Abstract
Free full text

Split-Protein Systems: Beyond Binary Protein-Protein Interactions
Abstract
It has been estimated that 650,000 protein-protein interactions exist in the human interactome [1], a subset of all possible macromolecular partnerships that dictate life. Thus there is a continued need for the development of sensitive and user-friendly methods for cataloguing biomacromolecules in complex environments and for detecting their interactions, modifications, and cellular location. Such methods also allow for establishing differences in the interactome between a normal and diseased cellular state and for quantifying the outcome of therapeutic intervention. A promising approach for deconvoluting the role of macromolecular partnerships is split-protein reassembly, also called protein fragment complementation. This approach relies on the appropriate fragmentation of protein reporters, such as the green fluorescent protein or firefly luciferase, which when attached to possible interacting partners can reassemble and regain function, thereby confirming the partnership. Split-protein methods have been effectively utilized for detecting protein-protein interactions in cell-free systems, E. coli, yeast, mammalian cells, plants, and live animals. Herein, we present recent advances in engineering split-protein systems that allow for the rapid detection of ternary protein complexes, small molecule inhibitors, as well as a variety of macromolecules including nucleic acids, poly(ADP) ribose, and iron sulfur clusters. We also present advances that combine split-protein systems with chemical inducers of dimerization strategies that allow for regulating the activity of orthogonal split-proteases as well as aid in identifying enzyme inhibitors. Finally, we discuss autoinhibition strategies leading to turn-on sensors as well as future directions in split-protein methodology including possible therapeutic approaches.
Introduction
Methods for discovering as well as investigating macromolecular interactions in a complex milieu have seen tremendous progress. Two and three-hybrid approaches [2, 3] as well as biomolecular fluorescence resonance energy transfer (FRET)[4] are widely used to study protein-protein interactions, both qualitatively and quantitatively. More recently split-protein reassembly methods have emerged as a potentially simple and general solution for studying protein-protein interactions as well as a host of other macromolecules. The observation that fragments of proteins can reassemble into functional complexes was observed over 60 years ago in the context of ribonuclease and subsequently beta-galactosidase [5,6]. However, conditional split-protein reassembly was put on the map in 1994 by the seminal discovery by Johnsson and Varshavsky that appropriate fragments of a monomeric protein, ubiquitin, could be conditionally reassembled when attached to interacting protein pairs that raise the local concentration of the protein fragments [7]. Split-ubiquitin was a major technological leap as it provided a direct method to link the non-covalent interaction of two proteins to the function of the split-reporter protein. This method obviates the requirement for nuclear localization of the interacting proteins or the host translational machinery as in the widely used yeast two-hybrid systems. For the successful creation of a split-reporter protein, a few criteria must be met. Each protein fragment by itself should not exhibit any activity, the affinity of the fragments in the absence of attached interacting proteins should be negligible, and ideally the reassembled split-protein must provide an easily measurable read out (Figure 1). In principle, most proteins can be dissected into such fragments, however despite its deceptive simplicity the identification of appropriate dissection sites within a protein that satisfy the criterion for use in split-protein assays is limited. A case in point is the dissection of firefly luciferase utilizing incremental truncation [8], wherein, the optimal protein fragments possess an 18 amino acid overlap, which would be difficult to anticipate by rational design. Split-protein pairs that are now widely used include those derived from ubiquitin [7], GFP and its variants [9, 10], dihydofolate reductase (DHFR) [11], ß-lactamase [12], firefly [13] and other luciferases [14, 15]. More recently, the tobacco etch virus (TEV) protease [16], thymidine kinase [17] and chorismate mutase[18] have also been co-opted. Currently split-reporter proteins offer a variety of readouts and can detect transient as well as irreversible protein-protein assemblies. Split protein reassembly methods have also been used in directed evolution experiments to discover new as well as improving existing protein partnerships [19-23]. Herein, we will focus primarily upon recent advances that go beyond binary protein-protein interactions.
