Abstract
Purpose
To estimate the maximum-tolerated dose (MTD), describe dose-limiting toxicities (DLTs), and characterize pharmacokinetic properties of MK-0752, a gamma secretase inhibitor, in children with refractory or recurrent CNS malignancies.Patients and methods
MK-0752 was administered once daily for 3 consecutive days of every 7 days at escalating dosages starting at 200 mg/m(2). The modified continual reassessment method was used to estimate the MTD. A course was 28 days in duration. Pharmacokinetic analysis was performed during the first course. Expression of NOTCH and hairy enhancer of split (HES) proteins was assessed in peripheral-blood mononuclear cells (PBMCs) before and following treatment with MK-0752.Results
Twenty-three eligible patients were enrolled: 10 males (median age, 8.1 years; range, 2.6 to 17.7 years) with diagnoses of brainstem glioma (n = 6), ependymoma (n = 8), medulloblastoma/primitive neuroectodermal tumor (n = 4), glioblastoma multiforme (n = 2), atypical teratoid/rhabdoid tumor (n = 1), malignant glioma (n = 1), and choroid plexus carcinoma, (n = 1). Seventeen patients were fully evaluable for toxicity. No DLTs occurred in the three patients enrolled at 200 mg/m(2)/dose. At 260 mg/m(2)/dose, DLTs occurred in two of six patients, both of whom experienced grade 3 ALT and AST. There were no grade 4 toxicities; non-dose-limiting grade 3 toxicities included hypokalemia and lymphopenia. Population pharmacokinetic values (% coefficient of variation) for MK-0752 were apparent oral clearance, 0.444 (38%) L/h/m(2); apparent volume of distribution, 7.36 (24%) L/m(2); and k(a), 0.358 (99%) hr(-1).Conclusion
MK-0752 is well-tolerated in children with recurrent CNS malignancies. The recommended phase II dose using the 3 days on followed by 4 days off schedule is 260 mg/m(2)/dose once daily.Free full text

Phase I Trial of MK-0752 in Children With Refractory CNS Malignancies: A Pediatric Brain Tumor Consortium Study
Abstract
Purpose
To estimate the maximum-tolerated dose (MTD), describe dose-limiting toxicities (DLTs), and characterize pharmacokinetic properties of MK-0752, a gamma secretase inhibitor, in children with refractory or recurrent CNS malignancies.
Patients and Methods
MK-0752 was administered once daily for 3 consecutive days of every 7 days at escalating dosages starting at 200 mg/m2. The modified continual reassessment method was used to estimate the MTD. A course was 28 days in duration. Pharmacokinetic analysis was performed during the first course. Expression of NOTCH and hairy enhancer of split (HES) proteins was assessed in peripheral-blood mononuclear cells (PBMCs) before and following treatment with MK-0752.
Results
Twenty-three eligible patients were enrolled: 10 males (median age, 8.1 years; range, 2.6 to 17.7 years) with diagnoses of brainstem glioma (n = 6), ependymoma (n = 8), medulloblastoma/primitive neuroectodermal tumor (n = 4), glioblastoma multiforme (n = 2), atypical teratoid/rhabdoid tumor (n = 1), malignant glioma (n = 1), and choroid plexus carcinoma, (n = 1). Seventeen patients were fully evaluable for toxicity. No DLTs occurred in the three patients enrolled at 200 mg/m2/dose. At 260 mg/m2/dose, DLTs occurred in two of six patients, both of whom experienced grade 3 ALT and AST. There were no grade 4 toxicities; non–dose-limiting grade 3 toxicities included hypokalemia and lymphopenia. Population pharmacokinetic values (% coefficient of variation) for MK-0752 were apparent oral clearance, 0.444 (38%) L/h/m2; apparent volume of distribution, 7.36 (24%) L/m2; and ka, 0.358 (99%) hr−1.
Conclusion
MK-0752 is well-tolerated in children with recurrent CNS malignancies. The recommended phase II dose using the 3 days on followed by 4 days off schedule is 260 mg/m2/dose once daily.
