Abstract
Free full text

Enhancer and promoter interactions — long distance calls
Abstract
In metazoans, enhancers of gene transcription must often exert their effects over tens of kilobases of DNA. Over the last decade it has become clear that to do this, enhancers come into close proximity with target promoters with the looping away of intervening sequences. In a few cases proteins that are involved in the establishment or maintenance of these loops have been revealed but how the proper gene target is selected remains mysterious. Chromatin insulators had been appreciated as elements that play a role in enhancer fidelity through their enhancer blocking or barrier activity. However, recent work suggests more direct participation of insulators in enhancer-gene interactions. The emerging view begins to incorporate transcription activation by distant enhancers with large scale nuclear architecture and sub-nuclear movement.
Introduction
Enhancers are regulatory elements that increase the transcriptional output of target genes. In metazoans enhancers and the genes they regulate can be as far as 2 or 3 Mbp distant from each other. This geometry produced lively debates on how the distant enhancers could activate their target genes. Models considered included looping and tracking and variations thereof [1]. The advent of new technologies, including 3C [2] confirmed the establishment of close proximity between enhancers and target genes. In the first example, loop formation between the β-globin locus control region enhancer (LCR) and gene was shown to accompany transcriptional activation [3–5]. While this new information did not rule out the possibility that a tracking mechanism contributes to gene activation by distant enhancers [6;7], it did establish a paradigm that was borne out in numerous other loci where developmentally regulated gene clusters and single genes are activated by a distant enhancer. These include the α-globin gene cluster, TH2, IFNG, MHC class II and IgH loci among others [8].
Genomes also contain insulators that modulate enhancer activity. These elements are protein-DNA complexes that prevent an enhancer from activating a gene when positioned between them and can act as barriers to the inappropriate spread of heterochromatin. Chromatin looping underlies their behavior as well (Figure 1). In vertebrates the only known insulator protein is CTCF, which recruits cohesin to many of its functional sites [9]. The cohesin complex forms a ring to embrace chromosomes during sister chromatid exchange and its role at insulator sites could be similar. Although insulators influence enhancer function and gene expression, these elements were thought be distinct; however, the distinctions are blurring. In this review we will discuss new attributes of enhancers and new direct roles for CTCF insulators in enhancer-promoter interactions and in broadly configuring the genome.
