mice (Fig. 1B).
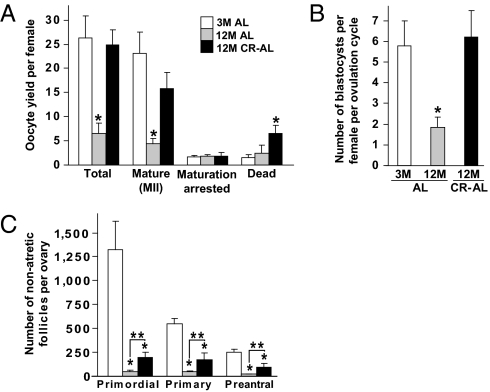
CR prevents the aging-related decline in ovulated oocyte numbers. (A) Yield and morphology of oocytes obtained after induced ovulation of 3-mo-old (3M) AL-fed (n = 6), 12-mo-old (12M) AL-fed (n = 12), and 12M CR–AL-fed (n = 6) mice (mean ± SEM; *P < 0.05 versus 3M AL-fed females). (B) Number of in vitro fertilized MII oocytes that developed to blastocysts per induced ovulation cycle per female (n = 11–16 mice per group; mean ± SEM; *P < 0.05 versus 3M AL-fed females). (C) Number of nonatretic immature follicles per ovary in 3M AL-fed, 12M AL-fed, and 12M AL–CR-fed mice (mean ± SEM, n = 9–14 mice per group; *P < 0.05 versus 3M AL-fed females; **P < 0.05).
To determine whether the beneficial effect of CR on maintaining oocyte yield from aging females was related to differences in body weight, we assessed superovulation rates in young AL-fed, aged AL-fed, and aged CR–AL-fed females on a mouse-by-mouse basis. We observed that differences in oocyte yield per mouse, which were greatest in the aged AL-fed group, were unrelated to variations in body weight among the three groups of mice (Fig. S2). Also notable was that the reserve of oocyte-containing follicles in ovaries of both 12-mo-old AL-fed and CR–AL-fed females was severely diminished compared with that of 3-mo-old mice (Fig. 1C). Thus, the ability of CR to maintain a high yield of MII oocytes from aged females does not appear linked to changes in body weight or maintenance of a follicle reserve equivalent in size to that of young females.
We next studied the quality of MII oocytes collected from aged AL-fed and CR–AL-fed females. Fully mature (MII) oocytes were selected for analysis because aging-related defects in oocytes are clearly evident at this maturational stage and because MII oocytes represent the fertilization-competent egg pool. To this end, we assessed chromosomal dynamics, spindle integrity, and mitochondrial dynamics, which are the most critical events involved in ensuring developmental competency of the egg. In MII oocytes collected from continuously AL-fed females, the incidence of hyperploidy (>20 chromosomes per cell; Fig. 2A) increased significantly from nondetectable levels at 3 mo of age to nearly 5% at 12 mo of age. In contrast, no hyperploidy was detected in MII oocytes from 12-mo-old mice maintained on CR (Fig. 2B). The incidence of hypoploidy (<20 chromosomes per cell) was also significantly elevated in MII oocytes from 12-mo-old versus 3-mo-old AL-fed females, and this was completely prevented by CR (Fig. 2B). A similar pattern in the incidence of premature sister chromatid separation (PSCS) was observed in mature oocytes among the three groups of mice, although these changes were not statistically significant (Fig. 2B).
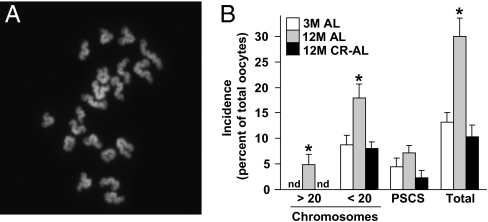
Aging-associated aneuploidy in MII oocytes is prevented by CR. (A) Example of a typical chromosome spread of an MII oocyte containing 20 chromosomes (DAPI staining of DNA shown in white). (B) Incidence of hyperploidy, hypoploidy, and PSCS (and total chromosomal defects from all three endpoints combined) in MII oocytes of 3M AL-fed, 12M AL-fed, and 12M CR–AL-fed females (mean ± SEM, n = 18–23 mature oocytes analyzed per group in each experiment replicated four times using a total of 20–34 mice per group; *P < 0.05 versus 3M AL-fed females; nd, none detected).
