Abstract
Background
Individuals with rheumatoid arthritis (RA) often are sedentary and have an increased risk of developing comorbid conditions. Women with RA are more likely to experience challenges in maintaining an active lifestyle over their life span than men with RA or people who are healthy. As the benefits of physical activity (PA) are well known, measuring PA accurately in this population is important.Objectives
The purposes of this study were: (1) to characterize PA as measured with the SenseWear Armband (SWA) in women with RA and (2) to determine the measurement time frame to obtain consistent estimates of PA and daily energy expenditure (EE) in women with RA.Design
This was a cross-sectional study.Methods
Participants wore the SWA for 7 days. Measurements of daily total energy expenditure (TEE), physical activity energy expenditure (PAEE) during activities at or above 1 metabolic equivalent (MET) level (PAEE≥1MET), PAEE during activities at or above 2 METs (PAEE≥2METs), PAEE during activities at or above 3 METs (PAEE≥3METs), and number of steps were obtained.Results
Fifty-three women participated. Complete data were obtained for 47 participants (89%). Daily usage of the SWA was 98% of the time (23:31 hours/24 hours). Means (SD) were 2,099 (340) kcal/d for TEE, 1,050 (331) kcal/d for PAEE≥1MET, 642 (309) kcal/d for PAEE≥2METs, 239 (178) kcal/d for PAEE≥3METs, and 7,260 (2,710) for number of steps. Results of intraclass correlation coefficient analyses and multiple linear regressions indicated that 2 days were needed to reliably estimate TEE; 3 days for PAEE≥1MET, PAEE≥2METs, and number of steps; and 4 days for PAEE≥3METs.Limitations
The sample was composed of well-educated women with RA who had mild to moderate difficulty performing daily activities.Conclusion
The SWA may be useful to quantify PA in women with RA and to monitor effectiveness of interventions aiming to increase PA levels. Minimizing the number of days necessary for data collection will reduce the individual's burden and may improve adherence in studies of PA behaviors.Free full text

Physical Activity Measured by the SenseWear Armband in Women With Rheumatoid Arthritis
Abstract
Background
Individuals with rheumatoid arthritis (RA) often are sedentary and have an increased risk of developing comorbid conditions. Women with RA are more likely to experience challenges in maintaining an active lifestyle over their life span than men with RA or people who are healthy. As the benefits of physical activity (PA) are well known, measuring PA accurately in this population is important.
Objectives
The purposes of this study were: (1) to characterize PA as measured with the SenseWear Armband (SWA) in women with RA and (2) to determine the measurement time frame to obtain consistent estimates of PA and daily energy expenditure (EE) in women with RA.
Design
This was a cross-sectional study.
Methods
Participants wore the SWA for 7 days. Measurements of daily total energy expenditure (TEE), physical activity energy expenditure (PAEE) during activities at or above 1 metabolic equivalent (MET) level (PAEE≥1MET), PAEE during activities at or above 2 METs (PAEE≥2METs), PAEE during activities at or above 3 METs (PAEE≥3METs), and number of steps were obtained.
Results
Fifty-three women participated. Complete data were obtained for 47 participants (89%). Daily usage of the SWA was 98% of the time (23:31 hours/24 hours). Means (SD) were 2,099 (340) kcal/d for TEE, 1,050 (331) kcal/d for PAEE≥1MET, 642 (309) kcal/d for PAEE≥2METs, 239 (178) kcal/d for PAEE≥3METs, and 7,260 (2,710) for number of steps. Results of intraclass correlation coefficient analyses and multiple linear regressions indicated that 2 days were needed to reliably estimate TEE; 3 days for PAEE≥1MET, PAEE≥2METs, and number of steps; and 4 days for PAEE≥3METs.
Limitations
The sample was composed of well-educated women with RA who had mild to moderate difficulty performing daily activities.
Conclusion
The SWA may be useful to quantify PA in women with RA and to monitor effectiveness of interventions aiming to increase PA levels. Minimizing the number of days necessary for data collection will reduce the individual's burden and may improve adherence in studies of PA behaviors.