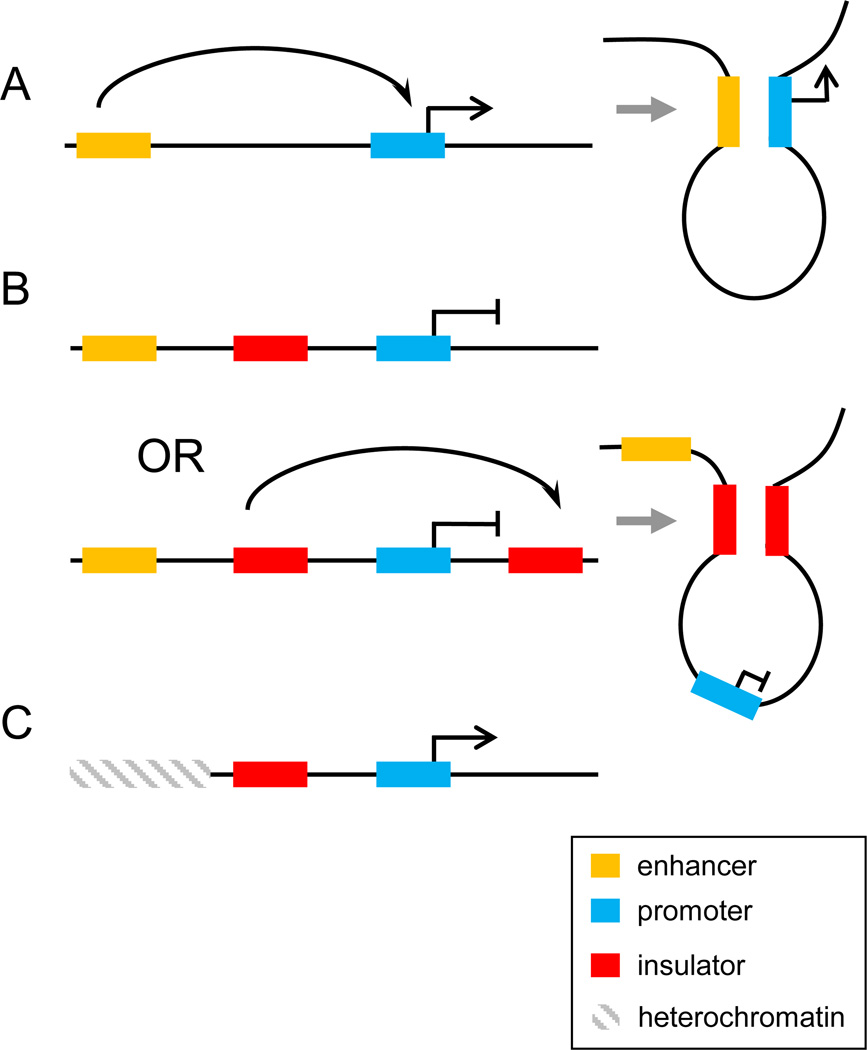
A. An enhancer is depicted as activating transcription from a target gene promoter through direct interaction over a large distance by creating a chromatin loop. B. An insulator located between an enhancer and a gene can block promoter-enhancer interaction by acting as a road block to a processive signal from the enhancer or by forming a loop with another insulator located distal to the gene. C. An insulator functions as a barrier to block the spreading of repressive chromatin into an inappropriate locus. This function is depicted as a road block but could also be carried out as in panel 1B by the insulator interacting directly with another insulator to form a loop encompassing the active gene. In all panels, the yellow rectangle is the enhancer and the blue rectangle is the gene. The red rectangle is the insulator. The hatched rectangle represents condensed heterochromatin.
Enhancer loops and functions—an update
Genome profiling of enhancers
Two studies localized putative enhancers genome wide by their signature of CBP/p300 binding and H3K4me1 modification [10;11]. However, discovering the targets of these enhancers is a formidable task. A different approach, Hi-C, has allowed investigators to capture long range interactions genome-wide by combining the classical 3C assay with high-throughput sequencing [12]. The resolution of the method was about 2 Mb but was sufficient to show that long range looping interactions underlie the co-localization of chromosomal domains based on functional state. Increased computing power has improved the resolution of Hi-C. Moreover, one could imagine combining this data with enhancer localization by CBP/p300 signature [10;11] to identify novel enhancers that function by long range interaction and their targets. This would allow an assessment of how general this phenomenon is.
In parallel with Hi-C, a different approach called ChIA-PET (chromatin interaction assay with paired end sequencing) was pioneered to investigate chromatin interactions on a genome-wide scale [13]. ChIA-PET is a ChIP-based assay allowing capture of long range chromatin interactions that are established by a specific protein of choice at high resolution. Fullwood et al. used an antibody to estrogen receptor-α (ER-α) to pull down chromatin interactions. Sequencing the resultant ChIA-PET library showed that remote ER-α enhancer-like sites interact with proximal promoters of target genes. It is of great interest to investigate the genome wide looping associations of other enhancer binding proteins.