Confocal analysis of α-tubulin and DNA distribution revealed that meiotic spindles in greater than 90% of MII oocytes collected from either 3-mo-old AL-fed or 12-mo-old CR–AL-fed females were regular in shape and size with distinct microtubule morphology; however, less than 39% of MII oocytes retrieved from 12-mo-old AL-fed mice exhibited normal meiotic spindles (Fig. 3 A and C). Furthermore, whereas 64% of MII oocytes from 12-mo-old AL-fed mice exhibited incomplete or aberrant alignment of chromosomes on the metaphase plate, 25% or fewer of the MII oocytes collected from either 3-mo-old AL-fed or 12-mo-old CR–AL-fed females exhibited chromosomal misalignment (Fig. 3 B and C).
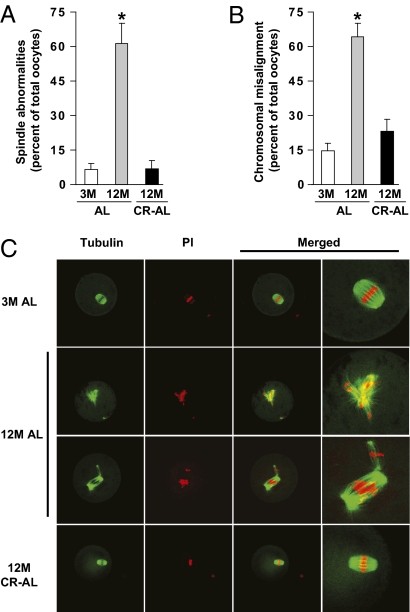
CR prevents spindle and chromosomal alignment defects in oocytes of aged females. (A and B) Incidence of spindle abnormalities (A) and chromosomal misalignment on the metaphase plate (B) in MII oocytes of 3M AL-fed, 12M AL-fed, and 12M CR–AL-fed mice (mean ± SEM, n = 3–20 oocytes analyzed per group in each experiment replicated four to seven times using a total of four to eight mice per group; *P < 0.05 versus 3M AL-fed females). (C) Representative examples of meiotic spindles in MII oocytes from the indicated mice (n = 22–72 oocytes analyzed per group), after labeling with α-tubulin antibody (green) and counterstaining of DNA with PI (red).
We then assessed whether mitochondrial aggregation, which has been linked to the decline in oocyte quality with advancing age (7), was affected by caloric intake. Confocal microscopic analyses of MII oocytes stained with MitoTracker revealed that over 90% of MII oocytes collected from 3-mo-old AL-fed females exhibited even and diffuse cytoplasmic distribution of mitochondria (Fig. 4 A and B). By comparison, nearly 50% of MII oocytes obtained from 12-mo-old AL-fed females exhibited extensive mitochondrial aggregation. However, more than 90% of mature oocytes collected from 12-mo-old CR–AL-fed females exhibited even and diffuse mitochondrial distribution, resembling that observed in MII oocytes retrieved from young females (Fig. 4 A and B). Paralleling these changes in mitochondria, the aging-related decline in ATP content in oocytes of aged AL-fed females was similarly prevented by adult-onset CR (Fig. 4C).
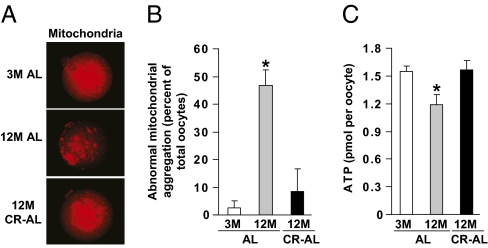
CR maintains normal mitochondrial dynamics in oocytes of aged females. (A) Representative mitochondrial distribution in MII oocytes from 3M AL-fed, 12M AL-fed, and 12M CR–AL-fed mice (MitoTracker staining shown in red). (B) Incidence of abnormal mitochondrial aggregation in MII oocytes from 3M AL-fed, 12M AL-fed, and 12M CR–AL-fed mice (mean ± SEM, n = 23–46 oocytes analyzed per group from three independent experiments using 4–11 mice per group; *P < 0.05 versus 3M AL-fed females). (C) Cytoplasmic ATP levels in individual MII oocytes from 3M AL-fed, 12M AL-fed, and 12M CR–AL-fed mice (mean ± SEM, n = 38–145 total oocytes analyzed per group from five to seven independent experiments using a total of 5–21 mice per group; *P < 0.05 versus 3M AL-fed females).
