Abstract
Free full text

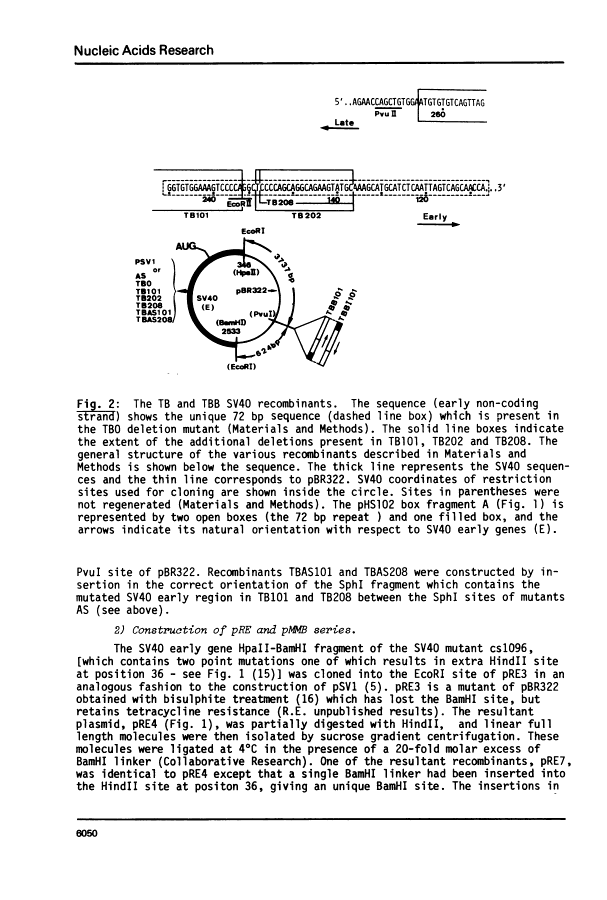
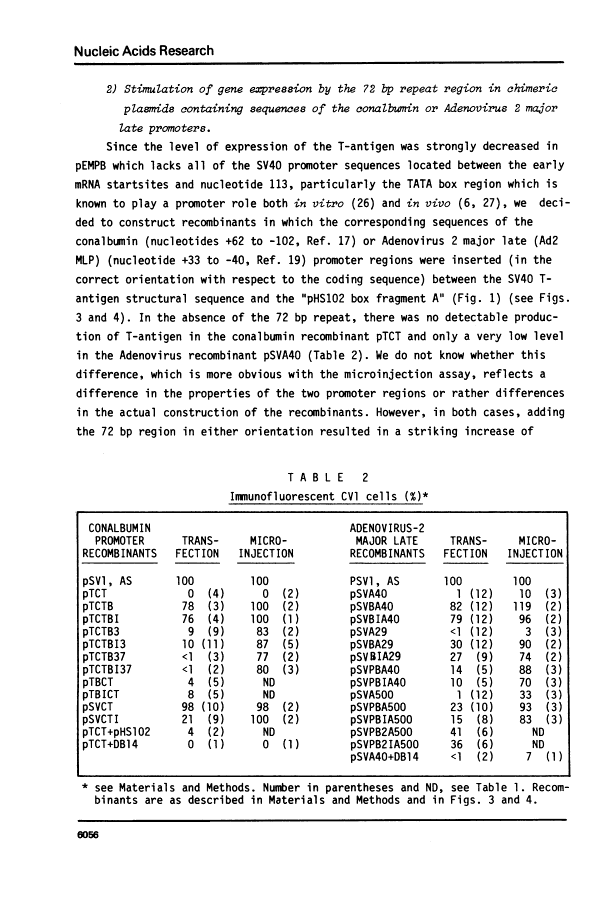
The SV40 72 base repair repeat has a striking effect on gene expression both in SV40 and other chimeric recombinants.