Individuals with rheumatoid arthritis (RA) often are sedentary and have increased risk of developing cardiovascular and cerebrovascular diseases at least in part due to the vascular consequences of chronic systemic inflammation.1–4 Rheumatoid arthritis is more prevalent in women, and women with RA are more likely than men with RA and people who are healthy to experience a challenge in maintaining an active lifestyle over the course of their lives.3,5–7 As physical activity (PA) is known to reduce the risk of comorbid conditions,8 low levels of PA in people with RA may increase their susceptibility to atherosclerotic diseases even further. Therefore, accurate estimation of PA and energy requirements in this population is important. Precise estimation of PA may improve the understanding of how PA affects health conditions and may allow accurate monitoring of the effectiveness of trials that aim to increase PA in this population.
In patients with RA, estimation of energy expenditure (EE) during PA traditionally has been done with self-reported questionnaires and diaries.3 Although these measures are inexpensive and easy to administer and can distinguish the types of activities performed,9 their validity is questionable. They are flawed by inconsistent subject recall, overestimation of PA, and underestimation of sedentary pursuits, all of which result in inadequate psychometrics.10 To overcome the limitation of these measures, portable activity monitors, such as pedometers, heart rate monitors, and accelerometers, were developed to collect real-time PA information. Among activity monitors, the accelerometer-based monitors have demonstrated superior reliability and validity.11–14 However, these monitors do not capture activities that do not involve ambulation, they underestimate EE during activities at low intensities,11,15,16 and they require considerable data processing and interpretation efforts.
An alternative option is the SenseWear Armband (SWA).* This monitor combines information from multisensors such as biaxial accelerometers, heat flux (heat being dissipated by the body), galvanic signal (onset, peak and recovery of maximal sweat rates), and skin temperature. The information is integrated and processed by software using proprietary algorithms utilizing an individual's demographic characteristics (sex, age, height, and weight) to provide minute-by-minute estimates of EE during different levels of PA. The SWA has good psychometric properties compared with other portable monitors17–20 and has the advantage of quantifying PA at low intensities and during activities that do not involve ambulation, as well as unstructured or intermittent PA. These characteristics are important because patients with RA may perform most of daily activities at low intensities, may perform work in very short bouts, and may execute activities that do not involve ambulation, all of which may not be captured by accelerometers or pedometers.
To date, several studies have used portable monitors to characterize PA in diverse populations. However, only 3 studies used accelerometers in RA: 1 study investigated methods for processing accelerometry data,21 and 2 studies with very small sample sizes (fewer than 20 participants) attempted to characterize patterns of PA.5,20 The 2 studies that used accelerometers to characterize PA in people with RA did not report on activities performed at low intensity levels. There is a dearth of information about the characteristics of energy expended by patients with RA during low-intensity activities. In addition, studies have not determined the appropriate monitoring time frame for measuring free-living PA in people with RA. In other words, the minimum number of days wearing the accelerometer that are necessary to obtain consistent measurements of PA remains undefined. This limitation should be considered, as the monitoring time frame may be different for patients with RA because they are less active and their disease activity fluctuates. Determining a monitoring time frame is a critical issue in research when considering surveillance, sampling costs, and subject burden. Physical activity is known to vary considerably across the day (more activity during working hours) and the week (less activity during weekends). Consequently, a monitoring time frame should consider the inherent day-to-day variability (intra-individual) and the differences in PA among individuals (inter-individual variability) to yield stable estimates. Studies on this topic have been either in young adults or children who were healthy,22–25 and the results cannot be generalized to patients with RA because the variability in patterns of PA is likely not similar. The purposes of this study were: (1) to characterize daily energy requirements and PA level in women with RA measured with the SWA and (2) to determine the measurement time frame to obtain consistent estimates of PA and daily EE in women with RA.
Method
Participants
This was a cross-sectional study. Participants were recruited from an original cohort that assessed 104 women with RA for subclinical cardiovascular disease and associated risk factors.26 This ancillary study was planned before the 5-year follow-up visit of the parent study, and all women who came back for the follow-up visit were invited to participate. Of the 56 women who returned for follow-up, 53 agreed to participate. The 3 women who declined stated that they had insufficient time to complete the study. The study took place from November 2007 to July 2008. Eligibility criteria were age older than 30 years, diagnosis of RA in accordance with the American College of Rheumatology criteria27 for at least 2 years, and no cardiovascular events (myocardial infarction, angina, or stroke) prior to recruitment. Study participants signed informed consent statements approved by the University of Pittsburgh Institutional Review Board.