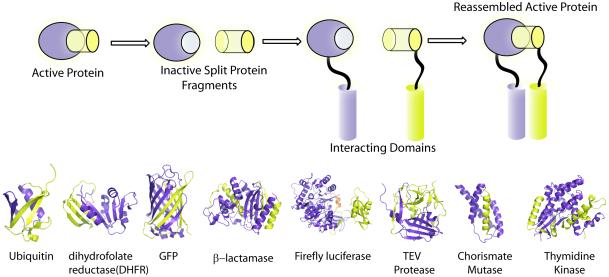
Illustration of conditional split-protein reassembly. A generic split-protein system is shown where a functional protein is dissected into two inactive fragments, purple and yellow. The attachment of two interacting proteins or protein domains brings the inactive fragments into close proximity and overcomes the entropic cost of fragmentation. This leads to the reassembly or complementation of the fragments thus providing a direct readout for the partnership between the interacting domains. Crystal structures of representative proteins which have been shown to be amenable to interaction dependent reassembly, where the N-terminal and C-terminal fragments are shown as purple and yellow respectively.
Imaging Protein-Protein Interactions
Spit-protein reassembly has established itself as a valuable tool to image and probe dynamic cellular processes. Most existing fluorescent and luminescent proteins have been co-opted to create split-reporters and have been extensively applied for imaging a variety of interactions, which has been recently reviewed [24,25]. It is worth noting that the irreversible nature of the reassembled GFP-like fluorescent proteins [26] limits their ability to report on temporal information, although they are useful for trapping low affinity interactions [27]. The irreversibility of the split-GFP system has been cleverly used for improving the thermodynamic stability of a variant of the B1 domain of protein G by directed evolution [28]. Many of the split-luciferase reporters on the other hand have been shown to be reversible [29] and offer advantages such as low background, as no exogenous light is required. However, increasing light output and substrate availability inside cells or tissues is an ongoing challenge. In order to expand the scope of split-luminescent reporters much like the formidable palette of fluorescent proteins, Ozawa and co-workers have described multicolor luciferases [30] with improved sensitivity. To go beyond absorbance, fluorescence, and luminescence based modalities, Gambhir and co-workers have recently utilized Positron Emission Tomography (PET) based imaging as their readout for protein-protein interactions [17]. A new split-thymidine kinase was discovered that when activated, phosphorylates (9-(4-[18F]-fluoro-3-hydroxymethylbutyl)-guanine) and localizes it to only those cells expressing reassembled split-thymidine kinase. This approach couples a sensitive radioactivity based imaging technique, such as PET, with split protein reassembly and can potentially provide a new approach for clinical imaging of protein-protein interactions in live animals. As a proof of principle, split-protein dependent PET was used to monitor the complexation of hypoxia-inducible factor-1α with the von Hippel-Lindau tumor suppressor protein.
Recently, split-protein approaches have been paired with resonance energy methods, wherein a reconstituted split fluorescent protein can serve as an acceptor for resonance energy transfer from a fluorescent (FRET) or bioluminescent protein (BRET). Indeed, such a strategy was utilized to demonstrate the association of four different proteins as part of a G protein-coupled receptor signaling complex [31]. Taking advantage of this strategy, Bouvier and co-workers discovered an unexpected asymmetric organization of the calcitonin gene-related peptide (CGRP) receptor [32]. In a FRET-based strategy, Hu and co-workers visualized ternary complexes implicated in transcription, wherein the fluorescent protein cerulean fused to NFAT1, NF-kB and p65 allowed energy transfer to a fos-jun mediated reassembled split-venus complex [33].
Interrogating Small Molecule Inhibitors
Split-protein reassembly naturally lends itself for discovering and evaluating small molecule inhibitors, especially those that directly disrupt protein-protein interactions. Recently a cell-free split-luciferase enabled approach was established (Figure 2a) which allowed for the rapid interrogation of a large number of macromolecular interactions [29]. This methodology was used to test the small molecule specificity for a set of therapeutically relevant helix/receptor pairs. Eighteen helix/receptor pairs were first examined for specificity, which recapitulated the reported affinity of these helix/receptor pairs [34]. Further investigation of inhibition profiles of some well known inhibitors of these helix/receptor interactions, led to some surprising results. For example, significant inhibition of the p53/hDM2 interaction was also observed for a previously identified Bcl-2 family inhibitor, BH3I-3. In the near future, we expect that large protein-protein interaction (PPI) arrays using split-protein reporters will be available for interrogating new classes of inhibitors. Such methods would aid in rapidly establish the desired selectivity or promiscuity for PPI inhibitors as has been the case with kinase inhibitors. Besides probing the direct effect of small molecules on a specific protein-protein interaction, split-protein reassembly has also been used to interrogate the effect of small molecules on specific biochemical pathways, allowing for the delineation of potential off-target effects of drug candidates. In a detailed study, a cell based split-Venus system was used to carry out pharmacological profiling of a large array of downstream protein-protein interactions, that were known to report upon specific cellular pathways ranging from cell-cycle control to apoptosis [35]. In this screen, 107 known drugs representing many therapeutic areas were interrogated in tandem. Strikingly, four existing drugs were found to possess previously unknown anti-tumor activity. Thus the ease of implementation of the split-protein approach has the potential to rapidly uncover potentially promiscuous molecules, whether desired or undesired, as well as identify the off-target effects of drug targets in a pathway specific manner.