INTRODUCTION
The four NOTCH proteins constitute a family of heterodimeric receptors, each with a single transmembrane domain. NOTCH signaling plays a crucial role in normal tissue development, cell fate determination, proliferation, and survival.1,2 NOTCH signaling is activated following the binding of cognate ligands that include Delta1, Delta2, and Delta3 and Jagged1 and Jagged2. Ligand binding activates proteolytic cleavage mediated by ADAM family protease and gamma secretase. Gamma secretase, a multiprotein complex, is an important component in the NOTCH cleavage machinery that catalyzes the cleavage of receptor protein substrates within their transmembrane domain.3–5 Gamma secretase cleaves several substrates including amyloid precursor protein, NOTCH receptors, and ligands.6 Cleavage of the NOTCH receptor releases the receptor intracellular domain allowing it to translocate to the cell nucleus where it binds to the transcriptional regulator CSL and activates gene transcription.7 Transcriptional targets of NOTCH cell signaling include hairy enhancer of split (HES) and HES-related proteins gene families.1,8,9 NOTCH dysregulation has been associated with several cancers, including T-cell leukemia,10 breast cancer,11–13 colon adenocarcinoma,14 and cervical cancer.15,16 Increased NOTCH signaling also plays a role in salivary gland carcinomas.17
The NOTCH pathway plays a central role in normal neural stem-cell regulation and maintenance.15,18–20 Recent data indicate that most brain tumors, including gliomas,21 medulloblastoma,22 and ependymoma,23 are maintained by rare fractions of stem-cell–like cancer cells. NOTCH signaling appears to be important in the sonic hedgehog type of medulloblastoma24; NOTCH amplification has been identified in a subgroup of posterior fossa ependymomas,23 and high levels of NOTCH ligand expression (Delta1, Jagged1, and Jagged2) have been identified in high-grade gliomas.25
MK-0752 is an oral inhibitor of gamma secretase, which inhibits the cleavage of gamma secretase substrates such as amyloid precursor protein and Notch with a concentration that inhibits 50% (IC50) of 50 nmol/L.26 MK-0752 was initially developed as a treatment for Alzheimer's disease. When tested in animal models of cerebral amyloid formation and deposition, MK-0752 penetrated the brain parenchyma reducing plasmatic amyloid peptide levels by 50% at brain and plasma concentrations of 154 nmol/L and 594 nmol/L, respectively. In vitro, MK-0752 leads to G0→G1 arrest in gamma secretase in T-cell acute lymphatic leukemia cell lines carrying notch-activating mutations with IC50 of 6.2 μmol/L and has demonstrated activity in neuT transgenic mouse models of mammary tumors.27 In a phase I study in adults with recurrent solid tumor, the maximum-tolerated dose (MTD) of MK-0752 using the 3-days-on/4-days-off schedule was 450 mg/d with a recommended dose of 350 mg/d because of fatigue during extended administration. Dose-limiting toxicities (DLTs) included diarrhea, nausea, vomiting, constipation, and fatigue.26
We report the results of a phase I trial of MK-0752 in children with recurrent or refractory malignant CNS tumors. The primary objectives were to estimate the MTD of MK-0752 administered 3 consecutive days of every 7 days for each 28-day cycle. The secondary objectives were to describe the DLTs of MK-0752 by using the above schedule to characterize MK-0752 plasma pharmacokinetics and to explore the incidence of NOTCH receptor and ligand expression and pathway activation in recurrent or refractory CNS tumor samples and peripheral-blood mononuclear cells (PBMCs).
PATIENTS AND METHODS
Patient Eligibility
Eligible patients were age ≥ 3 and ≤ 21 years with a histologically verified malignant CNS tumor (histology was not required for diffuse intrinsic pontine gliomas) refractory to conventional therapy and Lansky or Karnofsky performance score ≥ 60. Patients were required to have recovered from acute toxic effects of prior therapy and not to have received growth factors within 7 days of study entry; myelosuppressive chemotherapy within 3 weeks (6 weeks if prior nitrosourea); biologic agent within 7 days; biologic/investigational agents with a prolonged half-life within 3 weeks; craniospinal or total-body irradiation within 6 months; local palliative radiotherapy within 2 weeks; or other substantial bone marrow irradiation within 6 weeks. Patients on corticosteroids must have been on a stable or decreasing dose for at least 2 weeks. Other requirements included adequate bone marrow (peripheral absolute neutrophil count ≥ 1,000/μL, platelet count ≥ 100,000/μL, transfusion independent hemoglobin ≥ 8.0 g/dL), renal (serum creatinine ≤ 1.5× upper limit of normal for age, or glomerular filtration rate ≥ 70 mL/min/1.73 m2), and liver (total bilirubin ≤ 1.5× institutional upper limit of normal for age, ALT ≤ 2.5× institutional upper limit of institutional normal for age, and albumin ≥ 2.5 g/dL) function. Pregnant or lactating females and patients on enzyme-inducing anticonvulsants were excluded from the study. Patients of childbearing or child fathering potential had to agree to use a medically acceptable form of birth control, including abstinence, while on this study.