Procedure
Participants were assessed in one testing session by trained study personnel who administered demographic, history, and self-report questionnaires and performed a clinical examination. Height and weight were measured with a scale and stadiometer; these measurements were used to estimate body mass index (BMI=weight in kilograms/height in square meters). Disease activity was measured by the Modified Disease Activity Score (DAS-28), a reliable and valid measure of disease activity in people with RA.28 The DAS-28 includes in its calculation the number of tender and swollen joints (of 28 joints), erythrocyte sedimentation rate, and the individual's self-assessment of disease activity using a 100-mm visual analog scale.28,29 Pain intensity was measured using a 100-mm visual analog scale.30 Disability was measured by the Health Assessment Questionnaire (HAQ-DI), a widely used and validated tool to quantify functional disability in people with RA.31,32 The HAQ-DI queries the individual about 20 activities of daily living, and the score is expressed on a scale from 0 (no disability) to 3 (severe disability).
Energy expenditure was measured by the SWA, a multisensor monitor worn on the back of the right upper arm (over the triceps muscle) as per manufacturer orientation. The SWA records continuous physiological data during daily life activities. The SWA has been shown to be valid for assessing EE in free-living conditions.14,33–35 Energy expenditure estimated by the SWA correlates strongly with estimates from doubly labeled water and indirect calorimetry than estimates from accelerometers.34–36 Differences in daily values of EE (in kilocalories per day) measured by the SWA and doubly labeled water ranged from 4% to 27%,14,33–35 and the difference between the SWA and indirect calorimetry was 9%.36 Although worn on the arm, the SWA has been shown to accurately measure step counts compared with accelerometers worn on the waist (difference ranging from 2% to 16%) and pedometers (0.2% difference).34,36
Participants were instructed to wear the SWA for 10 consecutive days, 24 hours per day. The goal was to obtain at least 7 consecutive days with 12 hours of daily data to fulfill requirements for the analysis plan. Participants were instructed to take the SWA off for showering and water exercises, as the SWA is not waterproof. They also were provided with written instructions to certify that the SWA was working properly after it was removed and returned to the individual's arm. When the participants were not wearing the device, they were asked to record the type and time of the activity on their log sheet. From all participants, 5 reported water activities (aerobics) performed in a swimming pool for around 45 minutes a week. Participants also were instructed to call the research coordinator if they experienced any problem wearing the SWA. After the 10-day period, participants returned the armband and the log sheet by mail, and the data were downloaded into the SWA software (SenseWear Professional software version 6.1).* Data about time off recorded on the log sheet were verified by inspecting the data downloaded into the SWA software. The software flags any minute that the SWA is not worn and gives the option to manually enter the activity performed during that time. Discrepancies between the time off recorded in the logs and the time off indicated by the software were resolved by calling the participants to clarify SWA usage.
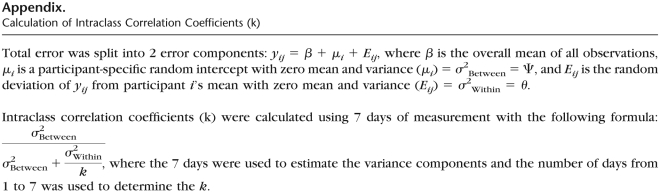
The SWA software allows researchers to select PA at any metabolic equivalent (MET) level. We explored daily averages of physical activity energy expenditure (PAEE) at 3 different intensity levels: (1) PAEE during activities at or above 1 MET (PAEE≥1MET), (2) PAEE during activities at or above 2 METs (PAEE≥2METs), and (3) PAEE during activities at or above 3 METs (PAEE≥3METs). The Figure exemplifies the measures of PA at different intensities. As the average person at rest has an oxygen consumption of 3.5 mL/kg/min (or 1 MET), the measure of PAEE≥1MET represents the daily energy spent at any activity at or above rest level, including very light activities such as watching television, writing, desk work, knitting, and driving. The measure of PAEE≥2METs represents the daily energy spent at or above light activities such as slow walking (~2 mph), light cycling (~5 mph), playing a musical instrument, bartending, cooking, scrubbing, and showering. Finally, the measure of PAEE≥3METs represents the daily energy spent at or above moderate activities such as brisk walking (~3 mph), cycling (~5.5 mph), bowling, canoeing, janitorial work, cleaning windows, and climbing stairs. Additional variables processed by the SWA were total energy expenditure (TEE) and number of steps. Total energy expenditure represents energy spent at any MET level, from sleeping peacefully to vigorous PA, which includes all values of PAEE.
