Abstract
Free full text

GM-CSF: a role in immune and inflammatory reactions in the intestine
Abstract
Granulocyte macrophage colony-stimulating factor (GM-CSF) is a cytokine that promotes myeloid cell development and maturation, and dendritic cell differentiation and survival in vitro. Growing evidence supports the notion that GM-CSF has a major role in some inflammatory and autoimmune reactions and in the host’s response to pulmonary infection, but few studies have addressed its functions and importance in the GI tract. Recent studies demonstrated that administration of GM-CSF can result in clinical improvement in patients with Crohn’s disease. Mice deficient in GM-CSF (GM-CSF−/−) developed more severe intestinal and systemic infection after an enteric infection, and more severe colitis in response to enteric exposure to dextran sodium sulfate. Both the severity of infection and colitis were largely prevented by GM-CSF administration. Such studies indicate that GM-CSF has an important role in the regulation of intestinal immune and inflammatory responses.
Granulocyte macrophage colony-stimulating factor (GM-CSF) is a hematopoietic growth factor originally discovered by its ability to generate in vitro granulocyte and macrophage colonies from bone marrow precursor cells [1]. In vitro, GM-CSF can promote the survival and activation of macrophages, neutrophils and eosinophils, in addition to dendritic cell (DC) maturation [2]. Nonetheless, GM-CSF-deficient mice (GM-CSF−/−) have normal numbers of bone marrow DC progenitors and mature DCs in the spleen, thymus and lymph nodes. This suggests a redundant role for GM-CSF in DC development and differentiation in those tissues under steady state or homeostatic conditions [3,4]. In the lung, however, maturation of alveolar macrophages is compromised in GM-CSF−/− mice and associated with the development of pulmonary alveolar proteinosis and increased susceptibility to certain pulmonary infections [4–6].
The biologic functions of GM-CSF and the consequences of GM-CSF deficiency in the human intestine are relatively unexplored. Current knowledge is based mainly on studies using human intestinal epithelial cells (IECs) in vitro [7–9], GM-CSF−/− mice [10–12], and either mice [13,14] or humans [15–19] administered GM-CSF therapeutically. T cells, macrophages, endothelial cells and fibroblasts are known to be major cell sources of GM-CSF [20]. However, GM-CSF expression by the various cell types in the intestinal mucosa has not yet been characterized. In this article, we discuss GM-CSF and GM-CSF receptor (GM-CSFR) expression in the human and mouse intestine, and examine the current knowledge of the role of GM-CSF in mucosal immune homeostasis, the intestine and gastrointestinal diseases including inflammatory bowel disease (IBD), gastrointestinal infection and colorectal cancer. Finally, we review the therapeutic potential of GM-CSF based on recent clinical trials.
GM-CSF receptor & signaling
Granulocyte macrophage colony-stimulating factor is secreted as a monomer by a variety of cell types in vitro (e.g., T cells, macrophages, endothelial cells, epithelial cells, mesothelial cells, chondrocytes and fibroblasts) in response to proinflammatory stimuli (e.g., LPS, IL-1 and TNF-α) [1,21].
The GM-CSFR on cells is a heterodimer that consists of an α-subunit specific for GM-CSF binding, and a signaling βc-subunit that is shared with the receptors for IL-3 and IL-5 in humans [2,22]. A recent model for the human GM-CSFR bound to GM-CSF, based on x-ray crystallography, revealed that, novel for cytokine–receptor complexes, it exists as a dodecamer that contains two hexameric complexes (each one composed of two GM-CSF molecules, two GM-CSFRα chains and two GM-CSFRβc chains) [23]. JAK2 tyrosine kinase plays an essential role in GM-CSF signaling. The dodecamer complex appears to be required for tyrosine phosphorylation of βc by JAK2, receptor activation and the initiation of signal transduction [23]. This complex structural organization of the receptor may explain, in part, the pleiotropic biologic functions of GM-CSF (e.g., protection from apoptosis, entry and progression of cells through the cell cycle, myelopoiesis, differentiation and maturation of target progenitors, and activation and motility functions in mature cells) [24]. Although it is not known how this segregation of functions is mediated, a number of possibilities have been suggested. For example, one mechanism could be that intermediate forms of receptor assembly might have different biological activities. Another mechanism could envision the segregation of function through differential phosphorylation of serine versus tyrosine residues that might differentially take place at different GM-CSF concentrations [25,26]. It should be noted that the binding of GM-CSF to its receptor is known to activate at least three signaling pathways: Janus kinase/signal transducer and activation of transcription (JAK/STAT), mitogen-activated protein kinase (MAPK), and phosphatidylinositol 3 (PI-3) kinase [22,27–33]. Furthermore, several studies on human GM-CSFRα have demonstrated that GM-CSFRα can exist in a soluble form that is able to bind to GM-CSF and block its function [34–37].