Mediators of loops between enhancers and genes
Transcription factors or their complexes are thought to mediate enhancer-promoter loop formation but the proteins involved have been functionally identified in only a few cases. In the TH2 cytokine locus, the Il4, Il5 and Il13 genes cluster in close proximity to the TH2 locus control region (LCR) in the poised or active state in T cells but these interactions do not occur in cells lacking the transcription factors STAT6 or GATA-3 [14]. In the β-globin locus, reduction of the erythroid factors GATA-1 and EKLF (KLF1) or the more widely expressed factor Ldb1showed that are required for β-globin activation and for looping between the gene and the β-globin LCR [15–17]. Ren et al now show that OCA-B and general transcription factor TFII-I are required for long range enhancer-promoter communication in the IgH locus [18]. The IgH promoter interacts with 3’ enhancers over 100 kb distant and depletion of OCA-B or TFII-I using RNAi reduced the interactions and IgH transcription. The architectural protein SATB1 also participates in long range enhancer interaction, binding to the BCL2 gene promoter and its distant enhancer to form a loop in cells where the gene is expressed [19]. SATB1 reduction compromised loop formation and transcription. This MAR binding protein engages in many interactions genome wide, making it difficult to rule out the contribution of indirect affects to this outcome [20]. There is a clear need to expand the repertoire of factors that participate in enhancer loop formation and/or maintenance and to decipher specifically what protein-protein contacts suffice for the long range interactions.
Enhancer-gene looping and transcriptional activation
Numerous enhancers have been described that loop to their target genes and increase transcription but how the transcriptional output is changed is unclear. The loop might increase the local concentration of factors that recruit RNA pol II or pol II might be transferred from an enhancer to a promoter through their proximity [21]. The loop might affect the nuclear localization of the enhancer-promoter pair to a favorable transcriptional compartment (see below). In any case, the enhancer-gene loop appears to be necessary for transcriptional up-regulation as gain or loss of a competing promoter [22;23], reduction of loop-associated proteins [15–18] or interruption of looping by an insulator [24;25] all affect transcriptional outcome. Is the enhancer loop a cause or effect of the onset of transcription? This is a challenging question but a recent study correlated nascent transcripts with proximity of the sonic hedgehog (Shh) promoter and the limb bud enhancer, supporting a causative role [26]. This is one area where careful time course studies might be revealing, perhaps in a system like mouse G1E cells where LCR-β-globin looping and transcriptional activation can be induced by GATA-1 expression [15].
Enhancers and locus migration
Gene re-localization in nuclei upon activation is a well documented finding although how general this phenomenon is remains in question [27–29]. In animal cells, the migration typically involves moving from a peripheral position to a more interior one. Thus, the β-globin locus moves away from the nuclear periphery in maturing fetal liver cell nuclei before becoming highly. Re-localization requires the β-globin LCR but also the protein factor Ldb1, required for looping [30;31]. Recent work shows that GATA-1, its co-factor FOG1, EKLF (KLF1) and chromatin remodeler Mi-2β are also required for migration of the β-globin locus to the nuclear interior [32]. Locus movement occurred before the appearance of key markers of erythroid differentiation, suggesting it is a pre-requisite and not a consequence of high level transcription. Interestingly, inactivation of FOG-1 and Μi-2β after re-localization did not return β-globin loci to the nuclear periphery [32].
Very recently it has been shown that the Eµ enhancer is required for movement of the IgH locus to the nuclear interior [33]. Enhancer dependent long range interactions involved YY1 binding to Eµ and to the sites with which it interacted. Taken together, these results implicate enhancers and factors required for enhancer loops to form in locus movement, raising the question whether looping precedes locus migration. The Eµ enhancer loops and IgH locus migration occur without recombination or transcription of the rearranged gene, arguing that looping and migration happen first (Figure 2A). Alternatively, looping might occur after association with a transcription factory, possibly as a result of transcription (Figure 2B). Further experiments will be necessary to distinguish these models and illuminate this fundamental question.