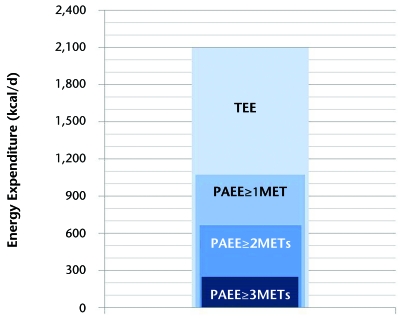
Schematic representation of energy expenditure values. Total energy expenditure (TEE) represents energy spent at any metabolic equivalent (MET) level, from sleeping peacefully to vigorous physical activity and includes all values of physical activity energy expenditure (PAEE). PAEE≥1MET represents energy spent during physical activities at or above 1 MET, PAEE≥2METs represents energy spent in physical activities at or above 2 METs and PAEE≥3METs is the energy spent in physical activities at or above 3 METs.
Data Management and Analysis
We determined adherence to wearing the SWA by calculating the number of participants who wore the SWA for 7 or more consecutive days with at least 12 hours of data daily and the proportion of hours in the day the device was worn out of 24 possible hours. The number of complaints related to wearing the SWA was collected from information in the log sheets and from telephone calls.
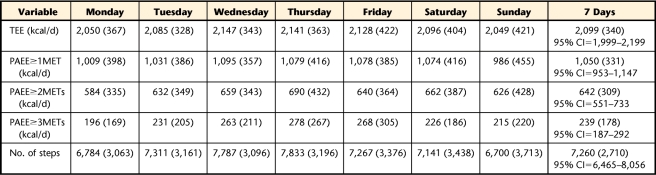
Data for the 7 days of the week were obtained from the 10-day period by: (1) excluding the first and last days of data collection, as they represented only half-days, and (2) from the remaining 8 days, organizing the data to obtain the 7 days of the week, starting with Monday (Monday through Sunday) and deleting the remaining day. For the characterization of TEE, PAEE at 3 different levels, and number of steps, we calculated descriptive statistics of each day of the week and the average of the 7 days of wearing the SWA. We then conducted a repeated-measures analysis of variance (ANOVA) to determine whether TEE, PAEE≥1MET, PAEE≥2METs, PAEE≥3METs, and number of steps differed across the 7 days of the week.
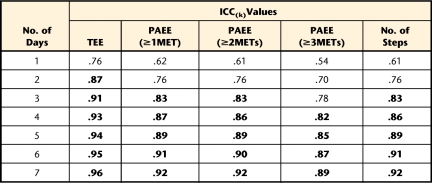
To determine the minimum number of days needed to obtain consistent estimates of TEE, PAEE≥1MET, PAEE≥2METs, PAEE≥3METs, and number of steps, we performed 2 analyses. First, multiple linear regression models37 were built to determine the minimum number of days needed to predict the average of 7 days of the week of TEE, PAEE at 3 different levels, and number of steps. The average of 7 days of the week was the dependent variable. The independent variables were all possible 42 combinations of days of the week (7 combinations of 1 day, 7 combinations of 2 days, and up to 7 combinations of 6 days). Each combination started at a particular week day (eg, Monday, Tuesday, and so forth). For example, for the combinations of 2 days, with starting day on Tuesday (ie, Tuesday and Wednesday), the average of these 2 days was compared with the average of the 7 days to examine how much of the variance in the 7 days was explained by 2 days. Therefore, 42 models were built for each of the measures of EE (TEE, PAEE≥1MET, PAEE≥2METs, PAEE≥3METs, and number of steps). The R2 value for each model was adjusted by the number of independent variables. In the second analysis, intraclass correlation coefficients (ICCs) were calculated to obtain reliable estimates of the number of days38 using a one-way ANOVA model taking repeatability into account, as proposed by Tudor-Locke et al39 for measures of PA (Appendix). Adjusted R2 values and ICCs above .80 were considered adequate.40 We used SAS version 9.1† and Stata version 10‡ to perform the statistical analyses.
