Abstract
Free full text

Melanopsin Signaling in Mammalian Iris and Retina
Abstract
Lower vertebrates have an intrinsically-photosensitive iris and thus a local pupillary light reflex (PLR). In contrast, it has been a dogma that the PLR in mammals generally requires neuronal circuitry connecting the eye and the brain. We report here that an intrinsic component of the PLR is actually widespread in nocturnal and crepuscular mammals. In mouse, this intrinsic PLR requires the visual pigment, melanopsin. It also requires PLCβ4, the vertebrate homolog of the Drosophila NorpA phospholipase C mediating rhabdomeric phototransduction. The Plcβ4−/− genotype, besides removing the intrinsic PLR, also essentially eliminates the intrinsic light response of the M1-subtype of melanopsin-expressing, intrinsically-photosensitive retinal ganglion cells (M1-ipRGCs), by far the most photosensitive ipRGCs and with the largest responses. Ablating in mouse the expression of both TRPC6 and TRPC7, members of the TRP channel superfamily, likewise essentially eliminated the M1-ipRGC light response, but spared the intrinsic PLR. Thus, melanopsin signaling exists in both iris and retina, involving a PLCβ4-mediated pathway that nonetheless diverges in the two locations.
The discovery of ipRGCs has overturned the century-old belief that rods and cones are the only mammalian retinal photoreceptors1–6. These ganglion-cell photoreceptors serve primarily non-image visual functions, with one being the PLR. For lower vertebrates such as fish, amphibian and bird, in addition to the neurally-driven PLR, the iris itself is capable of autonomous, light-induced constriction7–11. For mammals, the PLR is thought to generally require neuronal circuitry through the brain, although sporadic reports7,12,13 and controversy exist of an intrinsic iridic photosensitivity in occasional species, including human. Even in lower vertebrates, the photopigment driving the intrinsic PLR remains unidentified. It has been suggested to be rhodopsin in amphibians and fish8,9, and the non-opsin-based cryptochrome in chicken11.
We have examined this unsettled question of an intrinsic PLR in mammals, and found the phenomenon to be surprisingly widespread. Moreover, the intrinsic PLR bears a close kinship to the ipRGCs in phototransduction.
Intrinsic PLR in mouse and other mammals
We found that bright light triggered a pupillary constriction in an intact eye freshly isolated from a dark-adapted pigmented mouse (Fig. 1a; 3 eyes; Methods). This photosensitivity disappeared within ~1 min after eye-isolation perhaps due to anoxia, with the pupil remaining constricted thereafter. This PLR persisted in a reduced preparation with just the eye’s anterior chamber and iris8,11,12 under L-15 medium (Fig. 1b; 3 eyes; Methods), even after blocking any potential parasympathetic activity to the iris with 0.5% atropine; however, the PLR again faded after a few light trials.
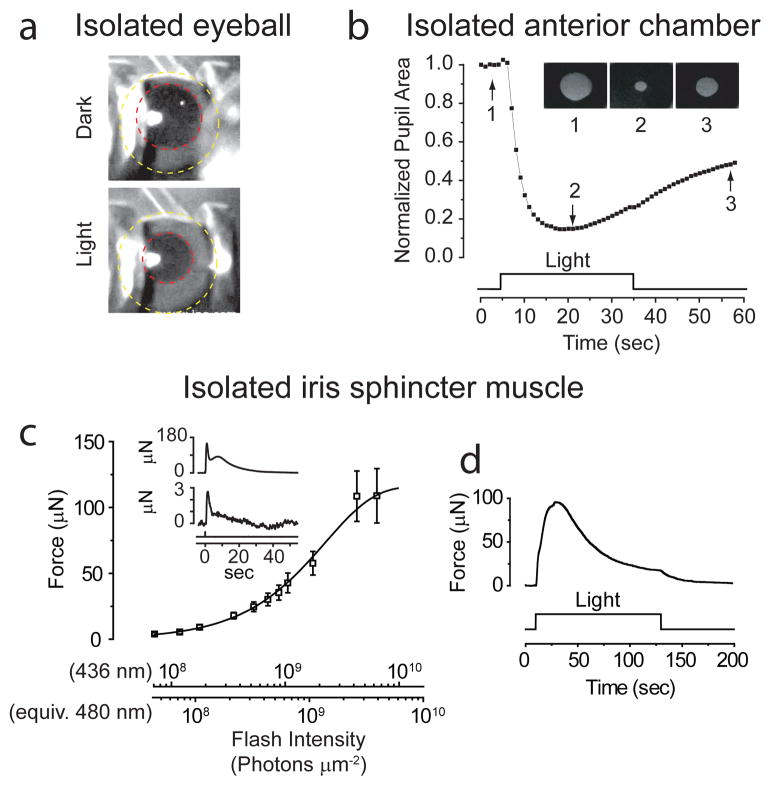
Intrinsic pupillary light reflex (PLR) of mouse. a, Constriction of pupil (red circle) in freshly isolated eye (yellow circle), elicited by 1.2 × 10−3 μW μm−2 of white Xe light for ~3 sec over iris. 23°C. b, Intrinsic PLR in isolated anterior chamber, plotted as pupil area normalized to dark state. 23°C. Inset shows pupil at times indicated by arrows. White Xe light (30 sec of 4 × 10−4 μW μm−2) over entire iris. c, Flash intensity-response relation for sphincter-muscle force at transient peak of response (mean ± SEM, 7 muscles). 35–37°C. Fit is Rmax (1− e−I/I0) with Rmax = 116 μN, I0 = 2.3 ×109 photons μm−2 (436-nm Hg light except for brightest two intensities, which were white; 3-mm spot covering entire muscle). Flashes were 12–400 ms in duration. Inset shows sample responses to a dim and a bright flash delivering (at time 0) 7.2 ×107 and 6.5 ×109 photons μm−2 (436 nm), respectively. d, Muscle-force response to a light step (6.1 ×109 photons μm−2 s−1 at 436 nm) to indicate adaptation. Flash intensities are also expressed in equivalent 480-nm photons, given that melanopsin is the signaling pigment (see Fig. 3). All WT mice here and in subsequent figures were C57BL/6J, unless indicated otherwise.