Informed consent was obtained from patients, parents, or guardians, and assent was obtained as appropriate at the time of protocol enrollment. The institutional review boards of each Pediatric Brain Tumor Consortium institution approved the protocol before initial patient enrollment, and continuing approval was maintained throughout the study.
Drug Administration and Study Design
MK-0752 (Merck, West Point, PA) supplied as 10-, 50-, and 200-mg dry filled, hard gelatin capsules was administered orally once daily for 3 consecutive days of every 7 days. Each course was 28 days in length. MK-0752 was given in the morning with or without food, with 8 ounces of water. For patients with difficulty swallowing, the capsules could be opened with active pharmaceutical ingredients (powder) released into a nonacidic beverage (eg, water or milk), as a slurry (sugar could be added to the beverage to enhance compliance), or thoroughly mixed into ice cream. The volume for mixing could be as little as 1 mL, and the preparation could be mixed with either a metal or plastic spoon.
Once mixed, the preparation would remain stable in this form for at least 5 to 10 minutes, and the entire preparation had to be administered. The starting MK-0752 dosage was 200 mg/m2/dose once daily (approximately 75% of the MTD of 450 mg). Dosage levels for subsequent patient cohorts were escalated in 30% increments after at least two patients were treated and monitored for one course at each dosage level. Patients could receive up to six courses in the absence of disease progression.
The MTD, defined as the dosage at which 25% of patients would be expected to experience a DLT, was estimated via the modified continual reassessment method (CRM).28 Toxicities were graded according to the National Cancer Institute Common Toxicity Criteria for Adverse Events (version 3.0). Hematologic DLT was defined as grade 4 neutropenia or thrombocytopenia. Nonhematologic DLT was defined as any grade 3 or 4 nonhematologic toxicity with the specific exclusion of grade 3 nausea or vomiting of less than 5 days duration responsive to antiemetic therapy; grade 3 diarrhea responsive to optimal use of antidiarrheal agents; grade 3 fever or infection of less than 5 days duration; or grade 3 hypophosphatemia, hypokalemia, or hypomagnesemia responsive to oral supplementation. Any clinically significant or sufficiently intolerable toxicity that led to the withholding of more than one dose of drug by the clinician was also considered a DLT.
Pretreatment evaluations included a history, physical examination with a thorough neurologic examination, performance status, disease evaluation, CBC, electrolytes, renal and liver function tests, and a pregnancy test for female patients of childbearing age. CBCs were obtained weekly during course 1, every 2 weeks during course 2, and before each subsequent course. History, physical examinations, and serum chemistries were obtained weekly in course 1 and before each subsequent course.
Disease evaluations were obtained at baseline, after course 2, and after every other course thereafter. Tumor response was defined as follows: complete response, disappearance of all measurable lesions on magnetic resonance imaging; partial response, ≥ 50% reduction in tumor size by bidimensional measurement on a stable or decreasing dose of corticosteroids accompanied by a stable or improving neurologic examination and maintained for at least 6 weeks; progressive disease, worsening neurologic status or more than 25% increase in the bidimensional measurement, or appearance of new lesions, or increasing corticosteroids doses; stable disease (SD), magnetic resonance imaging response did not meet the criteria for other categories, with stable neurologic examination and corticosteroid dose.29
Pharmacokinetic Studies
Blood samples (2 mL) were collected from consenting patients in heparinized tubes before the MK-0752 dose and at 1, 2, 3, 6, 8 (± 2), and 24 (± 2) hours after the first dose of course 1. The plasma concentrations of MK-0752 were determined by using a validated liquid chromatography tandem mass spectrometry method.30 The lower limit of quantitation for MK-0752 was 50 ng/mL. The within-day and between-day precision (% coefficient of variation [CV]) values and accuracy values met standard assay validation criteria.