Results
From the 53 participants, complete data (≥7 consecutive days with at least 12 hours of data daily) were obtained on 47, representing 89% adherence with respect to obtaining complete data. For 1 participant, data were not available because the SWA's battery was placed incorrectly, and for another individual data were unavailable due to a malfunction of the device. In the other 4 participants, data were obtained for fewer than 6 days due to the following complaints: 2 participants had skin rashes in the area of the Velcro strap§ of the SWA, and 2 participants had discomfort while wearing the device during sleep. The 47 participants wore the SWA for an average (SD) of 23.5 (0.6) hours a day.
Analyses included only the 47 participants with complete data to provide an accurate representation of PA during all days of the week. Sample demographics and biomedical characteristics are depicted in Table 1. Measurements of daily TEE, PAEE at 3 different levels, and number of steps are reported in Table 2. Results of the repeated-measures ANOVA demonstrated that there were no significant differences among measurements of PA across days of the week (F value for TEE=0.54, P=.78; F value for PAEE≥1MET=0.50, P=.81; F value for PAEE≥2METs=0.37, P=.90; F value for PAEE≥3METs=0.84, P=.54; and F value for number of steps=0.84, P=.54).
Table 1.
Sample Demographics and Biomedical Characteristics (N=47)a
Table 2.
Descriptive Characteristics of Energy Requirements During the Days of the Week (N=47)a
Results of the multiple linear regressions (Tab. 3) showed that any combination of 2 days of data collection predicted at least 86% of variability in TEE. Any combination of 3 days of data collection predicted at least 87% of variability in PAEE≥1MET, 86% of variability in PAEE≥2METs, and 87% of variability in number of steps. Any combination of 4 days of data collection predicted at least 84% of variability in PAEE≥3METs. These results were consistent across all days of the week. The ICC analysis (Tab. 4) revealed that 2 days wearing the SWA would be required to reliably estimate TEE (ICC=.87); 3 days would be required to reliably estimate PAEE≥1MET (ICC=.83), PAEE≥2METs (ICC=.83), and number of steps (ICC=.83); and 4 days would be required for achieving reliable estimation of PAEE≥3METs (ICC=.82).
Table 3.
Summary of the Linear Regression Models to Determine the Number of Days Required to Predict the Average of 7 Weekdaysa
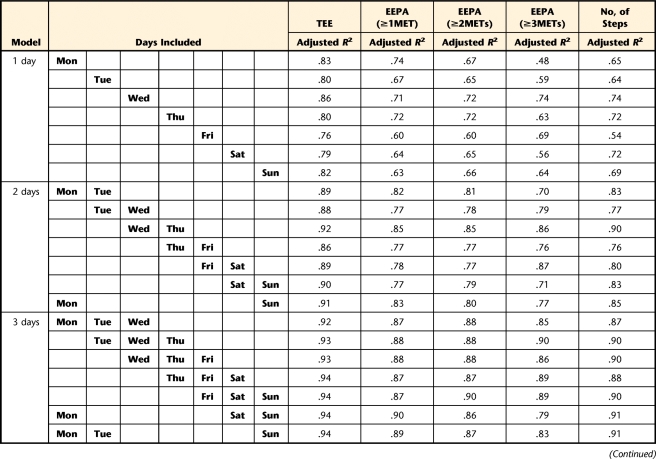
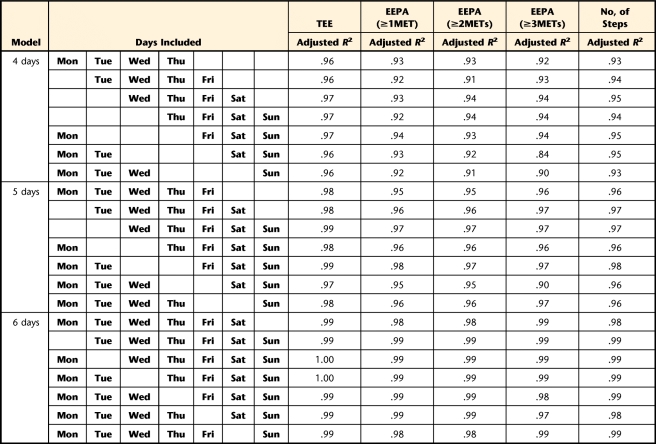
Table 4.
