Abstract
Free full text

Boc and Gas1 each form distinct Shh receptor complexes with Ptch1 and are required for Shh-mediated cell proliferation
Associated Data
SUMMARY
Hedgehog (Hh) proteins regulate important developmental processes including cell proliferation and differentiation. While Patched acts as the main Hh receptor, in Drosophila, Hh signaling absolutely requires the additional Hh-binding proteins Ihog and Boi. Here we show that, unexpectedly, cerebellar granule neuron progenitors (CGNPs) lacking Boc and Cdon, the vertebrate orthologs of Ihog and Boi, still proliferate in response to Hh. This is because in their absence, Gas1, a Hh-binding protein not present in Drosophila, mediates Hh signaling. Consistently, only CGNPs lacking all three molecules Boc, Cdon, and Gas1 have a complete loss of Hh-dependent proliferation. In a complementary manner, we find that a mutated Hh ligand which binds Patched1 but neither Boc, Cdon, nor Gas1 cannot activate Hh signaling. Together, this demonstrates an absolute requirement for Boc, Cdon, and Gas1 in Hh signaling and reveals a distinct requirement for ligand-binding components that distinguishes the vertebrate and invertebrate Hh receptor systems.
INTRODUCTION
Hh proteins are key molecules for diverse tissue patterning processes in both invertebrates and vertebrates. For example, in Drosophila, Hh is crucial for the development of a segmented body plan and the patterning of imaginal tissues, whereas in vertebrates Sonic hedgehog (Shh) functions to pattern limb buds and promote cell fate specification, proliferation, and axon guidance in the central nervous system (Charron and Tessier-Lavigne, 2005; Dessaud et al., 2008; Ingham and Placzek, 2006; Jiang and Hui, 2008). Shh is synthesized as a 45 kDa pro-protein and post-translational modifications generate a biologically active 19 kDa N-terminal fragment. Shh initiates signaling by binding the 12-pass transmembrane protein Patched1 (Ptch1). Upon Shh binding, the inhibition exerted by Ptch1 on the 7-pass transmembrane protein Smoothened (Smo) is relieved, eliciting a signaling cascade which ultimately leads to Gli-mediated transcription.
In addition to Ptch1, several membrane-associated proteins are thought to function as accessory receptors that promote Shh signaling. The related molecules Cdon (cell-adhesion-molecule-related/downregulated by oncogenes) and Boc (biregional Cdon-binding protein) positively regulate Shh signaling, promoting Shh-dependent cell fate specification and axon guidance (Okada et al., 2006; Tenzen et al., 2006; Yao et al., 2006; Zhang et al., 2006). They are single-pass transmembrane proteins of the Immunoglobulin (Ig) superfamily and contain an extracellular region consisting of four (Boc) or five (Cdon) Ig repeats and three Fibronectin type 3 repeats (FNIII). In Drosophila, Ihog (Interference hedgehog) and Boi (Brother of Ihog), the orthologs of Boc and Cdon, are redundant with one another and are absolutely required for Hh-dependent patterning (Camp et al., 2010; Zheng et al., 2010). However, whether Boc and Cdon are absolutely essential for Shh signaling in vertebrates is unknown.
Furthermore, additional membrane-associated proteins have been shown to positively modulate Shh signaling in vertebrates. Growth Arrest Specific 1 (Gas1), a GPI-linked protein bearing no structural resemblance to Boc and Cdon and with no orthologues in Drosophila, binds Shh and regulates ventral specification of neural progenitors during neural tube development by promoting Shh signaling (Allen et al., 2007; Martinelli and Fan, 2007a, b). Interestingly, analysis of Gas1 mutant mice shows that it is a positive regulator of cerebellar size and CGNP proliferation (Liu et al., 2001), two processes that are normally driven by Shh during early postnatal development. However, whether Gas1 acts in a Shh-dependent manner in CGNP proliferation has yet to be determined.