For each patient, the maximum concentration (Cmax) and the time to maximum concentration (Tmax) were the observed values. A one-compartment model was fitted to the MK-0752 plasma concentrations by using mixed effects nonlinear regression (NONMEM version VI) and the first-order conditional estimation method with interaction. Model parameters for each patient were used to simulate the plasma concentration-time profile, from which area under the plasma concentration-time curve (AUC) zero to infinity was calculated by using the log-linear trapezoidal method.
Notch Receptor Expression and Signal Activity
Expression of NOTCH1 (antibody #3608, Cell Signaling Technology, Danvers, MA), HES1 (#5702, Millipore, Billerica, MA), and HES5 (#5708, Millipore) was analyzed in pretreatment formalin-fixed paraffin-embedded tumor sections by using standard immunohistochemical (IHC) techniques as described previously.31,32 Expression of the cleaved NOTCH1 intracellular domain (NICD1; antibody #4147, Cell Signaling), HES1, and HES5 (antibodies as for IHC) proteins in PBMCs were quantified by Western blot analysis before and 8 and 24 hours following treatment with MK-0752. Changes in expression of NOTCH-cleaved forms and NOTCH pathway target genes were correlated with MK-0752 systemic exposure (eg, Cmax and AUC).
Neural stem cells were transduced with retrovirus-encoding human NOTCH1 intracellular domain (mouse stem cell virus—NOTCH1 intracellular domain—red fluorescence protein [MSCV-NCID-RFP]) or control virus (empty vector MSCV-RFP). NICD protein and associated upregulation of HES1 was detected in NICD-transduced but not control cells.
RESULTS
Patient Characteristics
Twenty-three patients were enrolled on the study. Table 1 summarizes the characteristics of the eligible patients. Twenty-one patients were evaluable for toxicity. Two patients were not evaluable for toxicity: they were unable to complete course 1 because of progressive disease. The median number of courses was 1 (range, 1 to 4).
Table 1.
Characteristics of Eligible Patients (n = 23)
| Characteristic | No. of Patients |
|---|---|
| Sex | |
    Male Male | 10 |
    Female Female | 13 |
| Age, years | |
    Median Median | 8.1 |
    Range Range | 2.6-17.7 |
| Diagnosis | |
    Ependymoma Ependymoma | 8 |
    Brain stem glioma Brain stem glioma | 6 |
    Medulloblastoma/primitive neuroectodermal tumor Medulloblastoma/primitive neuroectodermal tumor | 4 |
    Glioblastoma multiforme Glioblastoma multiforme | 2 |
    Malignant glioma (grade III to IV) Malignant glioma (grade III to IV) | 1 |
    Choroid plexus carcinoma Choroid plexus carcinoma | 1 |
    Atypical teratoid/rhabdoid tumor Atypical teratoid/rhabdoid tumor | 1 |
| Prior therapy | |
    Radiotherapy only Radiotherapy only | 3 |
    Chemotherapy and radiotherapy Chemotherapy and radiotherapy | 18 |
    Chemotherapy, radiotherapy, and stem-cell transplantation Chemotherapy, radiotherapy, and stem-cell transplantation | 2 |
| Courses of MK-0752 | |
    Median Median | 1 |
    Range Range | 1-4 |
Toxicities
The observed DLTs are summarized in Table 2. At dose level 200 mg/m2, no DLTs were observed. At 260 mg/m2, one of the first three patients had DLTs of increased AST (326 U/L) and ALT (163 U/L; both grade 3). The cohort was expanded to include three more patients, one of whom experienced DLTs of grade 3 ALT (333 U/L) and AST (333 U/L). On the basis of the CRM, 260 mg/m2/dose was the estimated MTD and the recommended phase II dose. This cohort was expanded to include 12 additional evaluable patients with mandatory pharmacokinetic testing to ensure that pharmacokinetic data were available on at least 12 patients as well as to obtain additional toxicity information regarding this dose. Among the additional 12 patients, one other experienced a DLT of grade 3 ALT (171 U/L). The grade 3 toxicities resolved to baseline at a median of 7 days (range, 3 to 16 days).
Table 2.