GM-CSF in GI tract homeostasis
GM-CSF & GM-CSFR expression in the intestine
Small intestine
Among the four cell types in the small intestinal epithelium (i.e., absorptive epithelial cells, goblet cells, enteroendocrine cells and Paneth cells), GM-CSF has been identified exclusively in Paneth cells, as shown by reverse transcription-PCR and immunohistochemical staining in rats [38]. Unlike GM-CSF, however, GM-CSFRβ mRNA is expressed both by Paneth cells and other epithelial cells of the rat small intestine. This led to the hypothesis that GM-CSF, secreted by Paneth cells in rat, might act via an autocrine or paracrine mechanism on both Paneth cells and IECs [38], although that would require each of those cell types to also express the GM-CSFRα chain and generate a functional signaling GM-CSFR, which was not addressed in those studies. Since it is known that GM-CSF enhances the expression of costimulatory molecules in antigen presenting cells (APCs) [39], and GM-CSF stimulation of rat IECs induced the expression of costimulatory molecules CD80 and CD86 [38], these authors proposed that GM-CSF may have a role in mucosal immunity of the small intestine by inducing costimulatory molecules not only present in typical APCs, but also in IECs.
Granulocyte macrophage colony-stimulating factor has been proposed to mediate other functions in the small intestine. For example, isolated small IECs producing GM-CSF have been proposed to modulate functional activity of hematopoietic stem cells [40] and proliferative activity of crypt cells in mice [41]. Furthermore, a recent study in mice, using bone marrow chimeras, found that a loss of GM-CSF signaling in nonhematopoietic cells increased ileal injury in response to NSAIDs [42]. This suggests that GM-CSF signaling may have a role in maintaining intestinal barrier function in the small intestine, possibly by regulating survival and proliferation of IECs. In the small intestinal lamina propria in rats, GM-CSF protein has also been detected in some mononuclear cells [38].
Colon
In humans, the GM-CSFR was expressed in freshly isolated colon epithelial cells at levels similar to that of monocytes [7]. Moreover, the levels of expression of GM-CSFR were similar in surface (more differentiated) and crypt (less differentiated) epithelial cells [7].
Expression of both GM-CSF [8,9] and a functional GM-CSFR [8] has been described in various human colon carcinoma cell lines. However, results from studies using colon cancer cell lines as representative of normal IECs must be interpreted cautiously. In this regard, we did not find detectable levels of GM-CSF protein in normal mouse colon epithelium in vivo [10]. Interestingly, immunohistochemical studies that aimed to identify the nature of GM-CSF-producing cells in human colon surgical samples demonstrated positive cytoplasmatic staining in neoplastic cells (in some cases accompanied by staining in fibroblasts and lymphocytes), but no staining in nontransformed epithelial cells distant from the neoplastic focus was observed [9].