The mammalian iris has three main tissue layers, all pigmented with melanin: an anterior fibrovascular stroma, a middle smooth-muscle layer consisting of the circumferential sphincter muscle at the pupil perimeter and the radial dilator muscle across the iris, and a posterior epithelium7,14. In lower vertebrates, the sphincter muscle itself is thought to be the light-sensor7,14. Accordingly, we reduced the mouse preparation further to the small ring of sphincter muscle, and connected it to a μNewton strain gauge for measuring isometric tension under oxygenated Ames solution9,10 (35–37°C; Methods). The isolated sphincter muscle gave a light-induced contractile force reproducibly for hours. A relatively dim flash elicited a transient increase in force that grew linearly with increasing flash intensity, i.e., proportional to flash intensity and with a constant waveform (Fig. 1c and inset, bottom; Supplementary Fig. S1). This flash-induced contraction reached a transient peak in ~1 s but decayed much more slowly, not dissimilar to the in situ ipRGC-driven PLR15. The response to an intense flash often showed a hump during its decay (Fig. 1c inset, top). Although the force elicited by a dim flash decayed to baseline in ≤ 1 min, a second identical flash typically elicited a smaller response unless given in ≥ 8 min after the first. Likewise, a near-saturated response to an intense flash decayed in ~1 min, but a ~15-min delay was required for a second identical flash to elicit a comparable response. This light adaptation was also manifested during steady light as a relaxation of the force from a transient peak to a lower plateau level (Fig. 1d; 3 muscles). In contrast, acetylcholine-elicited contraction did not show this adaptation (Supplementary Fig. S2), suggesting that this adaptation resided in the photosignaling pathway upstream of the contractile mechanism.
The intrinsic PLR turns out to be widespread across nocturnal mammals (Methods). Albino mice showed the same phenomenon but their sphincter muscle was more photosensitive (not shown) presumably due to higher light transmission through the non-pigmented iris. Albino rat (Fig. 2a, left; 2 muscles; pigmented species not tested) and hamster (Fig. 2a, right; 3 muscles; pigmented, but with little iridic melanin in the pupillary margin) also tested positively, with sensitivities similar to the albino mouse but a much larger muscle force. Dog, cat, and rabbit (all pigmented) also showed an intrinsic PLR (2, 1, 3 muscles respectively), but with distinctly lower photosensitivity and force production; cat and rabbit required a light step instead of flash to elicit a detectable response (Fig. 2b). Although these three species are strictly speaking not nocturnal, they are crepuscular, i.e., active at dawn and dusk. One somewhat unusual species, Nile grass rat, has both diurnal and nocturnal tendencies16 but also tested positively (3 muscles, not shown). On the other hand, the response was absent for guinea pig (2), ground squirrel (4) and pig (2) (not shown). Ground squirrel is strongly diurnal, while guinea pig and pig are variably described as crepuscular or diurnal. None of four primate species tested showed this phenomenon, including rhesus monkey (Fig. 2b; diurnal, 7 muscles), marmoset (diurnal, 2), owl monkey (nocturnal, 4) and bush baby (nocturnal, 2) (latter three not shown). Thus, nocturnal/nocturnal-leaning sub-primate mammals tend to have an intrinsic PLR.
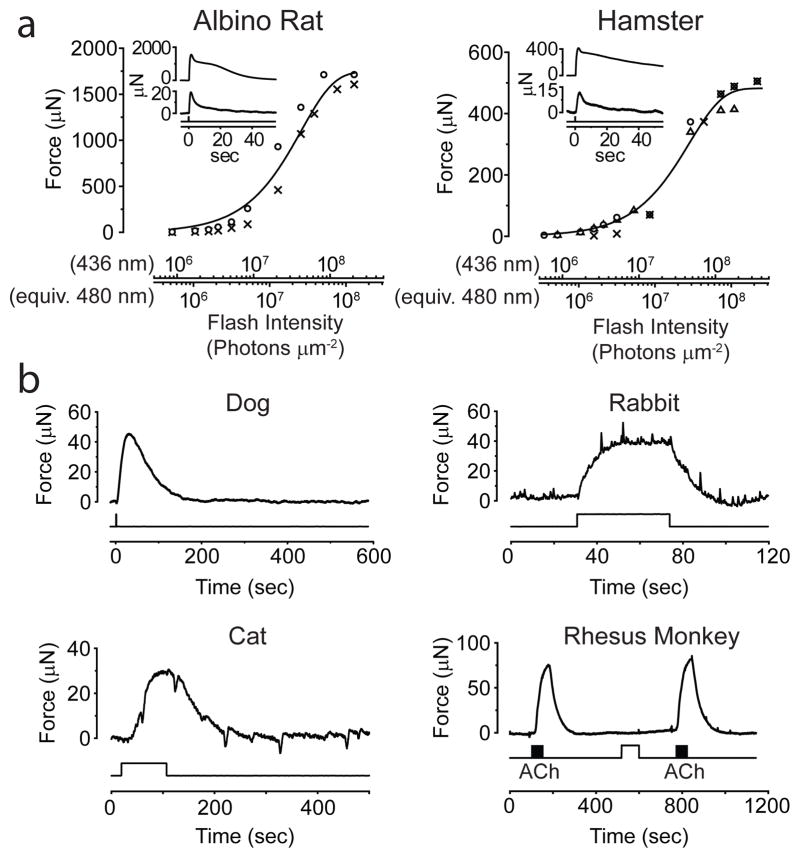
Intrinsic photosensitivity of iris sphincter muscle of other mammals. 35–37°C. a, Flash intensity-response relations from robust light responses of albino rat and pigmented hamster muscles (2 for rat and 3 for hamster). Same fits as in Fig. 1, with I0 (averaged across individual muscles) being 4.3×107 photons μm−2 and 4.6×107 photons μm−2 (436 nm) for rat and hamster, respectively. Flashes were 12–400 ms. Insets show sample responses from a muscle of each species to dim and saturating flashes. 3.4×106 and 2.1×108 photons μm−2 for rat and 1.6×106 and 3.6×108 photons μm−2 for hamster, respectively (436-nm Hg light). b, Similar experiments on dog (representative of 2 muscles), rabbit (3), cat (1) and rhesus monkey (7). All steps and the flash (600-ms) delivered 6.1×109 photons μm−2 s−1 (436-nm). Monkey muscle (pre-incubated with 30-μM 9-cis-retinal for 1 hr) gave no obvious light response, but responded to 50-μM acetylcholine in bath (black bars). Fast deflections in the rabbit and cat experiments reflected spontaneous muscle contractions/relaxations of unknown cause. 5-mm-diameter light spot, covering entire muscle of rat, hamster, cat and rabbit, but only partially the larger muscles of others.