Dose-Limiting Toxicity Summary (course 1)
| MK-0752 mg/m2* | No. of Patients Enrolled | No. of Patients Evaluable | No. of Patients With DLT | DLT |
|---|---|---|---|---|
| 200 | 3 | 3 | 0 | |
| 260 | 6 | 6 | 2 | Grade 3 AST (n = 2); grade 3 ALT (n = 2)† |
| 260 (expanded PK cohort) | 14 | 12 | 1 | Grade 3 ALT (n = 1) |
Abbreviations: DLT, dose-limiting toxicity; PK, pharmacokinetics.
Table 3 summarizes all adverse events at least possibly attributable to MK-0752 in all eligible patients. There were no grade 4 toxicities, and no patient required hospitalization for treatment-related complications.
Table 3.
All Toxicities Attributed to Therapy in Eligible Patients
| Toxicity | Grade | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | Total | |||||
| No. | % | No. | % | No. | % | No. | % | |
| Lymphopenia | 6 | 5 | 7 | 4 | 2 | 1 | 15 | 6 |
| Fatigue | 5 | 5 | 6 | 6 | 11 | 8 | ||
| Hypokalemia | 7 | 5 | 2 | 2 | 9 | 6 | ||
| Increased ALT | 3 | 3 | 2 | 2 | 3 | 3 | 8 | 6 |
| Nausea | 5 | 5 | 1 | 1 | 7 | 7 | ||
| Increased AST | 4 | 4 | 2 | 2 | 6 | 4 | ||
| Hypophosphatemia | 4 | 3 | 1 | 1 | 5 | 4 | ||
| Vomiting | 4 | 4 | 1 | 1 | 5 | 4 | ||
| Leukopenia | 5 | 4 | 5 | 4 | ||||
| Anorexia | 2 | 2 | 1 | 1 | 3 | 3 | ||
| Pain (headache) | 2 | 2 | 1 | 1 | 3 | 3 | ||
| Hyponatremia | 3 | 2 | 3 | 2 | ||||
| Constipation | 3 | 3 | 3 | 3 | ||||
| Neutropenia | 1 | 1 | 1 | 1 | 2 | 2 | ||
| Low serum bicarbonate | 2 | 1 | 2 | 1 | ||||
| Alkaline phosphatase | 2 | 2 | 2 | 2 | ||||
| Diarrhea | 2 | 2 | 2 | 2 | ||||
| Pain | 2 | 2 | 1 | 1 | 3 | 3 | ||
| Thrombocytopenia | 2 | 2 | 2 | 2 | ||||
| Weight loss | 2 | 2 | 2 | 2 | ||||
| Pruritus/itching | 1 | 1 | 1 | 1 | ||||
| Rash/desquamation | 1 | 1 | 1 | 1 | ||||
| Blurred vision | 1 | 1 | 1 | 1 | ||||
| Hypoalbuminemia | 1 | 1 | 1 | 1 | ||||
| Hyperbilirubinemia | 1 | 1 | 1 | 1 | ||||
| Cough | 1 | 1 | 1 | 1 | ||||
| Fever (in the absence of neutropenia) | 1 | 1 | 1 | 1 | ||||
| Hypermagnesemia | 1 | 1 | 1 | 1 | ||||
| Hypomagnesemia | 1 | 1 | 1 | 1 | ||||
Responses
No objective responses were reported. Most patients experienced progression of their disease after one or two courses. Prolonged SD (≥ three courses of therapy) was observed in only two patients: one patient with ependymoma (four courses) and one patient with glioblastoma multiforme (three courses).
Pharmacokinetics
After the first MK-0752 dose of course 1, pharmacokinetic studies were obtained in one patient receiving 200 mg/m2 dosage and 17 patients receiving 260 mg/m2 dosage (Table 4). Population pharmacokinetic values (% CV) for MK-0752 were apparent oral clearance, 0.444 (38%) L/h/m2; apparent volume of distribution, 7.36 (24%) L/m2; and ka, 0.358 (99%) hr−1. The observed Cmax and Tmax for the patient treated at 200 mg/m2 were 23 μg/mL and 12 hours, respectively. The median Cmax and Tmax for the patients treated at the 260 mg/m2 dosage level were 25 μg/mL (range, 16 to 39 μg/mL) and 6 hours (range, 2.2 to 17 hours). The AUC values for 200 and 260 mg/m2 were 469 μg/mL·hour and 413 μg/mL·hour (range, 299 to 655 μg/mL·hour). The median terminal half-life observed in these patients was 11.4 hours (range, 9.9 to 14.9 hours). Moderate interpatient variation in drug disposition was noted; however, only two dosages were studied, so it was not possible to relate dosage and MK-0752 systemic exposure (eg, Cmax or AUC). No correlation was noted between AUC and toxicities observed in patients.