GM-CSF functions in intestinal homeostasis
Intestinal homeostasis in the gut is maintained by a number of factors, including the epithelial surface mucus layer, IECs and their products, and intestinal mononuclear cells (including DCs, macrophages and lymphocytes) and their products [43]. However, little is known regarding the role of GM-CSF in intestinal homeostasis. DCs function as APCs and have an important role in maintaining intestinal homeostasis by maintaining the balance between tolerance to self-antigens and the activation of host pathways that protect against pathogenic bacteria. Two recent reports indicate that GM-CSF is a key factor in the differentiation of intestinal DCs in mice. One found that GM-CSF is important for the differentiation of CD11b+ CD103− DCs [44], whereas the other found that GM-CSF is required for the differentiation of CD11b+ CD103+ and CD11b− CD103+ DCs [45]. This difference may reflect different methodologies used to phenotype and separate the cell types. In either case, one mechanism by which GM-CSF may be an important contributor to intestinal homeostasis is through its influence on the differentiation of DCs. Furthermore, GM-CSF, together with IL-4, has a role in the induction of retinoic acid (RA) production by DCs in mice in vitro [46], and RA-producing DCs play a key role in gut homeostasis. Thus, RA-producing DCs imprint gut-homing specificity on T cells after antigen presentation and, in the presence of TGF-β induce the differentiation of naive T cells to T regulatory cells (Tregs) Foxp3+ in mice [47–49].
In addition, GM-CSF may contribute to intestinal homeostasis by maintaining the integrity of the intestinal epithelium. Several studies reported that GM-CSF can stimulate proliferation of freshly isolated murine IECs in vitro [41,50]. Furthermore, depending on its concentration, GM-CSF either stimulated or inhibited epithelial cell proliferation in mice (i.e., at 1 ng/ml, GM-CSF stimulated proliferation, whereas at 5 and 25 ng/ml it inhibited proliferation) [41]. By contrast, GM-CSF, at a dose similar to that which increased proliferation of freshly isolated epithelial cells, did not increase proliferation of human colon carcinoma cell lines [7].
Granulocyte macrophage colony-stimulating factor levels are low to undetectable in the healthy colon in vivo and the major reported steady-state phenotype in GM-CSF−/− mice is alveolar proteinosis. Therefore, one could posit that the most important functions of GM-CSF in the intestinal mucosa might reflect an influence on host responses to external stimuli (e.g., microbial infections) and its role in inflammatory or autoimmune reactions.
GM-CSF in gastrointestinal disease
GM-CSF in IBD
Inflammatory bowel disease includes Crohn’s disease (CD) and ulcerative colitis (UC). CD and UC are chronic inflammatory disorders of the GI tract. Current hypotheses suggest that genetic predisposition, environmental factors, a loss of tolerance to commensal gut flora, an aberrant or overactive immune response and an imbalance between pro- and anti-inflammatory cytokines have key roles in the pathogenesis of IBD [51–53]. In addition, a deficiency of host innate immunity may contribute to the pathogenesis of IBD [54,55].
Higher levels of GM-CSF secretion have been detected in mucosal lesions of CD and UC patients compared with normal control mucosa [56,57], and also in the colon lesions of mice in a dextran sulfate sodium (DSS)-induced colitis model [58]. By contrast, levels of GM-CSFR expression on IECs were similar in inflamed tissues from UC and CD patients and control patients [7].
Although the mechanism by which GM-CSF plays a role in IBD remains unknown, recombinant human GM-CSF (rhGM-CSF) has been used in clinical trials and was reported to result in improvement and remission in patients with CD (discussed later). To explore the mechanism by which GM-CSF can alter the course of intestinal inflammation, several groups used a murine model of acute colitis induced by DSS. Those studies have taken two different approaches: inducing colitis in the mice and administrating GM-CSF intraperitoneally as a therapy [13,14]; or alternatively studying the outcome of colitis in mice deficient in GM-CSF [12].
Studies in mice administered GM-CSF as a therapy
Two groups took the approach of administering GM-CSF as therapy in mice [13,14]. One study by Sainathan et al. found that GM-CSF administration to mice with DSS-induced colitis improved clinical parameters and histology by a mechanism presumed to involve increased type I interferon production [13]. Concurrent with this, plasmacytoid DCs (440c+) were expanded in the spleen, although the plasmacytoid DC population in the intestinal mucosa was not examined. In this study, GM-CSF treatment also resulted in decreased proinflammatory cytokines expression (TNF-α, IL-1β and IL-1α) in the colon [13]. Another study by Bernasconi et al. found a similar reduction in colitis severity after GM-CSF administration in DSS-treated mice, and reported that GM-CSF promoted accelerated ulcer healing in the colon and epithelial repair in vitro [14]. These effects were associated with a modified composition of the inflammatory infiltrate with reduced neutrophil numbers and increased monocytic subsets (CD11b+) [14]. However, the mechanism by which alterations in those cells contributed to ulcer healing was not addressed.