Intraclass Correlation Coefficients (ICCs) With Different Number of Days of Measurement, Using All 7 Days of Single Measurementa
Discussion
This is likely the first study to characterize PA at different intensity levels in women with RA and to establish a measurement time frame to obtain consistent estimates when using the SWA. Reporting on the characteristics of PA in women with RA will provide a point of reference to compare PA across studies and to help researchers to develop interventions designed to increase PA. Determining a measuring time frame will help to reduce patients' burden and improve adherence to interventions in future studies.
The mean (SD) value of TEE in our study (ie, 2,099 [340] kcal/d) was comparable to the results reported by Roubenoff et al5 using doubly labeled water to assess TEE (2,181 [319] kcal/d) in 20 women with RA. As doubly labeled water is considered the gold standard for assessing EE in free-living conditions, deriving similar values of TEE helps to support the validity of the SWA to assess TEE in women with RA. This finding is relevant for health care providers who are interested in energy balance (energy intake versus EE), weight management, and studies of the metabolic consequences of RA. On the other hand, we were not able to directly compare our results of PAEE with those of other RA studies. Existing studies estimated PAEE using different methods. Roubenoff et al5 used doubly labeled water and an accelerometer (Caltrac‖). Doubly labeled water estimates PAEE from an equation where resting EE and the thermic effect of food (10% of TEE) are subtracted from TEE. In the Roubenoff et al study, the mean (SD) PAEE was 681 (257) kcal/d, representing 31% of the participants' TEE.5 To make an indirect comparison, we subtracted the thermic effect of food in our study (10% of TEE=209 kcal/d) from the value of PAEE≥1MET: 1,050 − 209 kcal/d. Estimated using this approach, the value of PAEE in our study was 841 kcal/d, representing 40% of the participants' TEE. In our study, therefore, the PAEE was higher than the values reported by Roubenoff et al.5 The difference potentially can be explained by different study methods or by the fact that our participants were indeed somewhat more physically active.
Comparisons between PAEE estimated by accelerometry and SWA are not suitable because accelerometers do not measure activities that do not involve the lower extremities, such as playing video games, needlework, and desk work. In addition, results from accelerometry studies of people with RA have expressed PA in activity counts rather than kilocalories per minute.20,21 Three studies reported on moderate levels of PA (PAEE≥3METs) in adults using the SWA.34,41,42 One study in older adults (average age=75 years, BMI=26 kg/m2) reported a PAEE≥3METs value of 282 kcal/d.34 Another study in patients with type 2 diabetes (average age=55 years, BMI=34 kg/m2) reported a PAEE≥3METs value of 287 kcal/d.41 The third study assessed people with chronic obstructive pulmonary disease (COPD) during 2 days (12 hours per day) and reported a PAEE≥3METs value of 412 kcal/d.42 In our cohort, the PAEE≥3METs value was 239 kcal/d. The results suggest that our patients engaged in slightly less PA of moderate intensity than older adults and middle-aged individuals with diabetes and considerably less than individuals with COPD. The intermittent joint pain and fatigue experienced by patients with RA may explain the lower PA in our cohort study. It also is possible that some of the differences in PAEE≥3METs values may be explained because our study sample comprised only women, whereas the samples in the other 3 studies comprised men and women. Men are known to adopt a more active lifestyle than women.3,6 Because in the study of people with COPD the SWA was worn 12 hours for only 2 days, the higher value of PAEE≥3METs in this sample may reflect a short-lived motivation of the sample with COPD to be more active while wearing the monitoring device.
To our knowledge, no studies have investigated the differences between PA behavior on weekdays and weekend days in people with RA or other disabilities. The only studies on this topic have used populations of youth (5–16 years old),23,43–45 and adults who were healthy (mean age=38 years).46 These studies reported a significant difference, in that moderate to vigorous PA was observed more often during the weekends compared with the weekdays. Our results were not in agreement with these findings. Although our participants appeared to be more physically active in the middle of the week, this finding was not statistically significant. A potential explanation for this discrepancy between previously described findings and our own may be because the majority of our participants were regularly employed. Although youth and young adults who are healthy may use the weekend to exercise, our participants may have used the weekend to take a break and rest from the workweek. Their mild to moderate level of disability, reflected in the HAQ-DI scores, speaks to their ability to maintain employment.