Table 4.
Summary of MK-0752 Pharmacokinetic Parameters in Relation to MK-0752 Dosage for Day 1 of Course 1
| Dosage | No. of Patients | Parameter | Median | Range |
|---|---|---|---|---|
| 200 mg/m2 | 1 | AUC (μg/mL·h) | 469 | |
| Cmax (μg/mL) | 23 | |||
| Tmax (h) | 12 | |||
| 260 mg/m2 | 17 | AUC (μg/mL·h) | 413 | 299-655 |
| Cmax (μg/mL) | 25 | 16-39 | ||
| Tmax (h) | 6.0 | 2.2-17 |
Abbreviations: AUC, area under the plasma concentration-time curve; Cmax, maximum concentration; Tmax, time to maximum concentration.
NOTCH1 and HES Protein Expression
Pretrial tumor samples were available from 12 patients (four anaplastic ependymomas; two classic ependymomas; and one each of glioblastoma, atypical teratoid/rhabdoid tumor, choroid plexus carcinoma, malignant glioma [grade 3 or 4], medulloblastoma, and primitive neuroectodermal tumor). NOTCH1 expression was detected in all but three tumors: the medulloblastoma, malignant glioma (grade 3 or 4), and one anaplastic ependymoma were immunonegative. The greatest level of expression detected by IHC was observed in the choroid plexus carcinoma that displayed both intense nuclear (indicating activated NICD1) and membrane immunoreactivity (Fig 1A). All tumors displayed intense nuclear expression of HES1 (85% median nuclear positive) and HES5 (85% median nuclear positive). Thus, the NOTCH signal pathway appears to be broadly expressed and active in various relapsed pediatric brain tumors.
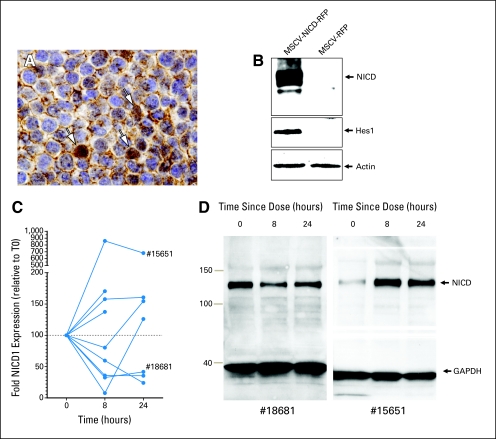
NOTCH pathway expression in patient tumors and peripheral-blood mononuclear cells. (A) NICD1 expression in choroid plexus carcinoma nuclei (arrows). (B) NICD1 Western blot analysis validation following cell transduction with human NICD1 virus (left lane) and control (right). Note hairy enhancer of split 1 (Hes1) induction. (C) Graph of fold change in NICD1 levels post treatment. (D) Western blot results in two patients (#18681 and #15651). GAPDH, glyceraldehyde 3-phosphate dehydrogenase; MSCV-NICD-RFP, mouse stem cell virus—NOTCH1 intracellular domain—red fluorescence protein.
As a first step to determine whether MK-0752 blocked gamma secretase inhibitor (GSI) –mediated cleavage of NOTCH1 in trial patients in vivo, we used Western blotting to measure the expression levels of NICD1 in PBMCs isolated from patients before and following dosing with the drug (Figs 1B to to1D).1D). PBMCs for at least one time point were obtained from 15 patients; baseline and at least one post-treatment PBMC pellet adequate for Western blot analysis were available for nine of these patients, all treated with 260 mg/m2 of MK-0752. NICD1 was readily detected in all PBMC pellets. Lower levels of NICD1 expression were observed in the PBMCs of five patients following treatment with MK-0752; in three of these patients, the reduced level was also observed at 24 hours (Figs 1C and and1D).1D). However, four patients demonstrated relatively higher levels of PBMC NICD1 expression post MK-0752 treatment. Furthermore, there was no evidence that the AUC or Cmax of MK-0752 correlated with the level of NICD1 expression.