Involvement of melanopsin in mammalian intrinsic PLR
The action spectrum for the isolated mouse sphincter muscle (Methods) fit an A1-pigment spectral template17 with λmax at 480 nm (Fig. 3a), suggesting melanopsin’s involvement3–6. Indeed, the melanopsin-knockout18 (Opn4−/−) muscle gave no light response (Fig. 3b). By RT-PCR, we detected melanopsin mRNA in the isolated wild-type (WT) mouse iris (Fig. 3c; Methods). Immunostaining (in an albino background for viewing immunofluorescence) also suggested melanopsin’s presence in the sphincter muscle of WT, but not Opn4−/−, iris (Fig. 3d; Methods). A BAC-transgenic mouse expressing the fluorescent protein, tdTomato, under the melanopsin promoter15 (Opn4:tdTomato, bred into an albino background) likewise showed fluorescence at least in the sphincter muscle (Fig 3e). For rat and hamster, their particularly robust sphincter-muscle photoresponses (see above) allowed characterization of the associated action spectra, which also fit a 480-nm spectral template (Supplementary Fig. S3). Strictly speaking, because melanopsin appears to be more widespread than in just the sphincter muscle (Fig. 3d), contraction is not necessarily initiated by just light absorbed in the muscle itself, but this is likely.
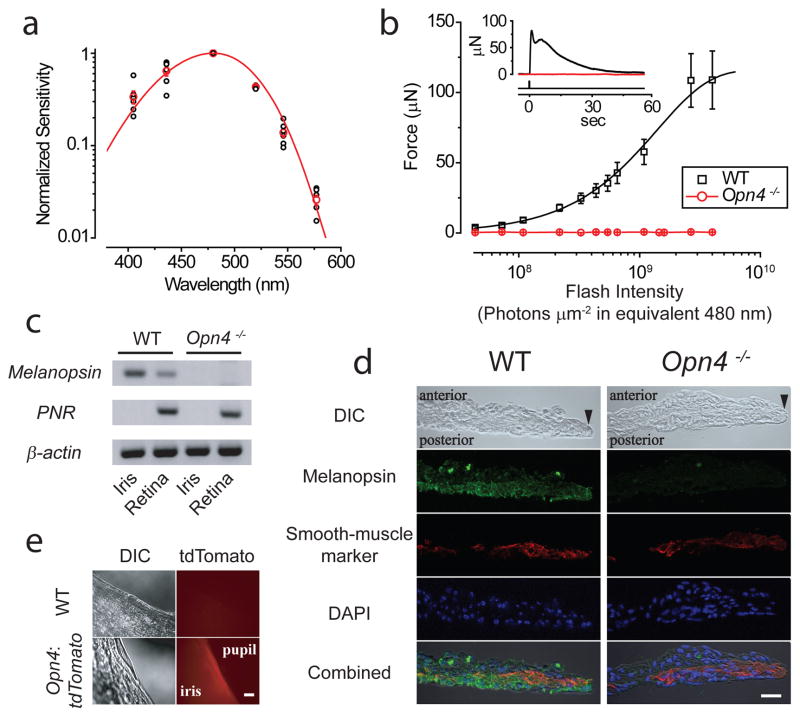
Dependence of intrinsic PLR on melanopsin. a, Action spectrum of mouse muscle (WT pigmented) (6 muscles), with sensitivity normalized to value at 480 nm in each experiment. Red circles are averages, and curve is an A1-pigment spectral template17 with λmax = 480nm. Hg light with interference filters used. b, Average flash intensity-response relation for Opn4−/− muscle (3 muscles). WT relation from Fig. 1c also shown for comparison. Inset shows sample responses of WT and Opn4−/− muscles to a saturating flash of 4.0×109 photons μm−2 (equivalent 480 nm). c, Melanopsin (Opn4) mRNA detected by RT-PCR in iris and retina from WT but not Opn4−/−. PNR (photoreceptor-specific nuclear receptor) mRNA used as control to rule out contamination from retina to iris. β-actin mRNA is a positive control. Difference in melanopsin mRNA signal between iris and retina presumably reflects different fractional total-tissue mRNA coding for melanopsin. d, Immunostaining of WT and Opn4−/− iris cross-sections for melanopsin (green), smooth-muscle α-actin (red, as muscle marker), and DAPI (blue). Anterior side (stroma) up and posterior side (posterior epithelium) down. Black arrowhead marks pupillary edge. The intensity of melanopsin immunofluorescence appeared lower in the iris than in ipRGCs (not shown). Additionally, although the Opn4−/− mouse contains the tau-lacZ marker gene replacing Opn418, β-galactosidase activity (by X-gal labeling) was not evident in the iris (not shown), presumably due to the low melanopsin-promoter activity. Scale bar: 20 μm. e, TdTomato signal detected in iris of Opn4:tdTomato but not WT mouse. Scale bar: 100 μm. Stimuli in b were 436-nm Hg light, and white for the two brightest intensities, although expressed in equivalent 480-nm photons. 3-mm-diameter spot covering entire muscle. All force measurements at 35–37°C. Mice in d and e were albino (C57BL/6J-Tyrc-2J/J).
For mouse, we were able to rule out any significant role of rhodopsin or cryptochromes in the sphincter-muscle photosensitivity (see Introduction); namely, besides a null contractile phenotype of the Opn4−/− muscle, the responses from Rho−/− (rhodopsin knockout19) and Cry1,2−/− (cryptochromes-1,2 double-knockout20) mice appeared normal (Supplementary Fig. S4a). We did find by RT-PCR rhodopsin mRNA in the dissected mouse iris (not shown; see also Ref. 21), but its significance is unclear.
By RT-PCR, we found melanopsin mRNA in the iris of rhesus monkey (not shown) and baboon (also diurnal; Supplementary Fig. S5), but the melanin-pigmentation confounded confirmation by immunohistochemistry. Melanopsin’s function in the primate iris is likewise unclear because there is no intrinsic PLR.