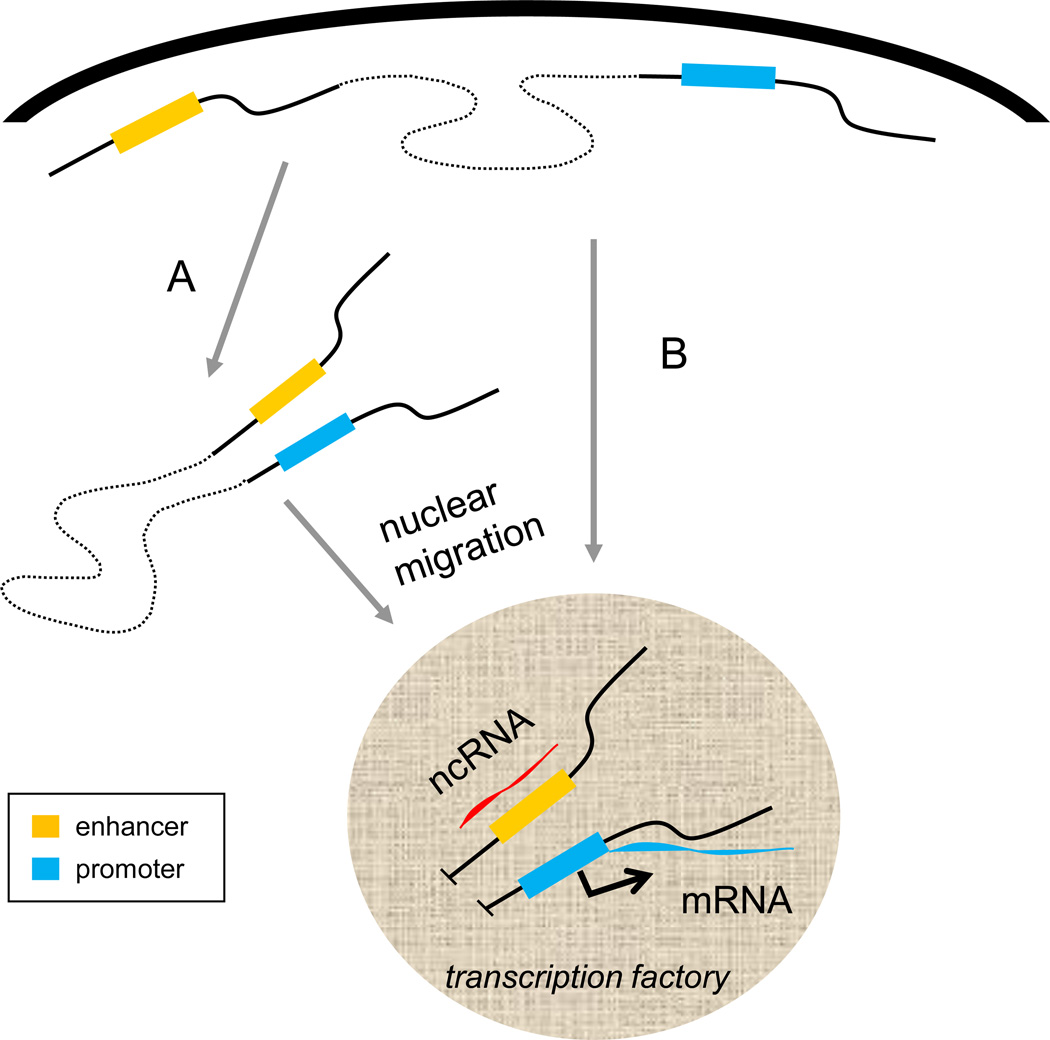
Before activation a locus occupies a position near the nuclear periphery and the enhancer and promoter do not yet interact with each other. A. In one view, after activation the enhancer interacts with the promoter which in turn leads to locus migration into the nuclear interior and localization in an RNA pol II transcription factory. Subsequently, transcription at the enhancer and promoter produces ncRNA that stabilize the interaction and mRNA respectively. B. Alternatively, the locus migrates to an interior position without communication between the enhancer and the target gene promoter and their interaction is established in the RNA polII factory, possibly with the participation of the ncRNA transcript. Designations are the same as in Figure 1.