Finally, we used gene mutant mice to explore whether deletion of PGC-1α, which has been linked to the actions of CR in other cell types (24, 31–33) and is expressed in oocytes (Fig. 5A and Fig. S3), influences the ability of CR to maintain oocyte quality with age. Consistent with past studies (27), an absence of PGC-1α increased mortality in mutant offspring (90 pups of 696 total generated by breeding heterozygotes were genotyped as knockouts at day 21). Assessment of null females that survived to 12 mo (36 of 47 total) showed that PGC-1α deficiency in AL-fed mice recapitulated the beneficial effects of CR on ovulated oocyte yield (Fig. 5B), meiotic spindle formation (Fig. 5C), chromosomal alignment (Fig. 5D), and mitochondrial distribution within the cytoplasm (Fig. 5E). At 12 mo, AL-fed females lacking PGC-1α exhibited a slightly larger follicle reserve than their wild-type counterparts, but follicle numbers remained severely diminished compared with young adult animals of either genotype (Fig. S4). No further changes in oocyte numbers per ovary (Fig. S4), or in oocyte yield or quality (Fig. 5 B–D), were observed when mice lacking PGC-1α were subjected to CR.
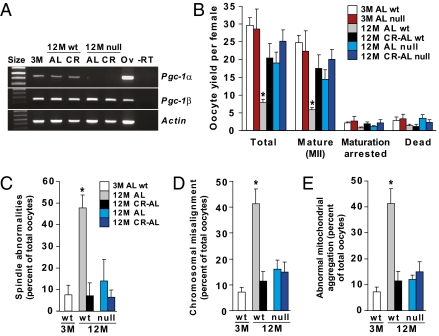
Loss of PGC-1α improves oocyte yield and quality in aging females. (A) RT-PCR analysis of Pgc-1α and Pgc-1β mRNA levels in isolated MII oocytes of 3M AL-fed wild-type (WT) mice, 12M AL-fed or CR–AL-fed WT mice, or 12M AL-fed or CR–AL-fed Pgc-1α–null mice (Actin, control gene for sample loading; size, molecular size marker; Ov, adult ovary RNA used as a positive control; −RT, RT-PCR analysis of ovary RNA without reverse transcriptase as a negative control). (B−E) Effects of PGC-1α deficiency in AL-fed and CR-AL-fed females on oocyte yield following superovulation (B), meiotic spindle formation (C), chromosomal alignment on the metaphase plate (D), and mitochondrial distribution (E). Definitions for D and E are the same as C. Data are the mean ± SEM (n = 20–117 oocytes analyzed per group for each endpoint from three independent experiments using a total of 3–14 mice per group; *P < 0.05 versus all other groups).
Discussion
Oocyte donation studies show that aging-related infertility in women can be effectively overcome through the use of oocytes from young adult donors (34, 35). Additionally, postmenopausal women can carry pregnancies to term as surrogates with success rates equaling those of younger patients undergoing ART with their own oocytes (36, 37). It is therefore believed that deterioration of egg quality is the single most important factor for determining pregnancy success in women of advanced reproductive age. Production of a developmentally competent egg requires that an oocyte successfully completes the reductive cell division program of meiosis. A full chromosome complement is then restored upon fusion of the egg with a haploid sperm at fertilization, initiating embryogenesis. Unfortunately, the meiotic cell cycle becomes highly prone to errors with age, which often results in a much higher proportion of aneuploid oocytes ovulated by older women (38, 39). One of the most widely known consequences of female reproductive aging is a dramatic rise in trisomy 21, which increases from around 2% of clinical pregnancies in women in their twenties to 30% or more of clinical pregnancies in women in their forties (2, 39). Our understanding of this maternal age effect remains limited; however, analyses of human and mouse oocytes have shown that aging disrupts the ability of oocytes to assemble and maintain meiotic spindles, which tightly align homologous chromosomes for segregation at anaphase. Other than chronic antioxidant treatment (10), which has significant limitations (11), efforts to prevent chromosomal or meiotic spindle abnormalities in oocytes of aging females have proven unsuccessful in any model system.