Thus, Boc, Cdon and Gas1 have each been proposed to function as positive modulators of Shh signaling (Allen et al., 2007; Martinelli and Fan, 2007a; Okada et al., 2006; Seppala et al., 2007; Tenzen et al., 2006). We hypothesised that they are obligate Shh receptors with Ptch1, and not simply modulators of the signaling cascade. If this model were correct, simultaneous disruption of all three molecules would completely abrogate Shh signaling. We tested this by investigating the proliferation of CGNPs in response to Shh in the developing cerebellum. We found that CGNPs express Boc and Gas1, but not Cdon. Interestingly, the cerebellum is smaller in Boc−/− mice, and Boc−/− CGNPs have lower proliferation than wild-type CGNPs in response to Shh. Similarly, Gas1−/− CGNPs are also less responsive to Shh, while Gas1−/−;Boc−/− CGNPs are completely unable to proliferate in response to Shh. We further demonstrated that Boc and Gas1 interact with Ptch1 and form distinct receptor complexes. Finally, we generated a Shh mutant protein that binds Ptch1 but not Boc, Cdon nor Gas1 and found that this molecule could not elicit Shh-dependent signaling and CGNP proliferation. Together, our data indicates that Boc, Cdon and Gas1 are necessary components of the Shh receptor complex and are essential for Shh signal transduction in vertebrates. While Ihog and Boi are necessary to mediate Hh signaling in Drosophila (Camp et al., 2010; Zheng et al., 2010), we show that Gas1 is an additional component of the Hh receptor system in vertebrates that can compensate for the absence of Boc and Cdon.
RESULTS
Boc, but not Cdon, is expressed in proliferating CGNPs of the cerebellum
To investigate the receptor requirements for the proliferative effect of Shh in CGNPs, we first analyzed Boc and Cdon expression in the developing cerebellum. CGNPs arise from the rhombic lip (RL) between embryonic day (E) 13.5–14.5 and migrate anteriorly over the cerebellar anlage, forming the highly proliferative external germinal layer (EGL) (Roussel and Hatten, 2011). Starting at E17.5 and continuing during early postnatal development, Purkinje cells (PCs) lining the EGL stimulate CGNP proliferation by secreting Shh (Dahmane and Ruiz i Altaba, 1999; Kenney and Rowitch, 2000; Wallace, 1999; Wechsler-Reya and Scott, 1999). Following a proliferative burst, CGNPs stop dividing, differentiate into granule neurons, migrate inwards past the PC layer and populate the internal granular layer (IGL).
We first examined Boc and Cdon expression in the cerebellar anlage of E14.5 mouse embryos. Immunostainings of sagittal sections showed that while Boc was expressed in the presumptive EGL, RL and the ventricular zone of the roof of the 4th ventricle, Cdon expression was restricted to the RL (Fig. 1A). At E18.5, a stage at which CGNPs proliferate in response to Shh, we detected Boc expression in the EGL and, albeit at lower level, in the PC layer of the developing cerebellum. In contrast, Cdon expression was limited to the tip of the RL.