Phototransduction mechanism underlying intrinsic PLR
Melanopsin shows a phylogenetic kinship to invertebrate rhabdomeric visual pigments22, thus possibly sharing a common phospholipase C (PLC)-mediated phototransduction pathway (see Ref. 23 for review). Moreover, PLC typically mediates membrane-receptor signaling in smooth muscles24,25. Indeed, the sphincter muscle from Plcβ4−/− mice26 was practically unresponsive to light (Fig. 4a; 5 muscles). Sometimes, we observed a tiny response that disappeared after several stimuli (red trace in Fig 4b, top; dim flash; 3 out of 5 muscles), unlike the much larger and persistent WT response (black trace in Fig 4b, top; dim flash). This small response could be mediated by a different PLCβ isoform or another, minor pathway. The Plcβ4−/−phenotype was not due to a defective contractile apparatus, because acetylcholine still elicited strong contraction via muscarinic receptors on the muscle14 (Fig. 4b, bottom). PLCβ4 is the closest vertebrate homolog26 of the Drosophila PLC (NorpA) mediating phototransduction in rhabdomeric photoreceptors23. Bath-applied U71322, a PLC-inhibitor, did not block the light-induced contraction of the WT sphincter muscle (not shown), but this may reflect poor drug penetration into the tissue (see below).
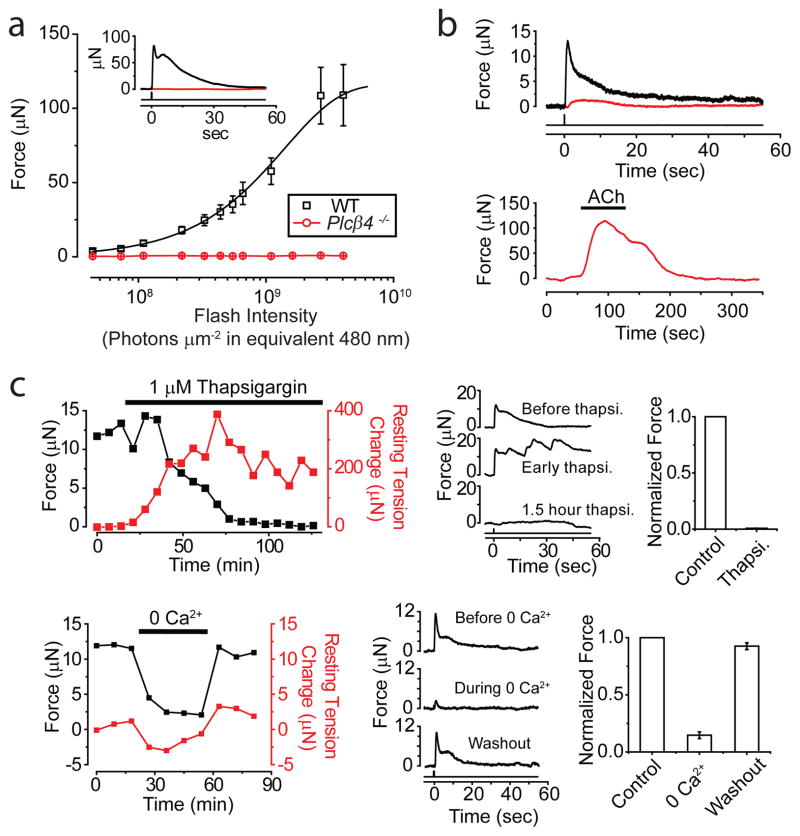
Phototransduction mechanism underlying intrinsic PLR. a, Average flash intensity-response relation for Plcβ4−/− muscle (5 experiments). WT relation from Fig. 1c shown for comparison. Inset shows sample responses of WT and Plcβ4−/− to a saturating flash, at 4.0×109 photons μm−2 (equivalent 480 nm). b, Top, Example of a Plcβ4−/− muscle (red) showing a tiny response (<3 μN) to first few dim flashes (7.3×107 photons μm−2) before becoming unresponsive. WT response (black) to same dim stimulus also shown for comparison. Bottom, Same Plcβ4−/− muscle nonetheless responded substantially to 10-μM acetylcholine. c, Thapsigargin and removal of extracellular Ca2+, respectively, greatly diminished light response. Left, Time course of effect on peak force (black) generated by dim flashes (1.1×108 photons μm−2). Resting muscle tension (red) arbitrarily set as 0 before thapsigargin or 0-Ca2+ application. Middle, Sample responses. Right, Collected data (4 muscles each). White Hg light for two brightest flashes in intensity-response relations of a; all other stimuli were 436-nm Hg light. All intensities expressed in equivalent 480 nm photons. Flashes delivered at time 0, as a 3-mm-diameter spot covering entire muscle. 35–37°C.
Smooth-muscle contraction often involves intracellular Ca2+ release (triggered by PLC via IP3 generation) in tandem with extracellular Ca2+ influx24,25. Indeed, blocking intracellular Ca2+ uptake with 1-μM thapsigargin to deplete the Ca2+-release pool gradually eliminated the muscle’s light response (Fig. 4c, top; 3 muscles). The muscle’s resting tension increased and oscillated during thapsigargin application, suggesting poor intracellular Ca2+-handling. Removing extracellular Ca2+ likewise reduced the light response by ~80% (Fig 4c, bottom; see also Ref. 9, 10, 12) and partially relaxed the resting tension. Because membrane depolarization is reportedly unnecessary for the intrinsic PLR in lower vertebrates9,10, Ca2+-permeable ion channels other than voltage-gated Ca channels are likely involved, with TRP channels – especially TRPC channels – being candidates25. However, neither the TRPC1/4/5 nor the TRPC3/6/7 subfamily was apparently involved because the Trpc1,4,5−/− and Trpc3,6,7−/− genotypes appeared normal (Methods and Supplementary Fig. S4b). A role of TRPV4 in smooth muscle has also been described25, but the muscle from Trpv4−/− mice27 was also normal (Methods and Supplementary Fig. S4b). With the apparent complexity of Ca2+ release and Ca2+ influx of unknown proportions10,28, we did not attempt to dissect the mechanistic details further.