Intra-nuclear migration during transcription activation correlates with entry into a transcription factory [34]. These entities are independent nuclear sub-compartments that are repositories of hyper-phosphorylated RNA pol II [35]. The β-globin locus enters a transcription factory after migration away from the nuclear periphery [30]. If transcription is interrupted the LCR- β-globin loop is retained, arguing that at least the maintenance of this proximity does not require ongoing transcription or even residence in a transcription factory [35;36]. Furthermore, the choice of transcription factory is non-random [37]. EKLF-dependent genes, including β-globin, co-localize at a subset of “specialized” factories that are enriched for EKLF. The underlying mechanisms are unclear. Do EKLF regulated genes seek out the proper factory, or are genes regulated by EKLF first bound by the factor and then co-migrate to a factory making it “specialized”. The precise sequence of events and interrelationships among factor binding, enhancer looping and intra-nuclear migration remain to be established.
Enhancers and long, non-coding RNAs
Genome profiling revealed that it is not unusual for RNA pol II to localize at enhancers [10]. It has also been known for many years that RNA pol II localizes at LCRs and that sense and antisense transcripts arise from these regions, although the function of such transcripts is unknown [38–41]. Using the pol II hallmark and p300 and H3K4me1 localization, Kim et al used ChIP-seq to identify thousands of neuronal activity-regulated putative enhancers genome wide [42]. A subset of the enhancers was transcribed bi-directionally by pol II into enhancer RNAs (eRNAs) which were not poly-adenylated and were generally short (<2 kb). The eRNA levels correlated with mRNA synthesis at nearby genes, suggesting that eRNA transcription might be necessary for activation of the genes. Interestingly, eRNA transcription, although not pol II occupancy, required the presence of an intact target promoter, at least in the case of the Arc gene [42].
Orom et al took a different approach and looked directly at the function of long non-coding RNAs (ncRNAs) [43]. They began with the GENCODE annotation of the human genome and filtered out transcripts over-lapping protein coding genes or belonging to known classes of non-coding RNAs. The resulting collection of ncRNA loci displayed CBP/p300 and RNA pol II occupancy but the ncRNAs were polyadenylated and less than 1% of them were bi-directional, in contrast to eRNAs described by Kim et al. Knock down of a subset of the ncRNAs (termed ncRNA-a, for activating) resulted in decreased transcription of neighboring protein coding genes and, in one case, a gene 150 kb distant. The ncRNA-a itself was required for the enhancer effect and not just transcription of the ncRNA gene. A direct role for a broader class of large non-coding RNAs (lincRNAs) in gene regulation was suggested by isolation of a subset as part of chromatin regulatory protein complexes in ES cells [44]. However, a comprehensive survey of lincRNAs in ES cells revealed that 15% of them overlapped enhancers but only 1% did so in neuronal cells [45]. At this point, the enhancer function of eRNAa and nc-RNA-a requires considerable further study and validation.
Interestingly, for nc-RNA-a there were often genes intervening between the putative enhancer and the activated target that were unaffected [43]. Viewed from an enhancer-centric perspective, the strong suggestion is that these enhancers loop to their target genes to activate their transcription, skipping over intervening genes, although the study did not include 3C experiments. Possibly, eRNAs and ncRNAs-a have a structural role in establishing or stabilizing enhancer-promoter loops although this remains unclear (Figure 2).
Enhancer and insulator functions converge
CTCF insulators protect enhancer-promoter interactions in vertebrates and insects. The β-globin LCR and genes are encompassed within a CTCF-mediated loop [46]. While reduction of CTCF in precursor cells not yet transcribing the globin genes does not appear to affect the locus, reduction of CTCF in cells actively transcribing γ-globin results in decreased transcription and incursion of repressive histone modifications, consistent with insulator function for these CTCF sites [46;47]. Likewise, disruption of interaction among surrounding insulators by CTCF depletion negatively impacts interaction of an enhancer with the APO gene promoters [48]. These examples illustrate positive indirect effects of CTCF insulator loops on enhancer-mediated gene expression (Figure 3A).