Study of the outcome of colitis in mice deficient in GM-CSF
GM-CSF−/− mice were more susceptible to DSS-induced colitis. This was shown by clinical and histological parameters and by increased proinflammatory cytokine production [12]. However, the precise mechanism by which GM-CSF deficiency increased the severity of colitis was not addressed, although the possibility of impaired macrophage function was suggested.
Under steady-state conditions, GM-CSF levels in the circulation are low to undetectable, but may also be underestimated owing to the uptake of GM-CSF by cells such as polymorphonuclear neutrophils [59]. In humans, low levels of autoantibodies to GM-CSF have been reported in the serum of healthy individuals [60]. However, other studies found GM-CSF autoantibodies only in the serum of patients with pulmonary alveolar proteinosis or myasthenia gravis, but none or a very low percentage was found in healthy controls [61–64]. These different results may reflect differences in assay design, and the specificity of the detection methods [65]. Importantly, increased levels of GM-CSF autoantibodies have been found also in pediatric and adult patients with CD with ileal or ileocolonic involvement, compared with those with colon involvement only, UC patients and healthy controls. Increased levels of GM-CSF antibodies directly correlated with aggressiveness of the disease, and neutrophil phagocytic activity was inversely correlated with GM-CSF antibody levels [66]. Thus, it is possible that GM-CSF antibody can bind and neutralize GM-CSF, resulting in a phenotype of GM-CSF deficiency and more severe colitis. Of note, in this regard, GM-CSF deficiency in mice was associated with increased ileal permeability, increased bacterial translocation to mesenteric lymph nodes and increased mucosal damage in response to NSAIDs [42,66], suggesting that GM-CSF is important for maintaining intestinal mucosal integrity and barrier function.
GM-CSF in gastrointestinal infection
Granulocyte macrophage colony-stimulating factor is a growth factor that promotes the differentiation of granulocytes and macrophages, which are critically involved in the control of the host’s defense to pathogenic microbes. For this reason, the role of GM-CSF in various infectious diseases has been investigated [67]. GM-CSF is approved as a clinical therapy to prevent and control infection in patients with myelosuppression based on its ability to increase bone marrow myeloid cells and to enhance functions of neutrophils and macrophages. However, little is known thus far regarding the role of GM-CSF in human gastrointestinal infections.
Evidence from mouse models of infection indicate that GM-CSF can provide vital host defense against infection with some classes of pathogenic microbes. The finding that GM-CSF−/− mice and mice lacking GM-CSFR could develop alveolar proteinosis led to initial studies focusing on pulmonary infection [68]. GM-CSF−/− mice were shown to be more susceptible to pulmonary infection with group B Streptococcus [6] and Pneumocystis carinii pneumonia [5]. These reports emphasized the importance of GM-CSF in alveolar macrophage function in the lung.