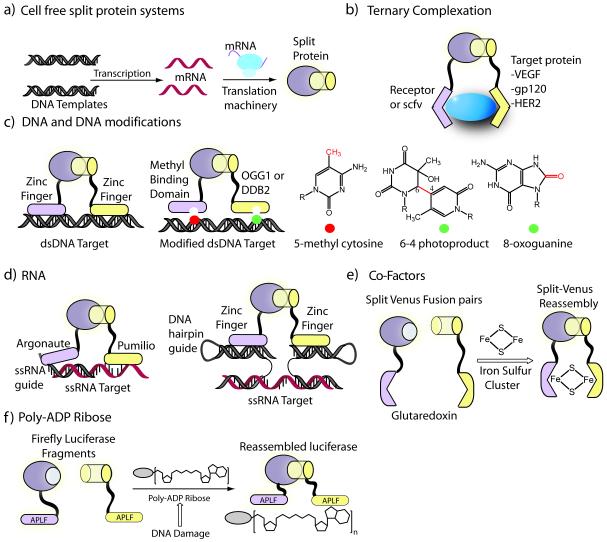
Detection of macromolecules using split-protein reassembly via ternary complexation. a) Schematics of split-protein translation in cell-free translation systems such as rabbit reticulocyte lysate or wheat germ extract, where interacting domains of interest can be rapidly produced and interrogated. b) Detection of unmodified native proteins via ternary complexation using receptor or single-chain antibody fragments attached to split-luciferase. c) DNA and DNA modifications, such as methylation, can be detected using appropriate fusions of split luciferase and user defined DNA binding domains. The genome can be scanned at all CpG methylated sites for specific types of modifications resulting from DNA damage. d) A quaternary strategy for detection of RNA utilizing RNA detection domains such as argonaute and pumilio domains. The pentameric assembly allows targeting any ssRNA or ssDNA utilizing a zinc finger binding hairpin guide sequence. e) Co-factors such as iron-sulfur clusters detected via split-venus and apoglutaredoxin fusion pairs that dimerize upon binding 2Fe2S. e) Monitoring the dynamics of poly-ADP Ribose (PAR) levels subsequent to DNA damage through attached PAR targeted APLF domains.
Ternary Complexation: Native Protein and Nucleic Acid Dependent Split-protein Reassembly
In split-protein systems, the proteins pairs being examined are attached to the fragmented reporters. Thus a question arises as to how may one detect an unmodified native target, whether protein, DNA, or RNA. A simple solution is to identify two protein domains that interact with the desired target, resulting in a ternary complex that drives the reassembly of the split-protein reporter (Figure 2b). Towards this goal, the split halves of firefly luciferase were fused to receptor fragments or single-chain antibodies [36]. The conditional reassembly of luciferase halves was induced in presence of several biologically relevant targets such as vascular endothelial growth factor (VEGF), and the human epidermal growth factor receptor-2 (HER2). Significantly, this ternary complexation method allows for rapid determination of the protein levels of cell-surface markers such as HER2 without any additional purification or handling steps.
The ternary split-protein reassembly strategy naturally lends itself to a convenient method for the sequence-specific detection of nucleic acids, particularly DNA. For example, designed and natural Cys2-His2 Zinc finger DNA binding domains have been fused to split fragments of a reporter protein such as GFP, ß-lactamase or firefly luciferase (Figure 2c). In the presence of target DNA, harboring specific adjacent DNA sites recognized by the zinc finger domains, the reporter protein activity is reconstituted providing an easily measurable “turn-on” fluorescent [37] colorimetric [38] or luminescent [29] signal. The modular nature of this system, allows for the detection of virtually any nucleic acid sequence. This can be achieved by replacing the zinc finger binding domains with alternate detection domains that target specific nucleic acid modifications. For instance, by utilizing a methyl binding domain (MBD) and Zif268, a natural zinc finger, DNA methylation can be detected in a sequence-specific manner [39]. More recently, turn-on sensors for the detection of UV or oxidation-dependent DNA damage has also been reported [40]. In this approach, the MBD domain can scan a large fraction of the genome (50-80% of the genome is methylated at CpG sites) while a damage specific protein domain can readout specific DNA modifications, such as 8-oxoguanine or pyrimidine dimer photoproducts (Figure 2c). Similarly, bipartite MBD domains have been used for measuring the activity of DNA methyltransferase inhibitors in live cells [41].