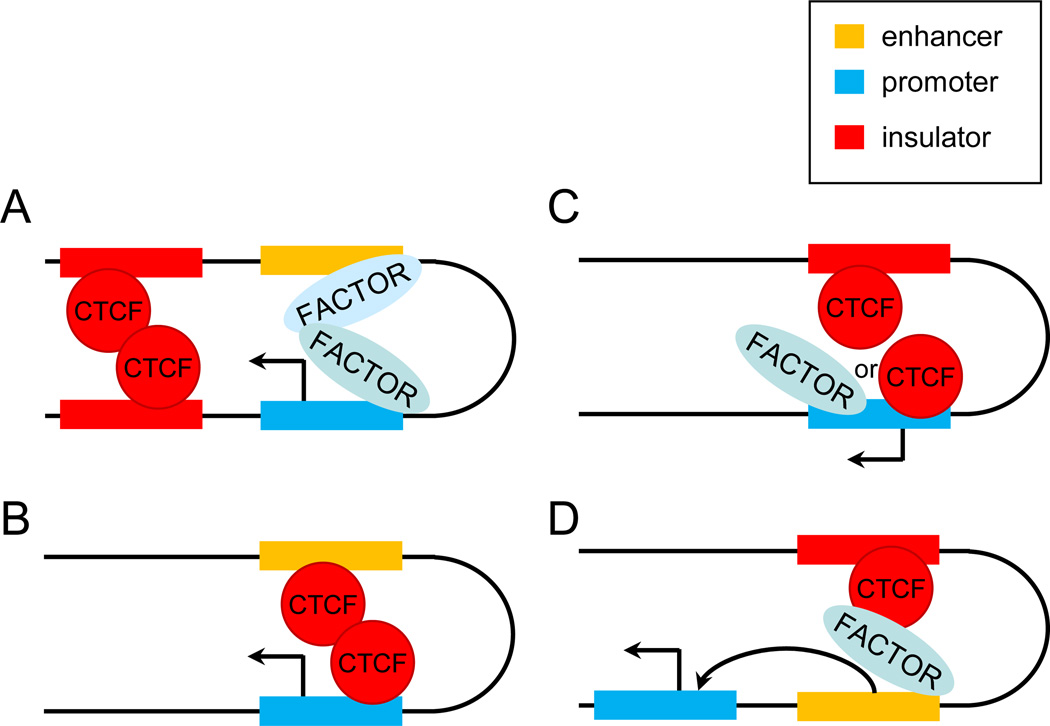
A. CTCF mediates interaction between two insulators which positively influences enhancer-promoter interaction. This model reflects the arrangement in the β-globin and APO loci [4;48]. B. CTCF interacts with an enhancer and target promoter and participates directly in long range interaction between them that leads to transcription activation. Examples of this arrangement have been recently been described [49]. C. CTCF interacts with an insulator and provides interaction with a promoter which in turn activates transcription. The INFG and MHC class II loci provide examples of this mechanism [51;53]. D. Insulator bound CTCF provides interaction with an enhancer which in turn interacts with a target promoter. This mechanism is utilized in the IgH locus [54;55]. Variants A, C and D can be incorporated into the concept of the active chromatin hub which provides an environment conducive to transcription activation. Designations are as in Figure 1.
Genome wide role of insulator loops
Genome profiling by Hi-C revealed that chromatin loops are central to the organization of active and silent chromatin into separate functional domains [12]. The emerging picture is that insulators are key contributors to this organization. Using ChIA-PET, new data show that CTCF mediates interaction between thousands of loci and organizes the genome into different functional compartments [49]. Handoko et al observed that many of the CTCF-mediated long range interacting sites coincided with an enhancer and promoter. Depletion of CTCF using RNAi reduced looping between the elements at select loci and reduced transcription of the gene involved. The suggestion is that CTCF facilitates enhancer-promoter interaction directly (Figure 3B). Thus, in addition to the classical view of domain separation by insulators to topologically isolate an enhancer and a proper gene target, we can envision other arrangements more directly involving insulators in this communication. Of note, insulator interaction with an enhancer and promoter can also be associated with a negative influence on transcription activation by the enhancer [50].