In contrast to respiratory tract infections, few reports have addressed the role of GM-CSF in GI tract infections. One study observed that GM-CSF−/− mice have impaired cytokine production in the intestine and delayed onset of inflammatory pathology in the spleen and liver during murine Salmonella-infection [11]. We recently investigated the specific role of GM-CSF in controlling intestinal inflammation and in host defense to enteric infection by Citrobacter rodentium [10]. C. rodentium is a naturally occurring mouse pathogen that is widely used to model infections with human pathogens that cause characteristic attaching-and-effacing lesions in the intestinal tract (e.g., enteropathogenic and enterohemorrhagic Escherichia coli). We observed that GM-CSF was induced after infection with C. rodentium (Figure 1) primarily in CD11c+ DCs and F4/80+ macrophages. Compared with infected control mice, infected GM-CSF−/− mice had a more severe infection with significantly greater colon mucosal inflammation, increased numbers of C. rodentium in feces, increased bacterial counts in the spleen and mesenteric lymph nodes and decreased serum antibody titers to C. rodentium [10]. This abnormal host response in GM-CSF−/− mice was rescued by GM-CSF administration [10]. C. rodentium-infected GM-CSF−/− mice had significantly fewer mucosal CD11c+ DCs than wild-type mice. By contrast, F4/80 macrophages and Gr1+ leukocytes were markedly increased in the colon mucosa of GM-CSF−/− mice, suggesting that GM-CSF regulates mucosal inflammation and immunity by controlling DC numbers and their localization in the intestine [10]. Consistent with this, selective DC depletion from control mice mimicked the abnormalities seen in GM-CSF−/− mice [10]. We found that GM-CSF enhanced DC survival by decreasing DC apoptosis after infection (Figure 1A) [10].
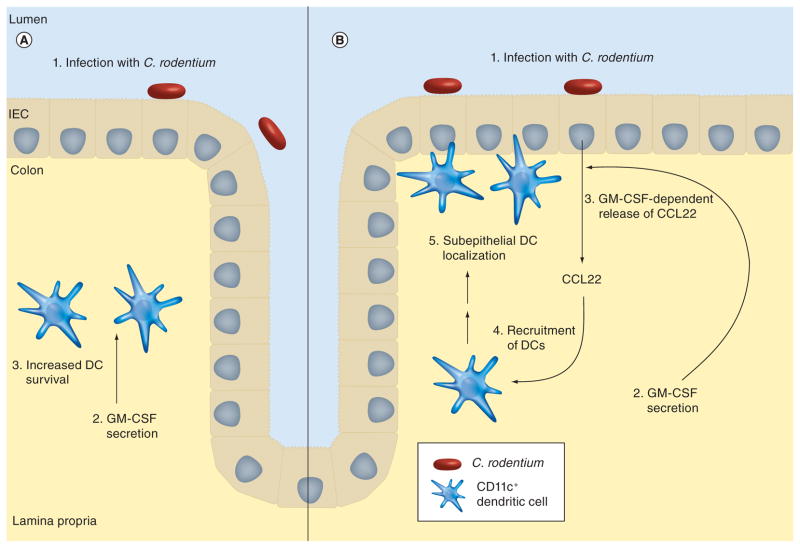
Citrobacter rodentium is a naturally occurring mouse pathogen that is widely used to model infections with attaching- and effacing lesion-inducing pathogens in humans (e.g., enteropathogenic and enterohemorrhagic Escherichia coli). After enteric infection of mice with this pathogen, GM-CSF is produced in the colon mucosa. (A) GM-CSF enhances DC survival by decreasing DC apoptosis. (B) GM-CSF, directly or indirectly, induces secretion of the chemoattractant cytokine CCL22 by intestinal epithelial cells, which has a major role in the recruitment and localization of CD11c+ DCs to the subepithelial region of surface epithelial cells.
DC: Dendritic cell; GM-CSF: Granulocyte macrophage colony-stimulating factor; IEC: Intestinal epithelial cell.
Chemokines have a major role in DC recruitment and localization in peripheral tissues. We found that a C-C chemokine CCL22 (also termed macrophage-derived chemokine) [69,70] was important for DC recruitment and subepithelial cell localization in the colon mucosa of C. rodentium-infected mice. CCL22 production by IECs in infected mice was dependent on GM-CSF (Figure 1B) [10]. Thus, GM-CSF regulation of mucosal DC survival, coupled with its influence on DC localization in the mucosa via CCL22, is important for host defense and the control of intestinal inflammation, such as that caused by an attaching- and effacing-pathogen.
Granulocyte macrophage colony-stimulating factor has a critical role in generating DCs in vitro, but the lack of apparent defects in DC numbers in uninfected GM-CSF−/− mice raised questions regarding its role in DC differentiation in vivo [3,4]. However, two recent studies that focused on the origin and differentiation of intestinal mucosal DCs in the steady state reported that GM-CSF has a nonredundant role in mucosal DC differentiation in vivo [44,45]. It now appears that GM-CSF is especially important for DCs in the intestinal mucosa where the balance between tolerance and immunity against enteric bacteria requires tight regulation by DCs.