Abstract
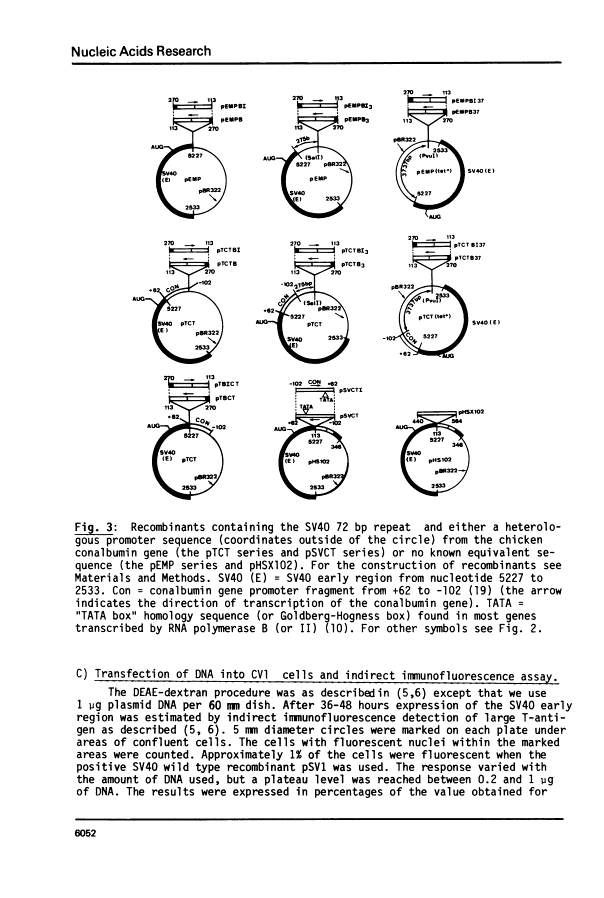
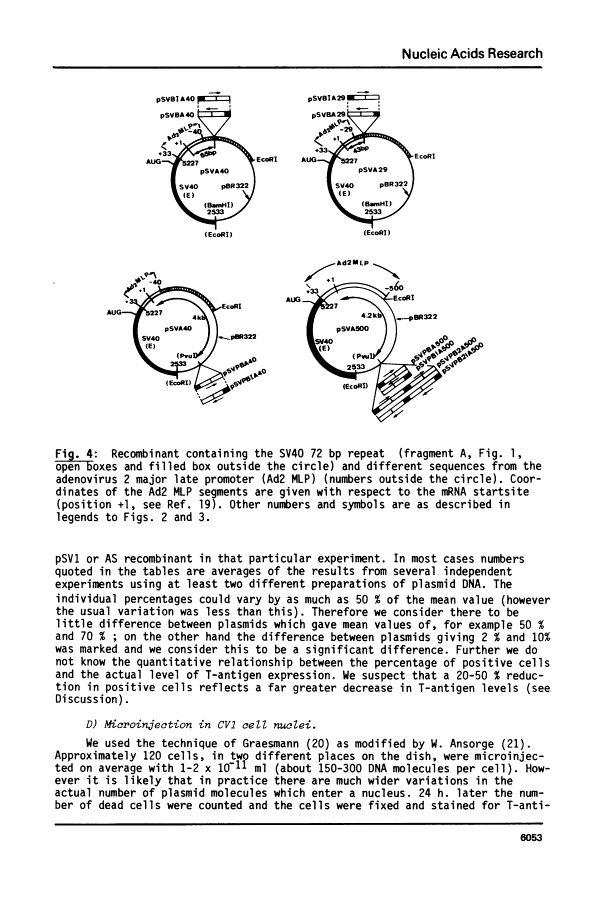
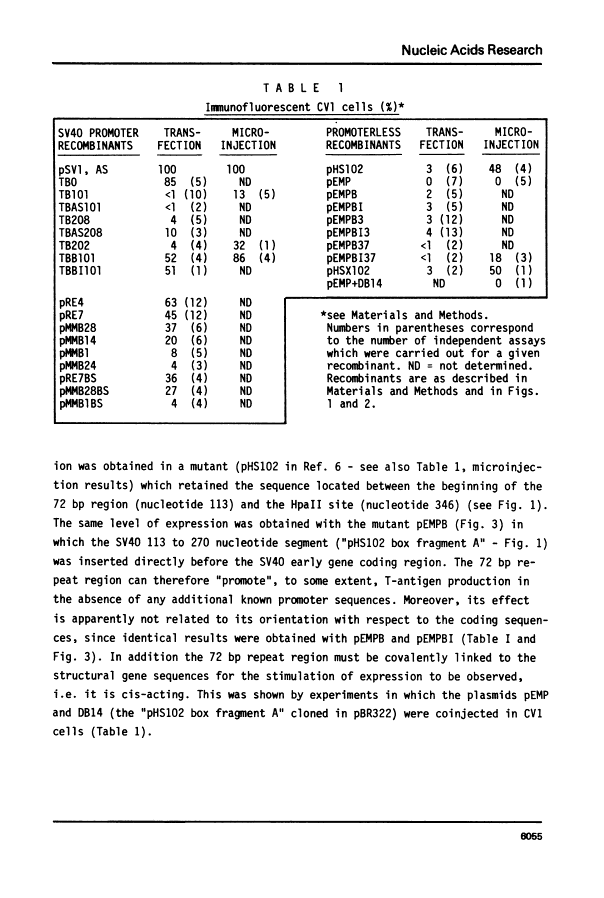
By introduction of recombinant plasmids into monkey CV1 cells, we have unambiguously demonstrated that sequences entirely within the 72 bp repeat, which is located upstream of the SV40 early region, are crucial for T-antigen expression in vivo. We have also shown that a DNA fragment containing the 72 bp repeat, inserted directly before chicken conalbumin or adenovirus-2 major late promoter sequences in chimeric plasmids where these promoters replace that of the SV40 early genes, caused a dramatic increase in the expression of T-antigen in vivo. This effect was independent of the orientation of the 72 bp repeat, but was sensitive to its location within the plasmid, when the 72 bp repeat was separated from the promoter sequences, T-antigen expression was reduced. Insertion of the 72 bp repeat into equivalent plasmids containing no known eukaryotic promoter sequences (plasmids which were not detectably expressed in vivo) gave rise to a measurable, but smaller level of expression. The stimulation of expression by the 72 bp repeat is cis-acting : it required covalent linkage to the recombinant. We discuss the possibility that the 72 bp repeat region in SV40 may act as a bi-directional entry site for RNA polymerase B such that promoter sequences linked to the repeat are more efficiently utilised.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.9M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- JACOB F, ULLMAN A, MONOD J. LE PROMOTEUR, 'EL'EMENT G'EN'ETIQUE N'ECESSAIRE 'A L'EXPRESSION D'UN OP'ERON. C R Hebd Seances Acad Sci. 1964 Mar 16;258:3125–3128. [Abstract] [Google Scholar]
- Scaife J, Beckwith JR. Mutational alteration of the maximal level of Lac operon expression. Cold Spring Harb Symp Quant Biol. 1966;31:403–408. [Abstract] [Google Scholar]
- Rosenberg M, Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. [Abstract] [Google Scholar]
- Siebenlist U, Simpson RB, Gilbert W. E. coli RNA polymerase interacts homologously with two different promoters. Cell. 1980 Jun;20(2):269–281. [Abstract] [Google Scholar]
- Benoist C, Chambon P. Deletions covering the putative promoter region of early mRNAs of simian virus 40 do not abolish T-antigen expression. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3865–3869. [Europe PMC free article] [Abstract] [Google Scholar]
- Benoist C, Chambon P. In vivo sequence requirements of the SV40 early promotor region. Nature. 1981 Mar 26;290(5804):304–310. [Abstract] [Google Scholar]
- Grosschedl R, Birnstiel ML. Spacer DNA sequences upstream of the T-A-T-A-A-A-T-A sequence are essential for promotion of H2A histone gene transcription in vivo. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7102–7106. [Europe PMC free article] [Abstract] [Google Scholar]