Direct interaction of enhancers and genes with insulators
Recent work documents CTCF occupied sites at insulators, within genes and in enhancers that participate in looping interactions and play a role in transcription activation. For example, the INFG gene has a CTCF site in the first intron that contacts two distant CTCF sites in the locus to form loops that are required for activation (Figure 3C) [51]. The INFG CTCF site interactions are modulated by recruitment of cohesin. In the MHC-II locus, a CTCF bound insulator site interacts with HLA-DRB1 and HLA-DQA1 promoters to form a chromatin loop [52]. These long range interactions depend on promoter-bound transcription factors CIITA and RFX and on CTCF whose reduction compromises gene transcription (Figure 3C). In addition, other insulator sites in the locus form long range associations among themselves that are dependent on CTCF but not on CIITA and RFX. Cohesin is also important for contact between the CTCF insulator site and MHC-II gene promoters [53].
In addition to promoters, enhancers can directly interact with insulators (Figure 3D). The Eμ recombination enhancer in the IgH locus of pro-B cells interacts with upstream and downstream CTCF insulators forming loops that are thought to contract the locus and facilitate recombination [54;55]. These interactions are CTCF and cohesin dependent and necessary for IgH locus functioning. At least one of the sites downstream from Eµ has enhancer blocking activity. Interestingly, one of the CTCF insulators downstream of Eμ (HS4) was found in the work of Ren et al (see above) to interact with the VDJ promoter in an OCA-B and TFII-I dependent fashion adding complexity to the overall locus structure. Very recent work using 4C details two sets of loops at the IgH locus; those dependent on CTCF but not on Eµ and those dependent on Eµ along with YY1 [33]. Several of the YY1 sites involved in Eµ looping bind CTCF and thus represent heterotypic interactions of the two factors [33;55].
In another example, T cell receptor rearrangement in the Tcra locus in mouse thymocytes involves CTCF and cohesin occupancy and long range interactions among the TEA gene, the Eα enhancer and the Tcra LCR. Deletion of the cohesin subunit Rad21 disrupted the long range interactions, accompanied by down-regulation of TEA, H3K4me3 depletion and aberrant TCRα rearrangement [56]. A regulatory element that engages in a similar mechanism is revealed in studies of the general transcription factor TAF3 [57]. Some distant TAF3 binding sites were found to colocalize with CTCF and cohesin and CTCF tethers these sites to TAF3 dependent promoters. However, the distant TAF3 sites were not otherwise tested for enhancer activity. The data discussed above collectively indicate that enhancer/insulator interplay is more complex that it was thought to be. In some cases, insulator protein CTCF and/or cohesin can facilitate enhancer-promoter interaction directly by interacting with these elements to bring them together.
Influence of cohesion on enhancer-promoter interactions
Cohesin is recruited to CTCF sites genome wide but a subset of sites is unique to cohesin [58;59]. New data has shed light on the function of these sites. ER-α binding sites colocalize with cohesin sites that lack CTCF [60]. It was proposed that ER-α together with cohesin provides long range interactions, although this was not tested directly by 3C. In another example, cohesin and the Mediator complex are implicated in enhancer-promoter interaction [61]. Kagey et al found that Mediator subunits colocalize with cohesin at enhancers and promoters and are necessary for loop formation between them. The data illustrate the potential role of cohesin as a physical tether mediating enhancer-gene proximity in combination with mediator, which may coordinate signals between enhancers and the general transcription machinery. What recruits cohesin to these non-CTCF sites and whether they have insulator function are unknown.