GM-CSF in colorectal cancer
There are few reports that have examined GM-CSF in the pathogenesis of colorectal cancer, although increased GM-CSF mRNA and protein have been found in many colon cancer cell lines, as well as in surgical specimens [8,9]. Expression of GM-CSFR is also documented in the SW403 colon cancer cell line [8]. Stimulation of colon cancer cells with GM-CSF activated tyrosine kinase(s) with molecular weights between 30 and 120 kDa, but did not increase proliferation of those cells [8,9,71]. We are not aware that GM-CSF−/− mice have been tested in the colon carcinogenesis models. Thus, the effect of GM-CSF in the promotion or progression of colon carcinogenesis is not known. Nonetheless, GM-CSF has been used in cancer vaccine therapy for colorectal cancer to enhance the maturation of DCs which, based on their antigen-presenting functions, are viewed as important for immunotherapy [71].
Therapeutic applications of GM-CSF in gastrointestinal disease
GM-CSF & CD
The use of GM-CSF for the treatment of CD was based on the notion that there may be an underlying defect in innate immunity in CD (i.e., involving macrophages), and that treatment with GM-CSF might correct this putative abnormality and prove therapeutically beneficial.
Recombinant human GM-CSF has been tested in CD patients in several clinical trials (Table 1). Initial findings based on a small number of patients suggested that patients with moderate-to-severe CD treated with GM-CSF had a high rate of clinical response and remission with minimal adverse effects [15]. Results from this initial open-label dose-escalation trial were followed up by a randomized double-blind placebo-controlled trial [16]. In this study, patients were randomly assigned in a 2:1 ratio to receive rhGM-CSF or placebo subcutaneously for 56 days. There was no significant difference between the drug and placebo groups in the primary end point, defined as a decrease of at least 70 points in the CD Activity Index (CDAI) on day 57, although there was a significant difference between these groups on day 29 of treatment. Furthermore, significance of a secondary end point was achieved both on day 29 of treatment and on day 57. In addition, the remission rate (CDAI <150) 30 days after end of the trial was significant in the rhGM-CSF treatment group, and there was a significant improvement in mucosal healing and measurements of health-related patient quality of life. Mild-to-moderate adverse effects were more frequent in the drug-treated group [16].
Table 1
Clinical trials using recombinant human granulocyte macrophage colony-stimulating factor as a treatment for Crohn’s disease.
| Study (year) | Patient population | Patients (n) | Design | Regimen | Results | Ref. |
|---|---|---|---|---|---|---|
| Dieckgraefe & Korzenik (2002) | Patients with moderate-to-severe CD (USA) | 15 | Uncontrolled open-label dose-escalation trial | 4, 6 or 8 μg/kg rhGM-CSF sc. daily for 8 weeks | Greater than 100 points decrease in CDAI in 12 patients. Eight achieved clinical remission (CDAI <150) with minimal adverse effects | [15] |
| Korzenik et al. (2005) | Patients with moderate-to-severe CD (USA) | 124 | Randomized double-blind placebo-controlled trial. Patients randomized at 2:1 ratio to rhGM-CSF or placebo | 6 μg/kg per day rhGM-CSF or placebo sc. for 8 weeks | No significant difference in primary end point of clinical response at end of trial. Secondary end points suggested rhGM-CSF decreased disease severity, increased remission and improved patient quality of life | [16] |
| Takazoe et al. (2009) | Japanese patients with moderate-to-severe active CD (Japan) | 5 | Open-label Phase I–II study of the tolerability and pharmacokinetics of rhGM-CSF | 6 μg/kg per day for 4 weeks to study tolerability and pharmacokinetics and for 8 weeks to study efficacy and safety | Pharmacokinetic studies show no systemic rhGM-CSF accumulation after repeated sc. administration. Clinical response (>100 point decrease in CDAI) in a maximum of two patients on day 43. Response not sustained at day 57 | [17] |
| Valentine et al. (2009) | Corticosteroid-dependent patients with CD (USA and Canada) | 129 | Randomized double-blind placebo controlled Phase II trial. Patients randomized at 2:1 ratio to rhGM-CSF or placebo | 6 μg/kg per day rhGM-CSF or placebo sc. for 22 weeks. The study consisted of an adjunctive phase, a forced corticosteroid tapering phase, and an observation phase | Treatment with rhGM-CSF resulted in corticosteroid-free remission 4 weeks after corticosteroid elimination in 18.6% of patients compared with 4.9% for placebo-treated patients | [18] |