Unlike zinc fingers and the recently discovered TAL-enhancers that recognize DNA, there are fewer avenues for co-opting sequence specific RNA recognition domains. Rackham and Brown utilized known RNA binding proteins tethered to a split-venus sensor to visualize ternary RNA-protein complexes in live mammalian cells [42]. More recently, several studies have now shown that natural and designed pumilio domains can be utilized for targeting specific RNA sequences, which may ultimately provide an inroad to targeting any RNA sequence of interest [43]. Towards the long-term goal of developing a general approach, for targeting RNA, a quaternary assembly strategy was examined, wherein RNA-binding Argonaut domains, which bind 2 nucleotide (nt) 3′ overhangs of short double stranded RNA, were fused to split-firefly luciferase [44]. The ternary assembly of the split-luciferase was then induced by Argonaut mediated recognition of nucleotide overhangs, facilitated by complementary ssRNA guide sequences. A pentameric assembly was also investigated, where designed zinc finger domains were appended to split-luciferase halves, such that a zinc finger binding hairpin containing guide sequence could target any ssRNA or ssDNA target (Figure 2d). In a similar approach, split-GFP has been directly conjugated to guide ssDNA oligonucleotides either covalently [45] or through biotin/streptavidin interactions resulting in DNA hybridization dependent GFP reassembly [46].
More recently, ternary split-protein reassembly has also been applied for the detection of other biologically relevant molecules. For example, in a clever ternary complexation strategy, Hoff and co-workers [47] developed a split-GFP based turn-on sensor for the detection of iron-sulfur clusters (2Fe-2S). Each of the fragment of split-Venus was appended to glutaredoxin 2 (GRX2), a glutathione dependent oxidoreductase. ApoGRX2 is monomeric, however, in the presence of 2Fe2S clusters, GRX2 dimerizes, bringing Venus fragments into close proximity, leading to complementation. Thus this approach allowed for imaging the biogenesis of a 2Fe2S cluster in live cells (Figure 2e). In another example, a poly-ADP ribose (PAR) binding protein was fused to both halves of split-luciferase and thus allowed for the detection of PAR [48]. This turn-on PAR sensor (Figure 2f) allowed for monitoring the dynamic change in PAR levels in several mammalian cell lines exposed to DNA-damaging agents.
Chemical Inducers of Dimerization (CID)
A desired goal of chemical biology is the conditional control of protein function with small molecules from both a therapeutic stand point as well as for delineating complex biological pathways. Since the seminal discovery of the small molecule rapamycin-dependent heterodimerization of FKBP12 (FK506 binding protein) with FRB (FKBP12 rapamycin binding protein), numerous applications have exploited the system for conditional control of cellular events [49, 50]. Not surprisingly, many split-protein reporters were initially validated using the FKBP/FRB CID system including ß-lactamase [12], firefly luciferase [13] and gaussia luciferase [14]. Small molecule-dependent protein fragment complementation is a particularly attractive technique, as it allows dose-dependent control of a functional protein inside a cell. For example, building upon the split-ubiqutin system [7], Muir and co-workers have recently described a clever strategy for controlling protein degradation in a CID-dependent fashion [51]. In this approach, a protein of interest is fused to one half of split-ubiquitin and FRB followed by a degron which tags the protein for destruction by the proteasome. The other half of split-ubiquitin is fused to FRBP, and upon addition of rapamycin, reconstituted ubiquitin rescues the protein of interest from degradation and thereby allows small molecule control of protein half-lives.
A split-TEV system was developed to monitor protein-protein interactions both at the membrane and in the cytosol of mammalian cells [16, 52]. More recently, Wells and co-workers engineered a conditional FKBP/FRB CID system using split-TEV [53] with decreased affinity of split fragments to identify and characterize substrates of specific caspases in a cellular context. In an elegant design, the authors generated TEV cleavage dependent caspase alleles, which could be turned on by activating CID dependent split-TEV (Figure 3a). Importantly, this approach led to the discovery of synergy between caspase activation and proteasome inhibition, suggesting negative regulation of proteasome activity by caspases during apoptosis, an observation which has therapeutic implications for the treatment of cancer.