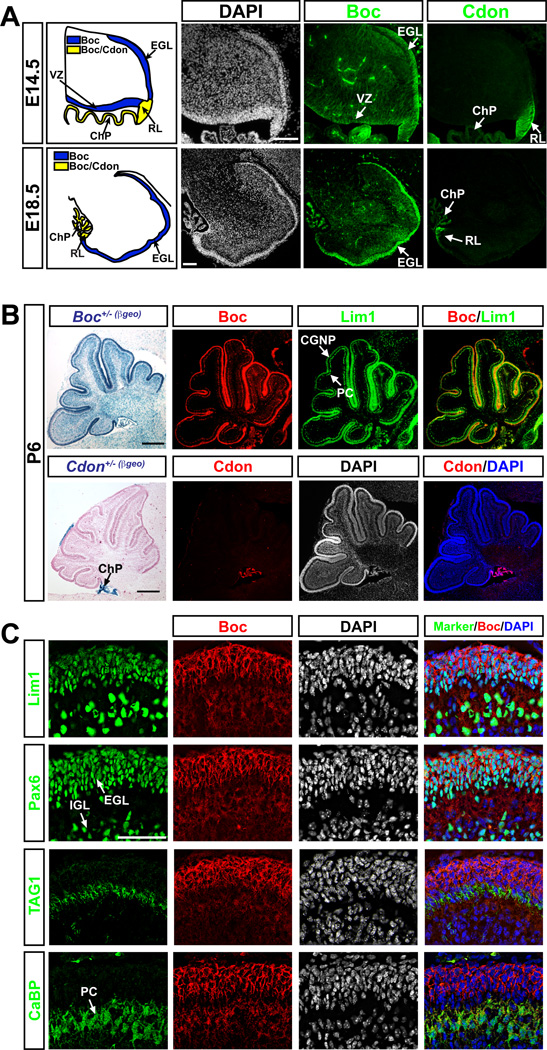
(A) Diagram of the developing cerebellum at E14.5 and E18.5. Immunostaining of Boc and Cdon in sagittal sections of the developing mouse cerebellum shows that Boc is expressed in the EGL, ChP and RL at E14.5 and expression is maintained in the EGL and ChP at E18.5. Cdon is expressed in the RL and ChP at E14.5 and E18.5. (B) At P6, Xgal staining (left, counterstained with nuclear fast red) and immunofluorescence on sagittal cerebellum sections from Boc+/- and Cdon+/- βgeo (β-galactosidase-neomycin) mice reveal Boc and Cdon expression. Boc is expressed in Lim1+ cells (B,C) and Cdon in the ChP (B). (C) P3 WT mouse cerebellum sections co-immunolabeled with Boc (red) and various cerebellar cell markers (green) showing high Boc expression in proliferating CGNPs (Lim1+, Pax6+) and lower expression in PC (CaBP+, Lim1+) and differentiated granule cells (Pax6+ in the IGL). Scale bars: (A) 100 µm (B) 250 µm, (C) 50 µm. EGL, external germinal layer; IGL, internal granular layer; ChP, choroid plexus; RL, rhombic lip; PC, Purkinje cells; CGNP, cerebellar granule neurons; CaBP, Calbindin; VZ, ventricular zone.
Analysis of post-natal day (P) 6 Boc+/- and Cdon+/- gene-targeted mice encoding a β-galactosidase (β-Gal)-neomycin reporter gene fusion (β-geo) (Okada et al., 2006) revealed strong β-Gal activity in Boc+/- cerebellum, but was limited to the choroid plexus of Cdon+/- cerebellum. Immunostainings confirmed this expression pattern and revealed that Boc localized to cells expressing Lim1, a marker for CGNPs and PCs (Fig. 1B,C). Interestingly, while highest levels of Boc were detected in the outer proliferative region of the EGL (Lim1+, Pax6+ and TAG1− cells), lower levels were observed in differentiated migratory granule cells (TAG1+ cells) and in PCs (Calbindin+ cells). These results show that Boc, but not Cdon, is highly expressed in proliferating CGNPs of the cerebellum.
Boc is important, but not absolutely required, for Shh-mediated CGNP proliferation
To investigate the role of Boc in cerebellum development, we examined the gross morphology of Boc−/− cerebella. While Boc−/− mice are viable and cannot be distinguished from their littermates, their cerebellum is smaller than Boc+/- or WT animals (Fig. 2A and data not shown). Boc−/− cerebella were 14.3±0.05% (p<0.001) lighter than that of Boc+/- cerebella (Fig. 2B). When the mass of the cerebellum was normalized to the body weight (p<0.001), the relative cerebellar mass was still reduced, indicating that this difference is not due to an overall decrease in total body weight (Fig. 2B). The cerebellum and IGL surface areas measured from sagittal sections of Boc−/− adult mice were also reduced when compared to Boc+/- animals (Fig. 2C,D; p<0.001 and 0.05, respectively). Although the IGL surface area is diminished in adult mice, migration of granule neurons and cerebellum foliation did not appear to be affected in Boc−/− mice.