Results of the multivariable regression and ICC analyses suggested that different numbers of days are needed to consistently estimate TEE, PAEE at 3 different levels, and number of steps. To be pragmatic, we suggest that a minimum of 4 consecutive days of SWA data collection would be necessary to achieve an ICC of at least .80 for all variables, regardless of the weekday on which the data collection begins. However, as the ICCs increase steadily by adding more days of data collection, Table 4 can be used to guide the decision on the number of days needed to collect PA data if greater reliability is sought. Our findings are in agreement with those of other studies that investigated monitoring time frame in adults. Coleman and Epstein,25 using the TriTrac accelerometer,# concluded that 4 days of assessment are required to achieve reliable measures of daily activity, with generalizability coefficients ≥.80. Matthews et al24 reported that 3 to 4 days of daily activity measures are necessary to achieve ICC≥.80 using the Computer Science and Applications accelerometer.** These results do not appear to be applicable to younger populations. Studies in children and adolescents have shown that anywhere from 4 to 9 days of data collection using accelerometry were necessary.22,24 This discrepancy between the numbers of days needed to reliably estimate PA in children compared with adults may have been due to a higher variability in the patterns of PA in younger individuals.
The health benefits of PA are known to occur with regular PA (30 minutes a day, 5 days a week) of moderate intensity or higher (PAEE≥3METs).8 For this reason, most measures of PA calculate energy spent during PA at moderate to vigorous levels. However, according to the 2008 Physical Activity Guidelines for Americans,8 the minimum amount of activity needed to produce a benefit cannot be stated with certainty. It was suggested that there is no PA threshold below which there are no health benefits. Particularly in people with RA, who spend more energy in activities below moderate intensity (Tab. 2), it is important to capture PA behaviors at workloads lower than 3 METs. Accurate measures of PA at lower levels may allow identification of changes in PA that would not be possible with other measuring methods. For example, in a person with a very low level of PA, an increase from walking very slowly inside the house for 20 minutes a day to walking outside for 45 minutes a day at 2 mph (absolute intensity of 2 METs), may represent a significant increase in PA. Being able to walk outside at 2 mph may allow the person to participate in community activities, and perhaps result in some health benefits. Therefore, we believe the clinical relevance of our study relies on its use of a portable activity monitor that captures PA at low workloads and that enables the researcher or clinician to assess improvements in time and energy spent at each intensity level.
Our results also suggested that patients with RA were comfortable wearing the SWA (89% adherence). Although the number of complaints was small, we believe adherence could have been further improved if we had instructed the participants to moisturize or cover the skin under the Velcro strap of the SWA or if we had advised them to remove the device at night if they experienced discomfort while sleeping. Our rate of adherence is comparable to or better than that of other studies that used portable activity monitors in other adult populations,33,34,46–51 suggesting that the SWA is a feasible device for assessing PA in women with RA.
Our study had some limitations. Although the characteristics of our sample appear to be similar to those reported in other studies of PA in people with RA,2–4,22 the generalizability of our findings should consider the relatively small number of participants studied, the inclusion of only women, the relatively high levels of education achieved, and the mild to moderate difficulty experienced when carrying out daily tasks (based on HAQ-DI scores). These factors should be considered when choosing a monitoring time frame of PA in samples of people with RA that include men, people with less education, and patients with severe physical disability. The relevance of real-time measurements of PA in patients with RA is further supported by the significant association between PAEE≥3METs and HAQ scores (r=−.38, P=.009), as reported in a previous article by our group.52 Although we investigated the appropriateness of reducing the number of days of data collection with the SWA, it does not imply that this device is superior to other accelerometers. Similar studies should be done to compare in parallel the performance of different activity monitors against a gold standard in people with RA. Finally, as we collected data only for 7 to 10 days, our data do not capture seasonal variability in PA. Future studies should collect data over multiple intervals throughout the year to confirm findings.
In conclusion, the SWA is a viable method of quantifying PA and may be useful to monitor effectiveness of interventions to increase PA in people with RA. A combination of any 4 days of the week predicted more than 80% of the variance across 7 days of data collection and allowed for consistent measurements with ICCs above .80. Minimizing the number of days necessary for data collection will reduce patients' burden and may improve adherence to studies of PA. The SWA may be considered for measuring PA in people with RA in future trials and may be a useful clinical tool to measure PA in patients with RA.