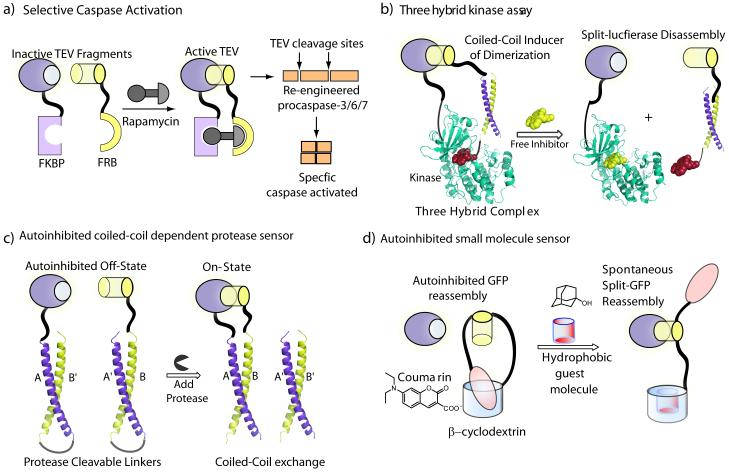
New strategies for split-protein systems. a) Selective activation of caspases incorporating TEV cleavage sites using a small molecule rapamycin triggered reassembly of split-TEV protease. b) Screening kinase inhibitors utilizing a three-hybrid strategy using a new peptide-small molecule chemical inducer of dimerization wherein a potential kinase inhibitor can be identified by split-luciferase disassembly. c) An autoinhibited coiled-coil split-luciferase turn-on protease sensor wherein the autoinhibition is relieved in presence of any protease of interest leading to split-luciferase reassembly. d) An autoinhibited strategy for detection of small molecules using supramolecular building blocks.
Beyond the classic FKBP/FRB CID system, a three-hybrid system has been reported wherein split-ubiquitin fragments were fused to glucocorticoid receptor (GR) and dihydrofolate reductase (DHFR) [54]. The reassembly of split-ubiquitin was conditionally induced in live cells by a bifunctional molecule, comprised of a classic dexamethasone and methotrexate, which bind GR and DHFR respectively. In a recent report a three-hybrid strategy for high-throughput profiling of protein kinase inhibitors was also reported using a new CID [55]. In this strategy the C-terminal half of split-firefly luciferase is fused to a protein kinase of interest while the N-terminal half of luciferase is attached to coiled coil Fos. The reassembly of split-firefly luciferase is then induced by the new CID, the peptide Jun covalently attached to staurosporine, a pan-kinase inhibitor (Figure 3b). Kinase inhibitors were rapidly identified by disrupting the staurosporine-kinase interaction with concomitant loss of firefly luciferase activity. The cell-free method was shown to be general across the kinome and also allowed for facile discrimination between competitive and allosteric kinase inhibitors.
Autoinhibited Turn-On Sensor Systems
The autoinhibition of protein activity is widely used in nature and also affords a design template for engineering split-protein sensors. A turn-on protease sensor has been designed (Figure 3c), where the reassembly of split reporter is auto-inhibited as the coiled-coils interact intramolecularly due to favorable entropy, however, upon protease mediated cleavage of the linker the two fragments can reassemble [56]. This method is tunable by modifying either the coiled-coils or protease cleavage site to monitor any protease of interest and afforded a complete panel of caspase sensors for determining caspase crosstalk [57]. This approach was also shown to serve as a platform for the construction of protein logic gates [56]. In another example of an autoinhibited system, host-guest interactions between ß-cyclodextrin and coumarin units were utilized to exercise supramolecular control over reassembly of split-GFP (Figure 3d). By incorporating both the ß-cyclodextrin and coumarin units in the same C-terminal GFP peptide fragment, the resulting intramolecular complex was autoinhibited and unable to bind the N-terminal GFP fragment [58]. The autoinhibition could be relieved by addition of an exogenous hydrophobic guest molecule with concomitant reassembly of the GFP fragments, which in effect creates a turn-on sensor for small molecules. Another self assembling autoinhibited split-GFP has also been described, wherein the autoinhibition is relieved by the action of a protease of interest instead of a small molecule [58]. In this approach, the C-terminal GFP fragment was incorporated as a loop in another protein with a protease cleavable linker, where the self assembly of split-GFP is inhibited until cleavage by a protease of interest. These autoinhibitory approaches marry biological as well as supramolecular building blocks to split-protein reporter outputs.