Phototransduction pathway in ipRGCs
We carried out the same interrogation on single ipRGCs (Methods). We focused on the M1-subtype, ipRGCs with by far the highest photosensitivity and largest responses3,4, and also strongly labeled in our Opn4:tdTomato BAC-transgenic line15. Indeed, dissociated Plcβ4−/−/Opn4:tdTomato M1-ipRGCs gave no detectable responses (Fig. 5a, left; 6 cells, 23°C). In flat-mount retina, Plcβ4−/− ipRGCs (with normal impulse-firing upon current injection) showed a tiny saturated response (2.7 ± 2.8 pA, 4 cells) to strong flashes (2.1–4.0 × 109 equivalent 480-nm photons μm−2), or ≤ 1% of WT (Fig. 5a, right and Fig. 5c; 23°C). As with sphincter muscle, this small response may involve another PLCβ isoform or a minor pathway. Also, as with iris, U71322 did not block in situ WT M1-ipRGC responses in flat-mount retina (not shown; see also Ref. 29), presumably due again to poor drug penetration29.
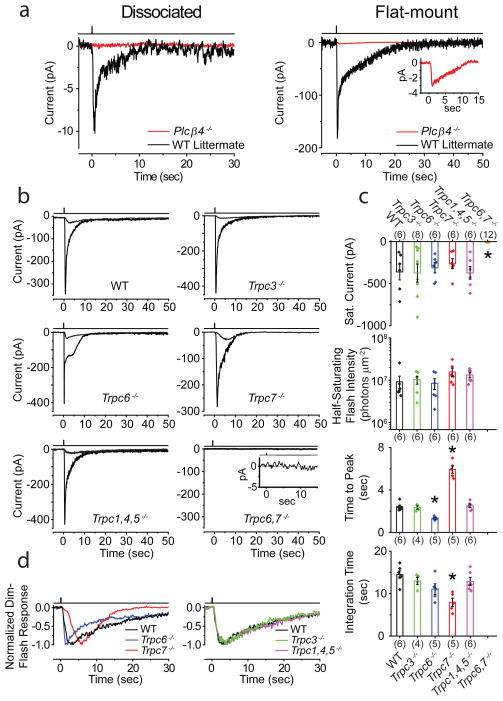
Phototransduction mechanism and components underlying ipRGC intrinsic light response. Cells identified for electrical recordings based on Opn4:tdTomato reporter background in all mouse lines. 23°C. a, Left, Dissociated Plcβ4−/− ipRGCs showed no detectable response to a saturating white Xe flash. Average of 4 trials for each response. Right, In flat-mount retina after synaptic block, Plcβ4−/− ipRGCs showed a tiny residual response (magnified in inset) to similar saturating flash. Single flash trial for WT-littermate, and average of 5 trials for Plcβ4−/−, explaining the low baseline noise in latter. 50–100 ms flashes delivering 1.97 – 3.94 × 109 photons μm−2 (equivalent 480 nm) in all cases. b, Intrinsic light responses from ipRGCs in flat-mount retina from WT, Trpc3−/−, Trpc6−/−, Trpc7−/−, Trpc1,4,5−/− and Trpc6,7−/− mice. Averaged responses to both dim (2.28–7.76×105 photons μm−2) and saturating flashes (2.05×108 photons μm−2) in each case are shown except Trpc6,7−/−, for which only response to brightest flash is shown (magnified in inset and essentially zero). Flashes were 100–300 ms of 505-nm LED light. c, Comparison of saturated response amplitude and dim-flash-response parameters from b. Half-saturating flash intensity (i.e., intensity eliciting a half-maximum response) is inversely proportional to sensitivity. Time-to-peak is the time lapse between flash and transient peak of dim-flash response. Integration time, ti, of the dim-flash response is a measure of its overall duration, given by ti = ∫ f(t)dt/fp, where f(t) is response profile and fp is its transient-peak amplitude. Collected data (mean ± SEM), where “*” indicates p<0.001 compared to WT by two-samples t-test. The number of cells examined for each parameter is in parentheses, and not always the same across parameters because not all parameters were obtainable for each cell. d, Normalized, averaged dim-flash responses from b for comparison of response waveforms. All intensities expressed in equivalent 480-nm photons, and delivered as a 730-μm-diameter spot centered on soma, sufficient for covering entire intact cell in retina. Light monitor above each trace. All recordings in perforated-patch, voltage-clamp mode (Vhold = −80 mV), with synaptic transmission blocked pharmacologically.
Interestingly, at 23°C for recording stability, M1-ipRGCs from Trpc6,7−/− mice (crossed into Opn4:tdTomato15 for cell identification; Methods) were not intrinsically photosensitive (Fig. 5b, c). Single-KO Trpc6−/− and Trpc7−/− M1-ipRGCs had similar sensitivities and saturated photocurrents as WT, albeit with distinct response kinetics (Fig. 5b–d). TRPC6 and TRPC7 likely form heteromeric ion channels in ipRGCs, although separate homomeric channels are remotely still possible (Supplementary Fig. S6). The Trpc1,4,5−/− and Trpc3−/− phenotypes were both like WT (Fig. 5b–d), while Trpc3,6−/− and Trpc3,6,7−/− (5 cells each) resembled Trpc6−/− and Trpc6,7−/−, respectively (not shown). The TRPC-subfamily is the closest vertebrate homolog of Drosophila TRP/TRPL channels mediating rhabdomeric phototransduction downstream of NorpA30.
At 35°C, M1-ipRGCs (further validated by intracellular dye-labeling after recordings; Methods) of Trpc6,7−/− genotype did give detectable, albeit tiny, responses (≤1% of WT) to strong flashes (1.1–1.9 × 109 equivalent 480-nm photons μm−2), as did Trpc3,6,7−/− cells (Supplementary Fig. S7). Presumably, these residual responses would be even smaller 15 and undetectable at 23°C. Opn4−/− cells, however, remained unresponsive at 35°C (Supplementary Fig. S7).