Here we provide evidence from studies in mice that not just chromosomal integrity but a spectrum of endpoints that impact on oocyte quality are all maintained in aged females by CR during adult life. Further, the following observations indicate that these endpoints may be intricately linked in the context of understanding how aging and CR affect oocytes. First, given the importance of a properly formed spindle to chromosomal alignment and segregation during meiosis, prevention of aging-related aneuploidy in oocytes by CR can logically be tied to dramatic improvements in meiotic spindle assembly and maintenance. Of all the endpoints assessed in our study, the increase in incidence of oocytes exhibiting spindle abnormalities, and consequently chromosomal misalignment, in AL-fed females from less than 10% to almost 65% between 3 and 12 mo of age offers the most prominent example of the negative influence of maternal aging on egg quality. Whereas there is some variation in the reported prevalence of these abnormalities in the literature, which may be due to strain- or methodology-related differences, the high rates of chromosomal and spindle abnormalities observed in our study are consistent with previous reports in humans and mice at ages close to the end of their reproductive lifespan (7, 8, 10, 40–43).
We also observed that adult-onset CR inhibited the aging-associated increase in mitochondrial aggregation in oocytes and maintained intracellular ATP concentrations at levels comparable to those detected in oocytes of young adult females. On the basis of prior studies with mouse and human oocytes showing that impaired mitochondrial function and lower ATP levels are associated with meiotic spindle abnormalities and failed conception (14, 15), the decrease in ATP availability in oocytes of aged AL-fed females is consistent with a critical need for adequate energy availability in proper assembly and maintenance of meiotic spindles.
Interestingly, PGC-1α deficiency in aged AL-fed mice reproduced the actions of CR on oocyte quality, and combining the two approaches (namely, PGC-1α–deficient mice maintained on CR) produced the same outcomes in oocytes as those obtained using each approach alone. On the basis of gene expression analysis, CR has been reported in somatic cells to act, at least in part, through activation of PGC-1α (24, 31). In addition, PGC-1α is thought to mediate gluconeogenesis in response to CR, although evidence for this conclusion derives from studies of acute fasting of animals or overexpression of PGC-1α using adenoviruses (25, 44) and not CR. Our study is unique, as far as we are aware, in subjecting mice lacking PGC-1α to a reduced calorie diet as a means to assess the functional role of this nuclear coactivator in mediating the actions of CR in any tissue with age. On the basis of correlations drawn from gene expression studies (24, 45), we initially expected that the effects of CR might be minimized, rather than reproduced, by PGC-1α deficiency. Although unanticipated, this outcome is similar to the unexpected increase in gluconeogenic gene expression in the livers of PGC-1α–deficient animals (27). Mice lacking PGC-1α are also surprisingly lean and do not develop diet-induced obesity or insulin resistance when maintained on a high-fat diet (25, 27, 29). Thus, whereas a positive correlation between CR and elevated PGC-1α expression in various tissues has been reported (45), some of the beneficial effects of CR in animals may be tied to reduced, rather than elevated, function of the PGC-1α pathway.
Because levels of PGC-1 protein remained essentially unchanged in ovaries of AL- or CR–AL-fed mice with age (Fig. S5), it does not appear that CR directly alters Pgc-1 gene expression in this organ. However, the finding that CR and PGC-1α independently produced the same outcomes in ovulated oocytes suggests that signaling pathways activated in the two models converge at a common downstream point that is essential to ensuring egg quality. Although more work will be needed to definitively establish this, coordination through mitochondria is a logical possibility for several reasons (46, 47). First, both PGC-1α deficiency and CR maintained an even and diffuse distribution of active mitochondria in oocytes of aged female mice, contrasting starkly with the abnormal aggregation of mitochondria observed in oocytes of AL-fed mice with age. This latter event was associated with a significant decline in oocyte ATP content, a threshold level of which is required for assembly and maintenance of the meiotic spindle (14). Second, both CR and PGC-1α interact with sirtuins as a means to control adaptive responses to energy availability (22–25). Recent findings have shown that multiple sirtuins isoforms are expressed in mouse eggs, and that loss of mitochondrial-associated sirtuin-3 in oocytes increases mitochondrial production of reactive oxygen species leading to impaired preimplantation embryonic development (48). Such an outcome is consistent with a primary role for accumulated oxidative stress as a driving force behind declining oocyte quality with age and with the known inverse relationship between CR and aging-associated increases in mitochondrial oxidant damage in the body (19–21).