| Magno et al. (2010) | Patients with fistulizing CD who had failed conventional therapy (Puerto Rico) | 3 | Uncontrolled study | 6 μg/kg per day of rhGM-CSF sc. for 8 weeks | rhGM-CSF was ineffective in all three patients. Small number of patients in the study precludes any conclusions | [19] |
CD: Crohn’s Disease; CDAI: Crohn’s Disease Activity Index; GM-CSF: Granulocyte macrophage colony-stimulating factor; rhGM-CSF: Recombinant human granulocyte macrophage colony-stimulating factor; sc.: Subcutaneous.
Another open-label Phase I–II study aimed to describe GM-CSF tolerability, pharmacokinetics, safety and efficacy in Japanese patients with active CD [17]. Whereas rhGM-CSF improved CDAI scores by greater than 100 points in two out of five patients on day 43, improvement was not apparent on day 57 (i.e., day following end of 56-day trial). Pharmacokinetic studies found no systemic accumulation of GM-CSF despite daily subcutaneous injections [17].
Finally, a randomized Phase II clinical trial demonstrated that rhGM-CSF was significantly more effective than placebo for obtaining a corticosteroid-free clinical remission 4 weeks after corticosteroid elimination, and significantly improved health-related quality of life in patients with corticosteroid-dependent CD [18].
Clinical trials support the notion that administration of rhGM-CSF in CD patients attenuates the severity of the disease and promotes an improvement of quality of life; however, the exact mechanism by which GM-CSF exerts its action remains to be elucidated.
Expert commentary
The possibility that GM-CSF has an important role in regulating immune and inflammatory responses in the intestinal mucosa was suggested by the observation that patients with CD, a chronic inflammatory bowel disease, improved on rhGM-CSF therapy. That finding initially seemed somewhat counterintuitive, as GM-CSF was generally regarded as a cytokine with more proinflammatory functions based on its activity on neutrophils and macrophages. Although specific mechanisms for the beneficial response to GM-CSF in CD were not defined, those studies suggested that GM-CSF can play a role in attenuating intestinal inflammation. To the extent that studies in mouse models reflect possible mechanisms by which GM-CSF may act to improve mucosal inflammation in CD, several possibilities warrant consideration. Some studies, discussed herein, suggest that GM-CSF has a role in the maintenance of intestinal mucosal barrier function, perhaps by directly or indirectly influencing epithelial cell proliferation and/or turnover. This may be relevant to the maintenance of mucosal integrity and mucosal defense against enteric commensal microbial flora that cross the mucosal barrier, particularly when conditions exist that are known to disturb that barrier (e.g., NSAIDs and infection). Furthermore, GM-CSF may promote increased innate host defense mechanisms within the intestinal mucosa by enhancing the activation and function of neutrophils and macrophages. Important for the nature of the host immune response, GM-CSF can influence characteristics of the DC populations in the intestinal mucosa and the interactions between DCs and T cells in the draining lymph nodes that determine proinflammatory versus anti-inflammatory characteristics of the acquired immune response. Studies reviewed here indicate that GM-CSF can have an important role in increasing mucosal defense by regulating DC survival and DC localization during microbial infection. Taken together, studies from animal models indicate that GM-CSF can have a key role in host defense and regulating inflammation in the intestinal mucosa, and that this is most evident when intestinal homeostasis is disturbed. Conversely, a relative deficiency of GM-CSF may render an individual more susceptible to the development of acute and/or chronic intestinal inflammation during, for example, intestinal infection or injuries that alter intestinal barrier function. It will be important in the future to clearly define the specific pathways, mechanisms and cellular interactions in the intestinal tract through which GM-CSF regulates host intestinal mucosal immune and inflammatory responses. It may be important, at that point, to revisit the potential manipulation of GM-CSF, its receptor and their downstream cellular, molecular and biochemical pathways as possible therapeutic targets in gastrointestinal disease.