- Struhl K. Deletion mapping a eukaryotic promoter. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4461–4465. [Europe PMC free article] [Abstract] [Google Scholar]
- Breathnach R, Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. [Abstract] [Google Scholar]
- Fiers W, Contreras R, Haegemann G, Rogiers R, Van de Voorde A, Van Heuverswyn H, Van Herreweghe J, Volckaert G, Ysebaert M. Complete nucleotide sequence of SV40 DNA. Nature. 1978 May 11;273(5658):113–120. [Abstract] [Google Scholar]
- Van Heuverswyn H, Fiers W. Nucleotide sequence of the Hind-C fragment of simian virus 40 DNA. Comparison of the 5'-untranslated region of wild-type virus and of some deletion Mutants. Eur J Biochem. 1979 Oct;100(1):51–60. [Abstract] [Google Scholar]
- Maxam AM, Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. [Abstract] [Google Scholar]
- DiMaio D, Nathans D. Cold-sensitive regulatory mutants of simian virus 40. J Mol Biol. 1980 Jun 15;140(1):129–142. [Abstract] [Google Scholar]
- Shortle D, Nathans D. Local mutagenesis: a method for generating viral mutants with base substitutions in preselected regions of the viral genome. Proc Natl Acad Sci U S A. 1978 May;75(5):2170–2174. [Europe PMC free article] [Abstract] [Google Scholar]
- Cochet M, Gannon F, Hen R, Maroteaux L, Perrin F, Chambon P. Organization and sequence studies of the 17-piece chicken conalbumin gene. Nature. 1979 Dec 6;282(5739):567–574. [Abstract] [Google Scholar]
- Corden J, Wasylyk B, Buchwalder A, Sassone-Corsi P, Kedinger C, Chambon P. Promoter sequences of eukaryotic protein-coding genes. Science. 1980 Sep 19;209(4463):1406–1414. [Abstract] [Google Scholar]
- Graessmann M, Graessman A. "Early" simian-virus-40-specific RNA contains information for tumor antigen formation and chromatin replication. Proc Natl Acad Sci U S A. 1976 Feb;73(2):366–370. [Europe PMC free article] [Abstract] [Google Scholar]
- Gruss P, Dhar R, Khoury G. Simian virus 40 tandem repeated sequences as an element of the early promoter. Proc Natl Acad Sci U S A. 1981 Feb;78(2):943–947. [Europe PMC free article] [Abstract] [Google Scholar]
- Subramanian KN, Shenk T. Definition of the boundaries of the origin of DNA replication in simian virus 40. Nucleic Acids Res. 1978 Oct;5(10):3635–3642. [Europe PMC free article] [Abstract] [Google Scholar]
- Waldeck W, Sauer G. Localization of a deletion in the DNA of a biologically active simian virus 40 strain. FEBS Lett. 1976 Dec 1;71(2):313–315. [Abstract] [Google Scholar]
- Van Heuverswyn H, Fiers W. Nucleotide sequence of the Hind-C fragment of simian virus 40 DNA. Comparison of the 5'-untranslated region of wild-type virus and of some deletion Mutants. Eur J Biochem. 1979 Oct;100(1):51–60. [Abstract] [Google Scholar]