Functional contribution of intrinsic PLR in mouse
Because of the intrinsic PLR, the overall pupil constriction in an illuminated eye should be stronger than the consensual constriction in the contralateral, unilluminated eye. Accordingly, we simultaneously monitored both pupils of a mouse while subjecting one eye to Ganzfeld illumination (2-min light step; Methods). For WT animals, the intensity-response (I-R) relations for the ipsilateral and contralateral PLRs were identical at the dimmest intensities but diverged thereafter, with the ipsilateral PLR indeed being always stronger within a given animal (Fig. 6a, b, top panel). For Opn4−/− animals, some bilateral asymmetry persisted but it was noticeably smaller especially at high intensities, with both I-R relations being broadly similar to WT except for a lower maximal PLR as found previously18 (Fig. 6a, b, second panel from top). For mice lacking rod and cone signals15 (Gnat1−/− cl), the I-R relation was shifted to much higher light-step intensities owing to exclusive signaling by melanopsin18, but the bilateral asymmetry at high intensities again became more obvious than Opn4−/− (Fig. 6a, b, third panel from top). The residual asymmetry in Opn4−/− may suggest slightly stronger rod/cone signals to the ipsilateral PLR as well, perhaps explaining some of the WT ipsi/contralateral disparity in PLR especially at lower intensities. Finally, Gnat1−/− cl Opn4−/− mice had practically no steady PLR (Ref. 31, but see Ref. 32) (Fig. 6a, b, bottom panel).
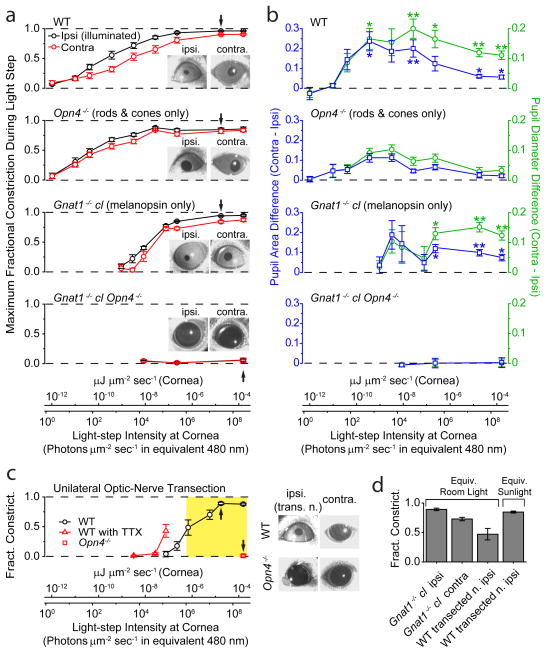
Simultaneous direct (ipsilateral) and consensual (contralateral) PLRs to unilateral illumination for different mouse genotypes in situ. 2-min of 505-nm LED light in a–c. PLR in ipsilateral, illuminated eye was measured at peak during this 2-min period, with contralateral PLR measured simultaneously. For a and b, conversion of light intensities into equivalent 480-nm photons applies strictly only to Gnat1−/− cl, but not to WT or Opn4−/− genotypes (which involved also rod/cone signals), because it required action spectrum of melanopsin. For this reason, light intensities are also given in the general unit of μJ μm−2 s−1 (same as μW μm−2). a, Average step intensity-response (I-R) relations for WT, Opn4−/−, Gnat1−/− cl, and Gnat1−/− cl Opn4−/− mice. PLR expressed as MFC (Maximum Fractional Constriction), where MFC = 1 (Normalized Pupil Area in Light) = 1 − (Pupil Area in Light/Pupil Area in Darkness). Inset shows exemplary bilateral PLRs at an intensity indicated by arrow on I-R relations. Number of animals were 7 (WT), 7 (Opn4−/−), 5 (Gnat1−/− cl) and 3 (Opn4−/− Gnat1−/− cl). b, Ipsi/contralateral difference in normalized pupil area and diameter. For area, this difference is simply the difference between the ipsi- and contralateral values in a. For diameter, normalized diameter is (Normalized Area)1/2 = (1- MFC)1/2, calculable from data in a; the ipsi/contralateral difference values can then evaluated (see Methods for significance of diameter). The area or diameter difference at a given intensity was then averaged over all animals of a given genotypic group. “**” indicates p<0.01, and “*” indicates p<0.05, when a WT or Gnat1−/− cl value is compared to corresponding Opn4−/− value by two-samples t-test. c, Direct (ipsilateral) PLR of eye with transected optic nerve to isolate the intrinsic component. WT mice in the absence or presence of TTX (5 μl of 600-μM TTX in water administered on the cornea) (same set of 5 animals), and Opn4−/− mice (4 animals). Images on right show exemplary bilateral PLRs (without TTX) at an intensity indicated by arrow on corresponding I-R relations. Opn4−/− animals with transected optic nerve gave essentially no PLR. Yellow shaded area indicates light-intensity range from room light (with a minimum measured as 1.0 × 10−6 μW μm−2, or 1.1 × 106 equivalent 480-nm photons μm−2 s−1) to direct sunlight (measured as 3.7 × 10−4 μW μm−2, or 3.9 × 108 equivalent 480-nm photons μm−2 s−1) (Methods). d, Pupil constriction triggered by white light (400–650-nm bandpass filter; Xe lamp), matched in power to average common room light and ambient sunlight (Methods) (2.2 × 10−6 and 4.2 × 10−5 μW μm−2, respectively), for Gnat1−/− cl without optic-nerve transaction (6 animals) and WT mice with optic-nerve transaction (5 animals). All light rendered adirectional with Ganzfeld diffusing sphere. Pupils video-recorded under dim infrared light that did not activate any photoreceptors. All error bars are S.E.M.
The above difference in bilateral asymmetry between WT and Opn4−/− PLRs cannot distinguish between an intrinsic iridic PLR and a bilateral asymmetry in ipRGC signaling to the PLR because both mechanisms involve melanopsin. To isolate the intrinsic PLR, we eliminated retinal signaling from one eye in the WT mouse by transecting its optic nerve (Methods). When the denervated eye was illuminated (at >7 days postsurgery), the intact contralateral eye failed to respond as expected, whereas the PLR persisted in the denervated eye (Fig. 6c, right panel), with the action spectrum of melanopsin (Supplementary Fig. S8). This residual component is the isolated intrinsic PLR. Its I-R relation on the intensity axis (Fig. 6c, left panel) relative to that for the ipsilateral PLR of non-operated WT animals (Fig. 6a, top panel) indicates that the intrinsic PLR begins to contribute when the normal overall PLR is ~90% complete. Nonetheless, the intrinsic PLR even by itself would have been able to drive the pupillary constriction 80–90% to completion over ~2.5 log units of light-step intensities. Furthermore, the intrinsic PLR has, in reality, an even lower light threshold (thus contributing even more to the overall PLR) because its I-R relation shifted by ~1 log unit to lower intensities after topical application of TTX to the cornea (Fig. 6c, left panel), which blocked any tonic autonomic inputs to the dilator and sphincter muscles. In short, the intrinsic component participates in the highest ~3.5 log units of the overall ~9-log-unit dynamic range of light intensities spanned by the normal PLR in mouse, at least during 2 min of steady illumination.