Conclusions
The very recent data summarized here strikingly illustrate that enhancer/insulator interplay in regulation of gene transcription is more complex than was previously appreciated. In some cases, enhancer-promoter interaction appears to be directly facilitated by CTCF/cohesin occupancy. In other cases, the promoter or the enhancer is occupied by CTCF/cohesin and additional factors cooperate to support interaction between them. It will be important to determine the exact geometry of the CTCF/cohesin binding sites vis-à-vis transcription factors when they co-occupy enhancers or promoters. In view of its interaction with potential enhancers [49], RNA pol II transcription factories [62] and the general transcription machinery [57], does CTCF have a direct role in transcription activation? Are all CTCF and cohesin sites insulator sites in the classic view? The genome wide architectural role of CTCF loops may provide a clue to how enhancer-promoter ensembles migrate within the nucleus. Almost nothing is known about this process although it seems to involve actin-myosin motors [63;64]. The novel convergence of enhancer-promoter pairs and CTCF insulators impacts important unresolved questions such as how enhancers and promoters establish a connection, how they move within the nucleus and how transcriptional output is increased by these events.
Acknowledgements
We would like to thank Dr. Gerd Blobel for helpful comments on the manuscript and members of our laboratory for their suggestions. Work in our laboratory is supported by the Intramural Program of NIDDK, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.gde.2011.11.001
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3342482?pdf=render
Citations & impact
Impact metrics
Article citations
Genetic characterization of a locus responsible for low pungency using EMS-induced mutants in Capsicum annuum L.
Theor Appl Genet, 137(5):101, 12 Apr 2024
Cited by: 1 article | PMID: 38607449 | PMCID: PMC11014816
Intrinsic protein disorder is insufficient to drive subnuclear clustering in embryonic transcription factors.
Elife, 12:RP88221, 26 Jan 2024
Cited by: 5 articles | PMID: 38275292 | PMCID: PMC10945700
Unveiling Alterations of Epigenetic Modifications and Chromatin Architecture Leading to Lipid Metabolic Reprogramming during the Evolutionary Trastuzumab Adaptation of HER2-Positive Breast Cancer.
Adv Sci (Weinh), 11(18):e2309424, 09 Mar 2024
Cited by: 1 article | PMID: 38460162 | PMCID: PMC11095153
DeepCORE: An interpretable multi-view deep neural network model to detect co-operative regulatory elements.
Comput Struct Biotechnol J, 23:679-687, 29 Dec 2023
Cited by: 1 article | PMID: 38292477 | PMCID: PMC10825326
Exploring the Functional Basis of Epigenetic Aging in Relation to Body Fat Phenotypes in the Norfolk Island Cohort.
Curr Issues Mol Biol, 45(10):7862-7877, 27 Sep 2023
Cited by: 0 articles | PMID: 37886940 | PMCID: PMC10605526
Go to all (133) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Boosting transcription by transcription: enhancer-associated transcripts.
Chromosome Res, 21(6-7):713-724, 01 Dec 2013
Cited by: 11 articles | PMID: 24178450 | PMCID: PMC3867273
Review Free full text in Europe PMC
Enhancer blocking activity of the insulator at H19-ICR is independent of chromatin barrier establishment.
Mol Cell Biol, 28(11):3767-3775, 31 Mar 2008
Cited by: 10 articles | PMID: 18378700 | PMCID: PMC2423287
Distant activation of transcription: mechanisms of enhancer action.
Mol Cell Biol, 32(24):4892-4897, 08 Oct 2012
Cited by: 69 articles | PMID: 23045397 | PMCID: PMC3510544
Review Free full text in Europe PMC
Transcription factors mediate long-range enhancer-promoter interactions.
Proc Natl Acad Sci U S A, 106(48):20222-20227, 18 Nov 2009
Cited by: 115 articles | PMID: 19923429 | PMCID: PMC2779200
Funding
Funders who supported this work.
Intramural NIH HHS (4)
Grant ID: ZIA DK075033
Grant ID: ZIA DK075033-02
Grant ID: ZIA DK015508
Grant ID: ZIA DK015508-23