In summary, this study has uncovered striking beneficial effects of adult-onset CR on chromosomal, spindle, and mitochondrial dynamics in mature oocytes of female mice at ages normally associated with poor reproductive parameters. These outcomes translate into vastly improved fertility in aged animals on the basis of recent work with mice showing that CR initiated during adulthood significantly extends reproductive lifespan and increases survival rate of offspring conceived by aging females (30). The present study not only establishes that CR sustains female fertile potential with age through significant improvements in oocyte chromosomal dynamics, but also identifies PGC-1α as a regulator of oocyte quality. More broadly, this study reinforces the idea that oocyte aneuploidy and spindle defects are not inevitable consequences of the aging process, thus opening prospects to safely circumvent the negative impact of aging on germline chromosomal segregation during meiosis. And whereas the effects of CR on ovarian function, oocyte dynamics, or germline aneuploidy in primates are currently unknown, recent studies have shown that rhesus monkeys maintained on CR into advanced age exhibit many of the same health benefits as those reported in mouse studies (18, 49, 50). Thus, it seems reasonable to now add prevention of oocyte aneuploidy and spindle defects, as a means to improve fertility and pregnancy outcomes in women of advanced reproductive age, to the growing list of human health endpoints that might one day become manageable through CR mimetics currently in development (51–55).
Materials and Methods
Animals.
B6D2F1 male mice were obtained from The Jackson Laboratory. Virgin C57BL/6 female mice were obtained from the National Institute on Aging or The Jackson Laboratory. Mice with a targeted disruption of the Pgc-1α gene (27) were obtained as heterozygous breeders from B. M. Spiegelman (Harvard Medical School, Boston, MA). All experiments were reviewed and approved by the institutional animal care and use committee of Massachusetts General Hospital.
Feeding Regimen.
We used an adult-onset 40% CR protocol developed by the National Institute on Aging (56). Females were housed individually in pathogen-free facilities and fed once daily with a rationed amount of fortified rodent diet (30, 56). The CR protocol was continued from 3.5 until 11 mo of age, at which time CR females were AL fed for 1 mo. Water was provided AL during the entire study. Effectiveness of the CR protocol was confirmed by monitoring body weight and estrous cyclicity (Figs. S6 and S7; SI Materials and Methods for details).
Oocyte Retrieval.
Mice were superovulated by injection of pregnant mare serum gonadotropin (PMSG, 10 IU; Sigma-Aldrich) followed by human chorionic gonadotrophin (hCG) (10 IU; Sigma-Aldrich) 46−48 h later. Oocytes were collected from oviducts 15−16 h after hCG injection, denuded of cumulus cells using hyaluronidase (Irvine Scientific), washed with human tubal fluid (HTF) (Irvine Scientific) supplemented with BSA (fraction V, fatty acid-free; Sigma-Aldrich), and classified as MII (first polar body in perivitelline space), maturation arrested (germinal vesicle breakdown with no polar body extrusion, or germinal vesicle intact), or degenerated.
IVF and Embryo Culture.
Sperm were collected from the cauda epididymides of male mice into HTF supplemented with BSA and then capacitated. Denuded MII oocytes or intact cumulus–oocyte complexes were mixed with 1−2 × 106 sperm/mL in HTF supplemented with BSA for 6−9 h, washed, and transferred to fresh medium. The number of two-cell embryos was used to measure IVF success rate, and blastocyst development rates from these embryos were recorded (57) (SI Materials and Methods for details).
Chromosomal Analysis.
A total of 795 mature (MII) oocytes collected from 3-mo-old AL-fed (n = 20 mice), 12-mo-old AL-fed (n = 34 mice), and 12-mo-old CR–AL-fed (n = 20 mice) females were fixed individually for chromosomal analysis using Tarkowski’s method (58, 59). Preparations were stained with 4',6-diamidino-2-phenylindole dihydrochloride (DAPI; Sigma-Aldrich) and scored for aneuploidy rates under a fluorescence microscope (SI Materials and Methods for scoring criteria).
Immunofluorescence.
Superovulated oocytes were denuded of cumulus cells, briefly incubated in acidified tyrode’s solution (Irvine Scientific) to soften the zona pellucida, and immunostained (SI Materials and Methods for details) using mouse anti–α-tubulin antibody (Sigma-Aldrich) followed by goat antimouse IgG conjugated with Alexa Fluor-488 (Life Technologies). Oocytes were mounted using Vectashield containing propidium iodide (PI; Vector Laboratories) and analyzed by confocal microscopy.
Mitochondrial Analysis.
Oocytes were denuded of cumulus cells, incubated in MitoTracker Red CMRox (Life Technologies), and processed for microscopic analysis (SI Materials and Methods for details). Levels of ATP in individual MII oocytes were determined using a commercially available bioluminescent assay kit under the manufacturer’s specifications (Sigma-Aldrich).