Footnotes
Dr Wasko and Dr Piva provided concept/idea/research design, fund procurement, and facilities/equipment. All authors provided writing. Mr Almeida and Dr Wasko provided data collection and project management. Dr Wasko provided participants. Mr Almeida provided clerical support. Dr Wasko, Mr Jeong, and Dr Moore provided consultation (including review of manuscript before submission).
This study was approved by the University of Pittsburgh Institutional Review Board.
This research was presented at the American College of Rheumatology Annual Meeting, October 24–29, 2008, San Francisco, California, and October 16–21, 2009, Philadelphia, Pennsylvania, and at the Combined Sections Meeting of the American Physical Therapy Association, February 6–9, 2009, Las Vegas, Nevada.
No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit on the authors or on any organization with which the authors are associated.
This study was partially supported by the Arthritis Foundation−Western Pennsylvania Chapter and MUH-CTRC-NIH/NCRR/CTSA grant UL1 RR024153.
*BodyMedia Inc, 420 Fort Duquesne Blvd, Pittsburgh, PA 15222.
†SAS Institute Inc, 100 SAS Campus Dr, Cary, NC 27513.
‡StataCorp LP, 4905 Lakeway Dr, College Station, TX 77845.
§Velcro USA Inc, 406 Brown Ave, Manchester, NH 03103.
‖Muscle Dynamics, 20100 Hamilton Ave, Torrance, CA 90502.
#Professional Products Inc, 960 17th St, Prairie Du Sac, WI 53578.
**Computer Science and Applications Inc, 2 Clifford Dr, Shalimar, FL 32579.
References
Articles from Physical Therapy are provided here courtesy of Oxford University Press
Full text links
Read article at publisher's site: https://doi.org/10.2522/ptj.20100291
Read article for free, from open access legal sources, via Unpaywall:
https://academic.oup.com/ptj/article-pdf/91/9/1367/17503240/ptj1367.pdf
Citations & impact
Impact metrics
Citations of article over time
Article citations
Cross-sectional and longitudinal neural predictors of physical activity and sedentary behaviour from a 6-month randomized controlled trial.
Sci Rep, 14(1):919, 09 Jan 2024
Cited by: 0 articles | PMID: 38195673 | PMCID: PMC10776740
Supporting physical activity for mobility in older adults with mobility limitations (SuPA Mobility): study protocol for a randomized controlled trial.
Trials, 24(1):769, 28 Nov 2023
Cited by: 0 articles | PMID: 38017467 | PMCID: PMC10685660
Four Days Are Enough to Provide a Reliable Daily Step Count in Mild to Moderate Parkinson's Disease through a Commercial Smartwatch.
Sensors (Basel), 23(21):8971, 04 Nov 2023
Cited by: 4 articles | PMID: 37960670 | PMCID: PMC10649244
Endothelial dysfunction in ME/CFS patients.
PLoS One, 18(2):e0280942, 02 Feb 2023
Cited by: 13 articles | PMID: 36730360 | PMCID: PMC9894436
Activity monitoring and patient-reported outcome measures in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome patients.
PLoS One, 17(9):e0274472, 19 Sep 2022
Cited by: 1 article | PMID: 36121803 | PMCID: PMC9484698
Go to all (56) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Study to determine the criterion validity of the SenseWear Armband as a measure of physical activity in people with rheumatoid arthritis.
Arthritis Care Res (Hoboken), 65(6):888-895, 01 Jun 2013
Cited by: 27 articles | PMID: 23213019
Profile of energy expenditure in people with rheumatoid arthritis.
Disabil Health J, 8(4):514-520, 31 Mar 2015
Cited by: 2 articles | PMID: 25953350
Comparison of methods to assess energy expenditure and physical activity in people with spinal cord injury.
J Spinal Cord Med, 35(1):35-45, 01 Jan 2012
Cited by: 34 articles | PMID: 22330189 | PMCID: PMC3240915
Computational methods for estimating energy expenditure in human physical activities.
Med Sci Sports Exerc, 44(11):2138-2146, 01 Nov 2012
Cited by: 26 articles | PMID: 22617402 | PMCID: PMC3475744
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NCRR NIH HHS (1)
Grant ID: UL1 RR024153
NICHD NIH HHS (1)
Grant ID: K01 HD058035