To translate into natural conditions, the yellow-shaded region in Fig. 6c, left panel, spans approximately from laboratory light to outdoor daylight (Methods). We also directly simulated ambient light with white xenon-arc light (400–650 nm) of matched power (Methods). For example, in room light, an ipsilateral (i.e., intrinsic) PLR of 0.47±0.10 fractional constriction was elicited from the denervated eye of WT mice (Fig. 6d, 5 animals), versus 0.89±0.02 when ipRGCs were also active (Gnat1−/− cl mice without transected optic nerve) (Fig. 6d, 6 animals). Thus, the intrinsic PLR contributes substantially to the melanopsin component of the overall PLR even in room light. Finally, exposing the bilaterally-denervated eyes of a dark-adapted mouse to genuine room or outdoor light indeed led to intrinsic PLRs of extents expected from above (not shown).
Similar denervation experiments on rhesus and owl monkeys revealed no intrinsic PLR (2 animals each; Supplementary Text), consistent with the above negative findings from their sphincter muscles and with clinical observations from human patients presenting complete unilateral optic neuropathy (resulting in loss of photosensitivity in the affected eye) (Supplementary Text).
Discussion
We have discovered a surprising, widespread intrinsic PLR among non-primate mammals, with a strong positive correlation between a nocturnal/nocturnal-leaning habitat and an intrinsic PLR. Teleologically, an intrinsic PLR benefits such mammals (with highly-photosensitive, rod-dominant retinas more susceptible to photodamage33) by sustaining a pupil constriction under strong steady light – a situation otherwise difficult to achieve with neural circuitry alone because of the meager light admitted through the tiny pupil to the retina. The reason for the loss of this feature in primates despite melanopsin’s continued presence in iris is unclear. Our work also reveals some bilateral asymmetry in the PLR of normal mouse (cf. 34, 35), which we now know to come partly from the intrinsic iridic photosensitivity in the higher-intensity range.
The intrinsic PLR is driven by melanopsin in mouse, probably also rat and hamster. By extension, the same presumably applies to other mammals showing an intrinsic PLR. As such, the function, if any, of the rhodopsin in mouse iris (see Results) is unknown. For amphibians and fish, the evidence for rhodopsin driving the intrinsic PLR was indefinite8,9 and probably should be re-examined given our present findings and melanopsin’s presence in the eyes of amphibians (retina and iris)22 and fish (Ref. 36; Hsi-Wen Liao and K.-W.Y., unpublished). In chicken, the intrinsic PLR reportedly has an action spectrum with λmax at <400 nm (Ref. 11; confirmed by us but not shown). This is interesting because chicken also has melanopsin in the retina and iris11,37.
Because melanopsin is phylogenetically related to rhabdomeric opsins22, it is perhaps not surprising that its photosignaling mechanism employs a PLC pathway. Previously, clues from heterologous expressions38,39, pharmacology, electrophysiology and/or immunohistochemistry on frog melanophores40, native ipRGCs29 and sub-vertebrate chordates41,42 have all implicated such a pathway. With gene-knockout mouse lines, we have now established this pathway more definitively and identified the signaling enzyme as PLCβ4, the closest homolog of Drosophila NorpA and the phospholipase C that mediates rhabdomeric phototransduction in fly. Although PLCβ4 is shared by iris and ipRGCs (at least the M1-subtype), the pathway diverges downstream. For M1-ipRGCs, the depolarizing light response results from the opening of presumably heteromeric channels formed predominantly by TRPC6 and TRPC7 (with potentially additional non-TRPC or non-critical TRPC subunits), gated possibly by phosphatidylinositol-4,5-bisphosphate, PIP2 (Ref. 29). This makeup of the native channel agrees with indirect suggestions from immunohistochemical43,44 and RT-PCR45 studies. For the sphincter muscle, however, TRPC3/6/7 or TRPC1/4/5 are apparently not involved in the light-induced contraction. An intracellular Ca2+ release is clearly important for the intrinsic PLR (see Results), but apparently not for the ipRGC light response29.
We have not yet identified the Gα-species signaling between melanopsin and PLCβ4. However, conventional wisdom30, pharmacological evidence29, and in vitro biochemistry46 suggest that it (they) should belong to the Gq-subfamily.
Finally, ordinary retinal ganglion cells (i.e., non-ipRGCs) become intrinsically photosensitive when transduced by virus to express melanopsin47. Because PLCβ4 and TRPC6 are both expressed generally in RGCs48,29,43,44, the same signaling pathway may well underlie this virus-induced intrinsic photosensitivity.
NOTE
After we had completed the experiments on ipRGCs, a paper appeared49 reporting that the Trpc3−/− and Trpc7−/− single-KO genotypes showed no effect on the ipRGC’s light response, while the Trpc6−/− genotype showed a smaller light response than WT. These data are not in quantitative agreement with ours reported here. We attribute this difference to the use of whole-cell recording by the other group, a recording configuration that, from our experience, does not monitor the light response of ipRGCs with the same fidelity as the perforated-patch recording adopted by us.
Methods Summary
All experimental details are provided in Supplementary Information. The experimental procedures on animals followed the guidelines of the Animal Care and Use committee of the Johns Hopkins University School of Medicine. All indicated errors are standard errors of the mean (SEM).