DISCUSSION
This pediatric phase I trial establishes the MTD of MK-0752 as 260 mg/m2/dose orally daily for 3 consecutive days of every 7 days. Observed DLTs included increased ALT and AST. Two of the initial six patients experienced DLTs at the MTD that was defined by CRM. The traditional 3 + 3 design would have resulted in finding this dose level to be too toxic and thus would have led to a de-escalation to 200 mg/m2/dose. However, the extended cohort of 12 patients at the 260 mg/m2/dose dose level led to only one additional DLT, thus confirming the safety of the recommended dose by CRM. Unlike in adult studies,33,34 dose-limiting diarrhea and GI symptoms were not observed in this study. A recent study by Real et al35 reported that, in a xenograft model of glucocorticoid resistant T-cell acute lymphatic leukemia, GSI treatment resulted in accumulation of goblet cells in the gut, while glucocorticoid therapy led to transcriptional upregulation of cyclin D2 and protected mice from developing the intestinal goblet cell metaplasia typically induced by inhibition of NOTCH signaling with GSIs. These data suggest that the use of steroids may reduce the gut toxicity of GSIs. In the current cohort, seven patients (30%) were receiving steroids while on study, which may in part explain the lack of GI toxicity noted in this study compared with that in adults. Although increased fatigue did occur in some patients, the fatigue was not dose-limiting, as reported in adult studies. No objective responses were observed, and only two patients had SD for ≥ 3 months.
To the best of our knowledge, this is the first description of MK-0752 disposition in children with cancer, and since no pharmacokinetic studies of MK-0752 have been published in adults, it is unknown how they compare. However, data from two meeting abstracts33,34 suggest that the disposition (eg, AUC, Cmax, Tmax) is similar. MK-0752 was absorbed slowly, with a range of 2.2 to 17 hours required to reach maximum plasma concentrations. Although some variability was observed in the maximum MK-0752 plasma concentration at the 260 mg/m2 dosage level, it was not as much as that observed for other phase I drugs (ie, 2.4-fold v > 10-fold).36–38 Finally, measures of MK-0752 systemic exposure (eg, AUC and Cmax) were related to pharmacodynamic measures, but no relation was observed. It should be noted that total drug (bound and unbound) was used in this analysis, and for a highly protein-bound drug such as MK-0752 (percent unbound, 0.4%; unpublished results), exposure to the unbound drug may be more informative.
IHC analyses confirmed frequent and high-level expression of the NOTCH family and active downstream signal intermediates in pediatric brain tumors. Particularly high-level expression of cleaved nuclear NOTCH1 was observed in a patient with choroid plexus carcinoma. These data are compatible with reports that Notch signaling critically regulates choroid plexus development39,40 and drives tumorigenesis in the choroid plexus.41 The mechanism that mediates this high level of NOTCH signaling in relapsed tumors and its pathogenic significance remain to be determined. Our study also confirms prior reports42 that cleaved NOTCH1 and its downstream targets can be readily detected in PBMCs; however, our initial studies indicate this is not likely to be a useful assay for detecting in vivo activity of MK-0752. Further work will be required to determine whether PBMC NICD1 correlates with in vivo drug activity in patients with brain tumors.
This study demonstrates that MK-0752 is well-tolerated in children at the dose and schedule studied. However, there were no objective responses, and only two patients experienced prolonged stabilization of disease for at least three cycles. Preclinical models indicate that a once-weekly regimen of MK-0752 is well-tolerated and efficacious with no significant difference in efficacy in rodent models between 3 days on and 4 days off and once weekly dosing.43,44 On the basis of these data, a once-weekly schedule for MK-0752 is currently being explored in adults with recurrent CNS malignancies.
Acknowledgment
We thank Christopher Smith, Rebecca Turner, Daniel Mink, and Michelle Rabanus for clinical research and regulatory support and Inga Luckett and Radhika Thiruvenkatam for their technical assistance.
Footnotes
Supported in part by National Institutes of Health Grant No. U01 CA81457 for the Pediatric Brain Tumor Consortium and by the American Lebanese Syrian Associated Charities.