Outlook and Conclusion
The range of biological activities and processes that can be monitored with a variety of readouts using split-protein reassembly both in an in vitro and in vivo setting is unprecedented. There has been a veritable explosion of applications utilizing split-protein reassembly and the long-term potential of this methodology is only beginning to be realized, as the existing split-protein outputs are being combined in a variety of ways to user-defined inputs under examination. We have already seen the ability to probe binary as well as ternary partnerships and the rapid interrogation of small molecules that perturb them. Certain technical milestones would help advance the field, such as the discovery of new split-fluorescent proteins that reversibly assembles and dissembles, or the discovery of split-luminescent proteins and substrates which allow for imaging in deep tissue. We have also witnessed that the ever expanding repertoire of split-proteins at the disposal of the chemical biologist provides a means for the temporal control over the outcome of various biomolecular processes. This has already enabled the discovery of new biology, such as the synergy between caspase activation and proteasome inhibition. In the not too distant future, we can envision the explicit control over the outcome of specific signal transduction pathways by utilizing fragmented protein kinases, phosphatases, acetylases or ligases engineered to be temporally controlled by a small molecule CID. Finally, we anticipate that the general split-protein ternary complexation approach may allow for the conditional reassembly of a split toxic protein inside a particular cell type, that responds to specific over-expressed proteins, nucleic acids, and other macromolecules and thereby provide new therapeutic and imaging modalities.
Acknowledgements
We thank past and present members of the Ghosh laboratory particularly Ben Jester and Jenny Furman for their thoughtful contributions. I.G. acknowledges support from the NSF (CHE-0548264) and NIH (CA141974 and CA143661).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
*of special interest
**of outstanding interest
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.cbpa.2011.10.014
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3237955?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1016/j.cbpa.2011.10.014
Article citations
Imaging interorganelle contacts at a glance.
J Cell Sci, 137(20):jcs262020, 23 Oct 2024
Cited by: 0 articles | PMID: 39440475
Review
Comprehensive Overview of Bottom-Up Proteomics Using Mass Spectrometry.
ACS Meas Sci Au, 4(4):338-417, 04 Jun 2024
Cited by: 2 articles | PMID: 39193565 | PMCID: PMC11348894
Review Free full text in Europe PMC
Exploiting protein domain modularity to enable synthetic control of engineered cells.
Curr Opin Biomed Eng, 31:100550, 02 Jul 2024
Cited by: 0 articles | PMID: 39430298
A putative design for the electromagnetic activation of split proteins for molecular and cellular manipulation.
Front Bioeng Biotechnol, 12:1355915, 28 Mar 2024
Cited by: 7 articles | PMID: 38605993 | PMCID: PMC11007078
Irreversible light-activated SpyLigation mediates split-protein assembly in 4D.
Nat Protoc, 19(4):1015-1052, 22 Jan 2024
Cited by: 0 articles | PMID: 38253657
Review
Go to all (96) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
A molecular imaging biosensor detects in vivo protein folding and misfolding.
J Mol Med (Berl), 94(7):799-808, 08 Jun 2016
Cited by: 2 articles | PMID: 27277823
Combining protein complementation assays with resonance energy transfer to detect multipartner protein complexes in living cells.
Methods, 45(3):214-218, 27 Jun 2008
Cited by: 27 articles | PMID: 18586102
An autoinhibited coiled-coil design strategy for split-protein protease sensors.
J Am Chem Soc, 131(42):15284-15290, 01 Oct 2009
Cited by: 42 articles | PMID: 19803505 | PMCID: PMC2783329
Engineering Aspects of Olfaction
CRC Press/Taylor & Francis, Boca Raton (FL), 05 Jun 2015
Cited by: 0 articles | PMID: 26042329
ReviewBooks & documents Free full text in Europe PMC
Funding
Funders who supported this work.
NCI NIH HHS (8)
Grant ID: CA141974
Grant ID: R21 CA141974-02
Grant ID: R21 CA141974-01
Grant ID: R21 CA143661-01
Grant ID: R21 CA143661-02
Grant ID: CA143661
Grant ID: R21 CA143661
Grant ID: R21 CA141974