Gene Expression.
Total RNA from five MII oocytes or one ovary was isolated using the RNeasy Plus Micro kit (Qiagen) or Tri-Reagent (Sigma-Aldrich), respectively, and reverse transcribed (Superscript II; Life Technologies) with random primers (Promega). The cDNA was amplified by PCR with gene-specific primers (Table S1).
Protein Analysis.
PGC-1 protein was localized in paraformaldehyde-fixed paraffin-embedded tissue sections using a rabbit anti–PGC-1 antibody (Calbiochem), as described (60). Protein samples (10 μg) were assessed by immunoblotting using antibodies against PGC-1 (Calbiochem) and panactin (Neomarkers) as a loading control (SI Materials and Methods for details).
Data Analysis.
All experiments were independently replicated at least three times. Quantitative data from experimental replicates were combined and are presented as the mean ± SEM. Statistical comparisons between mean values were performed using ANOVA and Student’s t test. P values <0.05 were considered significant.
Acknowledgments
We are grateful to P.A. Hunt for technical support in assessing oocyte aneuploidy, D. A. Sinclair for critical reading of the manuscript before submission, B. M. Spiegelman for providing Pgc-1α mutant mice, and D. C. Woods and R. Tyszkowski for technical assistance. This study was supported by National Institutes of Health’s Method to Extend Research in Time (MERIT) Award R37-AG012279, the Henry and Vivian Rosenberg Philanthropic Fund, the Sea Breeze Foundation, and Vincent Memorial Research Funds. K.S. is a recipient of a Massachusetts General Hospital’s Executive Committee on Research (ECOR) Fund for Medical Discovery Award.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/10.1073/pnas.1018793108/-/DCSupplemental.
References
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.1018793108
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3145697?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/102453178
Article citations
An aggregated mitochondrial distribution in preimplantation embryos disrupts nuclear morphology, function, and developmental potential.
Proc Natl Acad Sci U S A, 121(27):e2317316121, 25 Jun 2024
Cited by: 0 articles | PMID: 38917013 | PMCID: PMC11228517
The Antioxidant Salidroside Ameliorates the Quality of Postovulatory Aged Oocyte and Embryo Development in Mice.
Antioxidants (Basel), 13(2):248, 19 Feb 2024
Cited by: 0 articles | PMID: 38397846 | PMCID: PMC10886307
Oocyte Health and Quality: Implication of Mitochondria-related Organelle Interactions.
Results Probl Cell Differ, 73:25-42, 01 Jan 2024
Cited by: 0 articles | PMID: 39242373
Review
Polyamine metabolite spermidine rejuvenates oocyte quality by enhancing mitophagy during female reproductive aging.
Nat Aging, 3(11):1372-1386, 16 Oct 2023
Cited by: 21 articles | PMID: 37845508
Deletion of enzymes for de novo NAD+ biosynthesis accelerated ovarian aging.
Aging Cell, 22(9):e13904, 18 Jun 2023
Cited by: 10 articles | PMID: 37332134 | PMCID: PMC10497836
Go to all (121) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Mechanisms of oocyte aneuploidy associated with advanced maternal age.
Mutat Res Rev Mutat Res, 785:108320, 04 Jul 2020
Cited by: 90 articles | PMID: 32800274
Review
Chromosome Segregation in the Oocyte: What Goes Wrong during Aging.
Int J Mol Sci, 23(5):2880, 07 Mar 2022
Cited by: 21 articles | PMID: 35270022 | PMCID: PMC8911062
Review Free full text in Europe PMC
Activin Decoy Receptor ActRIIB:Fc Lowers FSH and Therapeutically Restores Oocyte Yield, Prevents Oocyte Chromosome Misalignments and Spindle Aberrations, and Increases Fertility in Midlife Female SAMP8 Mice.
Endocrinology, 157(3):1234-1247, 29 Dec 2015
Cited by: 3 articles | PMID: 26713784 | PMCID: PMC4769367
Granulosa cell mevalonate pathway abnormalities contribute to oocyte meiotic defects and aneuploidy.
Nat Aging, 3(6):670-687, 15 May 2023
Cited by: 5 articles | PMID: 37188792
Funding
Funders who supported this work.
NIA NIH HHS (4)
Grant ID: R37 AG012279
Grant ID: R37 AG012279-17
Grant ID: R37 AG012279-16
Grant ID: R37-AG012279