Five-year view
Empiric clinical trials of GM-CSF in CD did not meet their primary therapeutic end point. Nonetheless, recent, more mechanistic studies, show the importance of GM-CSF in the regulation of intestinal immune and inflammatory responses. Additional studies in animal and human models are needed to clearly define the mechanisms and pathways by which GM-CSF regulates those responses. Such studies will probably reap important dividends in terms of identifying key cellular and molecular targets useful for controlling mucosal immunity and inflammation.
Footnotes
Financial & competing interests disclosure
These studies were supported in part by NIH grant P01DK35108 and a grant from the William K Warren Foundation. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of the manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
Full text links
Read article at publisher's site: https://doi.org/10.1586/egh.10.73
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3291482?pdf=render
Free to read at FutureDrugs
http://www.future-drugs.com/doi/abs/10.1586/egh.10.73
Subscription required at FutureDrugs
http://www.future-drugs.com/doi/pdf/10.1586/egh.10.73
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1586/egh.10.73
Article citations
Strategies for engineering oncolytic viruses to enhance cancer immunotherapy.
Front Pharmacol, 15:1450203, 06 Sep 2024
Cited by: 0 articles | PMID: 39309012 | PMCID: PMC11413971
Review Free full text in Europe PMC
Focal Adhesion Kinase and Colony Stimulating Factors: Intestinal Homeostasis and Innate Immunity Crosstalk.
Cells, 13(14):1178, 11 Jul 2024
Cited by: 0 articles | PMID: 39056760 | PMCID: PMC11274384
Review Free full text in Europe PMC
Neuroinflammatory markers at school age in preterm born children with neurodevelopmental impairments.
Brain Behav Immun Health, 38:100791, 03 May 2024
Cited by: 0 articles | PMID: 38818370 | PMCID: PMC11137520
Intestinal stroma guides monocyte differentiation to macrophages through GM-CSF.
Nat Commun, 15(1):1752, 26 Feb 2024
Cited by: 0 articles | PMID: 38409190 | PMCID: PMC10897309
A Versatile Intestine-on-Chip System for Deciphering the Immunopathogenesis of Inflammatory Bowel Disease.
Adv Healthc Mater, 13(7):e2302454, 11 Feb 2024
Cited by: 1 article | PMID: 38253407 | PMCID: PMC11468350
Go to all (63) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Granulocyte macrophage colony-stimulating factor and the intestinal innate immune cell homeostasis in Crohn's disease.
Am J Physiol Gastrointest Liver Physiol, 306(6):G455-65, 06 Feb 2014
Cited by: 35 articles | PMID: 24503766
Review
The role of granulocyte macrophage-colony-stimulating factor in acute intestinal inflammation.
Cell Res, 18(12):1220-1229, 01 Dec 2008
Cited by: 78 articles | PMID: 19030026
Granulocyte-macrophage colony-stimulating factor elicits bone marrow-derived cells that promote efficient colonic mucosal healing.
Inflamm Bowel Dis, 16(3):428-441, 01 Mar 2010
Cited by: 51 articles | PMID: 19639560
GM-CSF produced by nonhematopoietic cells is required for early epithelial cell proliferation and repair of injured colonic mucosa.
J Immunol, 190(4):1702-1713, 16 Jan 2013
Cited by: 43 articles | PMID: 23325885 | PMCID: PMC3563922
Funding
Funders who supported this work.
NIDDK NIH HHS (3)
Grant ID: P01 DK35108
Grant ID: P01 DK035108
Grant ID: P01 DK035108-25