- Myers RM, Tjian R. Construction and analysis of simian virus 40 origins defective in tumor antigen binding and DNA replication. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6491–6495. [Europe PMC free article] [Abstract] [Google Scholar]
- Mathis DJ, Chambon P. The SV40 early region TATA box is required for accurate in vitro initiation of transcription. Nature. 1981 Mar 26;290(5804):310–315. [Abstract] [Google Scholar]
- Ghosh PK, Lebowitz P, Frisque RJ, Gluzman Y. Identification of a promoter component involved in positioning the 5' termini of simian virus 40 early mRNAs. Proc Natl Acad Sci U S A. 1981 Jan;78(1):100–104. [Europe PMC free article] [Abstract] [Google Scholar]
- Wasylyk B, Derbyshire R, Guy A, Molko D, Roget A, Téoule R, Chambon P. Specific in vitro transcription of conalbumin gene is drastically decreased by single-point mutation in T-A-T-A box homology sequence. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7024–7028. [Europe PMC free article] [Abstract] [Google Scholar]
- Wasylyk B, Chambon P. A T to A base substitution and small deletions in the conalbumin TATA box drastically decrease specific in vitro transcription. Nucleic Acids Res. 1981 Apr 24;9(8):1813–1824. [Europe PMC free article] [Abstract] [Google Scholar]
- Sassone-Corsi P, Corden J, Kédinger C, Chambon P. Promotion of specific in vitro transcription by excised "TATA" box sequences inserted in a foreign nucleotide environment. Nucleic Acids Res. 1981 Aug 25;9(16):3941–3958. [Europe PMC free article] [Abstract] [Google Scholar]
- Nelkin BD, Pardoll DM, Vogelstein B. Localization of SV40 genes within supercoiled loop domains. Nucleic Acids Res. 1980 Dec 11;8(23):5623–5633. [Europe PMC free article] [Abstract] [Google Scholar]
- Saragosti S, Moyne G, Yaniv M. Absence of nucleosomes in a fraction of SV40 chromatin between the origin of replication and the region coding for the late leader RNA. Cell. 1980 May;20(1):65–73. [Abstract] [Google Scholar]
- Jakobovits EB, Bratosin S, Aloni Y. A nucleosome-free region in SV40 minichromosomes. Nature. 1980 May 22;285(5762):263–265. [Abstract] [Google Scholar]
- Liu LF, Miller KG. Eukaryotic DNA topoisomerases: two forms of type I DNA topoisomerases from HeLa cell nuclei. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3487–3491. [Europe PMC free article] [Abstract] [Google Scholar]
- Sternglanz R, DiNardo S, Voelkel KA, Nishimura Y, Hirota Y, Becherer K, Zumstein L, Wang JC. Mutations in the gene coding for Escherichia coli DNA topoisomerase I affect transcription and transposition. Proc Natl Acad Sci U S A. 1981 May;78(5):2747–2751. [Europe PMC free article] [Abstract] [Google Scholar]