Presented in part at the 46th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 4-8, 2010.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00572182.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Tim Demuth, Merck (C) Consultant or Advisory Role: None Stock Ownership: Tim Demuth, Merck Honoraria: None Research Funding: Maryam Fouladi, Merck; James Olson, Merck; James M. Boyett, Merck Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Maryam Fouladi, Clinton F. Stewart, James Olson, Arzu Onar-Thomas, Mehmet Kocak, Roger J. Packer, Sridharan Gururangan, Tim Demuth, Larry E. Kun, James M. Boyett,Richard J. Gilbertson
Administrative support: Larry E. Kun, James M. Boyett
Provision of study materials or patients: Roger J. Packer,Sridharan Gururangan
Collection and assembly of data: Clinton F. Stewart, Lars M. Wagner, Arzu Onar-Thomas, Roger J. Packer, Amar Gajjar, Richard J. Gilbertson
Data analysis and interpretation: Clinton F. Stewart, Arzu Onar-Thomas, Mehmet Kocak, Roger J. Packer, Stewart Goldman, Sridharan Gururangan, Tim Demuth, James M. Boyett, Richard J. Gilbertson
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
Articles from Journal of Clinical Oncology are provided here courtesy of American Society of Clinical Oncology
Full text links
Read article at publisher's site: https://doi.org/10.1200/jco.2011.35.7806
Read article for free, from open access legal sources, via Unpaywall:
https://ascopubs.org/doi/pdfdirect/10.1200/JCO.2011.35.7806?role=tab
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1200/jco.2011.35.7806
Article citations
Targeted therapy of cancer stem cells: inhibition of mTOR in pre-clinical and clinical research.
Cell Death Dis, 15(9):696, 30 Sep 2024
Cited by: 0 articles | PMID: 39349424 | PMCID: PMC11442590
Review Free full text in Europe PMC
Notch signaling pathway in cancer: from mechanistic insights to targeted therapies.
Signal Transduct Target Ther, 9(1):128, 27 May 2024
Cited by: 11 articles | PMID: 38797752 | PMCID: PMC11128457
Review Free full text in Europe PMC
Metastasis and cancer associated fibroblasts: taking it up a NOTCH.
Front Cell Dev Biol, 11:1277076, 10 Jan 2024
Cited by: 0 articles | PMID: 38269089 | PMCID: PMC10806909
Review Free full text in Europe PMC
Exploring the dynamic interplay between cancer stem cells and the tumor microenvironment: implications for novel therapeutic strategies.
J Transl Med, 21(1):686, 02 Oct 2023
Cited by: 21 articles | PMID: 37784157 | PMCID: PMC10546755
Review Free full text in Europe PMC
The critical role of γ-secretase and its inhibitors in cancer and cancer therapeutics.
Int J Biol Sci, 19(16):5089-5103, 02 Oct 2023
Cited by: 3 articles | PMID: 37928268 | PMCID: PMC10620818
Review Free full text in Europe PMC
Go to all (99) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials
- (1 citation) ClinicalTrials.gov - NCT00572182
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Phase I trial of weekly MK-0752 in children with refractory central nervous system malignancies: a pediatric brain tumor consortium study.
Childs Nerv Syst, 31(8):1283-1289, 01 May 2015
Cited by: 19 articles | PMID: 25930724 | PMCID: PMC4681692
Sequential combination therapy of ovarian cancer with cisplatin and γ-secretase inhibitor MK-0752.
Gynecol Oncol, 140(3):537-544, 15 Dec 2015
Cited by: 43 articles | PMID: 26704638
Phase I trial of lapatinib in children with refractory CNS malignancies: a Pediatric Brain Tumor Consortium study.
J Clin Oncol, 28(27):4221-4227, 16 Aug 2010
Cited by: 45 articles | PMID: 20713864 | PMCID: PMC2953974
A phase 1 and pharmacokinetic study of enzastaurin in pediatric patients with refractory primary central nervous system tumors: a pediatric brain tumor consortium study.
Neuro Oncol, 17(2):303-311, 27 Nov 2014
Cited by: 8 articles | PMID: 25431212 | PMCID: PMC4288513
Funding
Funders who supported this work.
NCI NIH HHS (2)
Grant ID: U01 CA81457
Grant ID: U01 CA081457