Acknowledgments
We thank the following individuals for kindly providing knockout mouse lines (those with no indicated affiliation are all at Johns Hopkins School of Medicine): Drs. Michael Caterina (Trpv4−/−), Janis Lem (Tufts University) (Rho−/− and Gnat1−/−), Jason C. Chen (Virginia Commonwealth University) (Rho−/−), Jeremy Nathans (cl, also known as cone-DTA), Aziz Sancar (University of North Carolina at Chapel Hill) (Cry1,2−/−) and Lutz Birnbaumer (US National Institute of Environmental Health Sciences) (Trpc1−/−, Trpc3−/− and Trpc6−/−). We thank Drs. David Marshak (University of Texas Medical Center, Houston), Rudiger von der Heydt, Xiaoqing Wang, and Vivien Casagrande (Vanderbilt Medical School) for eyes from baboon, rhesus monkey, marmoset and bush baby, respectively, and Drs. Wei Li (US National Eye Institute), Ruifa Mi, Brian O’Rourke, Laurie Pipitone, Dawn Ruben, David Ryugo, Laura Smale (Michigan State University), and Gordon Tomaselli for eyes of the other animals. Experiments on bush baby were carried out in Dr. Vivien Casagrande’s laboratory with help and hospitality. We also thank Dr. Weidong Gao for suggestions on the force transducer, Drs. Fred Rieke (University of Washington at Seattle) and Alapakkam Sampath (University of Southern California) for suggestions on the design of the LED light system, Dr. Petri Ala-Laurila (University of Washington at Seattle) for the method of equivalent-480-nm-photon conversion, Dr. Hugh Cahill for the mouse-head-anchoring method, Dr. Xiaozhi Ren for help on western blots, Dr. Ofelia Garalde and Austin Chen for help in the monkey experiments, and Wendy W.S. Yue for help on RT-PCR. We also thank Terry Shelley for fabricating all custom equipment, Sue Kulason for help in data analysis, and Liusong Ding for mouse-genotyping support. Members of the Yau laboratory provided comments on the manuscript. This work was supported by U.S. NIH Grant EY14596 and the António Champalimaud Vision Award (Portugal) to K.-W.Y.
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Author Contributions. T.X., M.T.H.D. and K.-W.Y. designed the experiments. T.X. and M.T.H.D. did the isolated-eyeball experiments. T.X. carried out all muscle recordings and also Trpcs-KOs ipRGC recordings at 23°C (recordings at 35°C were by Z.J.), the mouse-optic-nerve transections, some of the in situ PLR measurements (the rest done by J.H.), some of the RT-PCR experiments on melanopsin (the rest done by H.C.W.), the tdTomato-fluorescence experiments, and the generation and maintenance of all double, triple and quadruple genetically-engineered mouse lines for this study. The recordings from ipRGCs of Plcβ4−/− mice and their WT littermates were done by M.T.H.D. (23°C) and Z.J. (35°C). T.X., M.T.H.D and J.H. designed the head-fixed, dual-PLR-recording instrument. H.C.W. and T.X. did the anterior-chamber measurements. H.C.W. did the immunocytochemistry and X-gal labeling. S.L.M. and D.W. did the surgery of transecting the optic nerve in anesthetized monkeys, and tested the PLR with T.X. and M.T.H.D. T.Y. participated in the monkey experiments. A.R. in D.E.C’s laboratory made the TRPC7−/− mouse line and did the associated characterization (retinal RT-PCR done by T.X.). P.W., S.S., V.F. and M.F. made the TRPC5−/− line and did the associated characterization, and also provided the Trpc4−/− and Trpc4,5−/− lines. M.I.S. provided the Plcβ4−/− line. T.X., M.T.H.D. and Z.J. analyzed the data with assistance from J.H., and, together with K.-W.Y., wrote the paper.
Reprints and permissions information is available at www.nature.com/reprints.
References
Full text links
Read article at publisher's site: https://doi.org/10.1038/nature10567
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3270891
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1038/nature10567
Article citations
TRPC5 controls the adrenaline-mediated counter regulation of hypoglycemia.
EMBO J, 07 Oct 2024
Cited by: 0 articles | PMID: 39375537
Visible light accelerates skin wound healing and alleviates scar formation in mice by adjusting STAT3 signaling.
Commun Biol, 7(1):1266, 05 Oct 2024
Cited by: 0 articles | PMID: 39367154 | PMCID: PMC11452386
Direct retino-iridal projections and intrinsic iris contraction mediate the pupillary light reflex in early vertebrates.
Commun Biol, 7(1):993, 14 Aug 2024
Cited by: 1 article | PMID: 39143195 | PMCID: PMC11324758
Introduction and reflections on the comparative physiology of sleep and circadian rhythms.
J Comp Physiol B, 194(3):225-231, 10 Jun 2024
Cited by: 1 article | PMID: 38856727 | PMCID: PMC11233284
Circadian rhythms in the Drosophila eye may regulate adaptation of vision to light intensity.
Front Neurosci, 18:1401721, 30 May 2024
Cited by: 0 articles | PMID: 38872947
Go to all (164) article citations
Other citations
Wikipedia
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
A role for the ciliary marginal zone in the melanopsin-dependent intrinsic pupillary light reflex.
Exp Eye Res, 119:8-18, 05 Dec 2013
Cited by: 26 articles | PMID: 24316157
C-terminal phosphorylation regulates the kinetics of a subset of melanopsin-mediated behaviors in mice.
Proc Natl Acad Sci U S A, 114(10):2741-2746, 21 Feb 2017
Cited by: 23 articles | PMID: 28223508 | PMCID: PMC5347544
Intrinsically photosensitive retinal ganglion cells detect light with a vitamin A-based photopigment, melanopsin.
Proc Natl Acad Sci U S A, 102(29):10339-10344, 12 Jul 2005
Cited by: 92 articles | PMID: 16014418 | PMCID: PMC1177370
Intrinsically photosensitive retinal ganglion cells.
J Neuroophthalmol, 27(3):195-204, 01 Sep 2007
Cited by: 55 articles | PMID: 17895821
Review
Funding
Funders who supported this work.
Howard Hughes Medical Institute
NEI NIH HHS (11)
Grant ID: R01 EY006837-23
Grant ID: R37 EY006837-13
Grant ID: R01 EY006837
Grant ID: R01 EY014596
Grant ID: R37 EY006837-15S1
Grant ID: R01 EY006837-24
Grant ID: R37 EY006837-14
Grant ID: R37 EY006837
Grant ID: R01 EY006837-22
Grant ID: EY14596
Grant ID: R37 EY006837-15
NHLBI NIH HHS (1)
Grant ID: T32 HL007572
NIDCD NIH HHS (4)
Grant ID: R01 DC006904
Grant ID: R01 DC006904-07
Grant ID: R01 DC006904-09
Grant ID: R01 DC006904-08