- Hsieh T, Brutlag D. ATP-dependent DNA topoisonmerase from D. melanogaster reversibly catenates duplex DNA rings. Cell. 1980 Aug;21(1):115–125. [Abstract] [Google Scholar]
- Capecchi MR. High efficiency transformation by direct microinjection of DNA into cultured mammalian cells. Cell. 1980 Nov;22(2 Pt 2):479–488. [Abstract] [Google Scholar]
- McKnight SL, Gavis ER, Kingsbury R, Axel R. Analysis of transcriptional regulatory signals of the HSV thymidine kinase gene: identification of an upstream control region. Cell. 1981 Aug;25(2):385–398. [Abstract] [Google Scholar]
Associated Data
Articles from Nucleic Acids Research are provided here courtesy of Oxford University Press
Full text links
Read article at publisher's site: https://doi.org/10.1093/nar/9.22.6047
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc327583?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Non-canonical functions of enhancers: regulation of RNA polymerase III transcription, DNA replication, and V(D)J recombination.
Trends Genet, 40(6):471-479, 19 Apr 2024
Cited by: 0 articles | PMID: 38643034
Enhancer selectivity in space and time: from enhancer-promoter interactions to promoter activation.
Nat Rev Mol Cell Biol, 25(7):574-591, 27 Feb 2024
Cited by: 7 articles | PMID: 38413840
Review
Enhancer Arrays Regulating Developmental Genes: Sox2 Enhancers as a Paradigm.
Results Probl Cell Differ, 72:145-166, 01 Jan 2024
Cited by: 0 articles | PMID: 38509257
From Genotype to Phenotype: How Enhancers Control Gene Expression and Cell Identity in Hematopoiesis.
Hemasphere, 7(11):e969, 08 Nov 2023
Cited by: 1 article | PMID: 37953829 | PMCID: PMC10635615
Review Free full text in Europe PMC
In Silico Verification of Predicted Potential Promoter Sequences in the Rice (Oryza sativa) Genome.
Plants (Basel), 12(20):3573, 14 Oct 2023
Cited by: 0 articles | PMID: 37896036 | PMCID: PMC10609952
Go to all (488) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The repeated GC-rich motifs upstream from the TATA box are important elements of the SV40 early promoter.
Nucleic Acids Res, 11(8):2447-2464, 01 Apr 1983
Cited by: 150 articles | PMID: 6304653 | PMCID: PMC325895
Mutational dissection of the 21 bp repeat region of the SV40 early promoter reveals that it contains overlapping elements of the early-early and late-early promoters.
Nucleic Acids Res, 12(2):915-932, 01 Jan 1984
Cited by: 56 articles | PMID: 6320125 | PMCID: PMC318545
A novel eukaryotic promoter element: the simian virus 40 72 base pair repeat.
Fed Proc, 43(2):226-234, 01 Feb 1984
Cited by: 1 article | PMID: 6319202
Regulation of eukaryotic gene expression by transactivating proteins and cis acting DNA elements.
Biol Cell, 50(3):203-216, 01 Jan 1984
Cited by: 41 articles | PMID: 6235875
Review