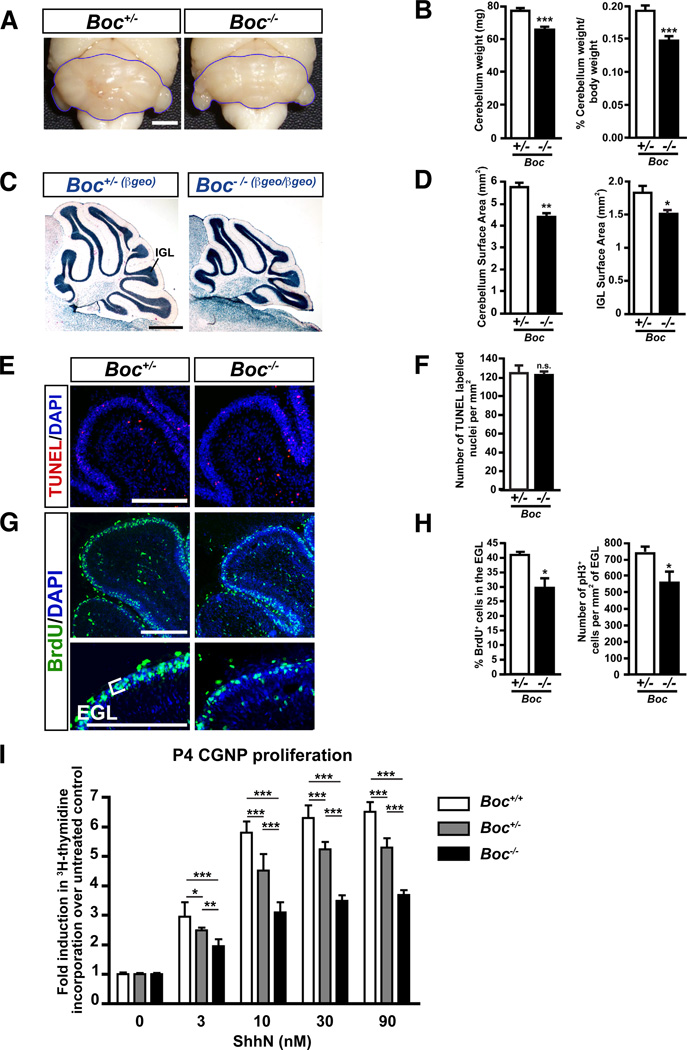
(A) Comparison of whole cerebella and (C) sagittal sections from adult Boc+/- and Boc−/− mice. (B) Cerebellum weight and normalized cerebellum weight relative to body weight. n=11 cerebella/group. (D) Cerebellum surface area (left) and IGL surface area (right) measured from three medio-lateral matching levels. n=4 cerebella/group. (E) Apoptotic CGNPs from P3 Boc+/- and Boc−/− mice visualized by TUNEL staining. (F) Number of apoptotic cells/mm2 in the EGL from TUNEL-stained sections from 5 Boc+/- and 4 Boc−/− mice. (G) Proliferating CGNPs in the EGL from P3 Boc+/- and Boc−/− mice visualized by anti-BrdU staining. (H) (left) Percentage of BrdU+ cells in the EGL. n=4 animals/group. (right) Number of pH3+ cells/mm2 in the EGL of 5 Boc+/- and 4 Boc−/− P3 mice. (I) CGNPs purified from Boc+/+, Boc+/- and Boc−/− mice cerebella at P4 were cultured in the presence of 0–90 nM ShhN. Proliferating cells were measured by 3H-thymidine incorporation. Data is represented as fold induction in CGNP proliferation compared to untreated cells. Scale bars: (A) 2mm, (C) 1mm, (E,G) 250 µm. IGL, internal granular layer; EGL, external germinal layer. p valules measured from Student’s t-test (B,D,F,H) and two-way ANOVA (I).
The decrease in cerebellum size in the absence of Boc could be due, at least in part, to reduced cell proliferation and/or enhanced cell death. TUNEL staining showed no significant difference in the number of apoptotic cells between Boc−/− and Boc+/- cerebella (Fig. 2E,F). In contrast, measurement of BrdU incorporation in the EGL of Boc−/− and Boc+/- mice showed that 40±1% of Boc+/- CGNPs were actively dividing, compared to only 30±3% of Boc−/− CGNPs (p<0.05) (Fig. 2G,H). Phospho-histone H3 (pH3) staining also showed a significant reduction in the number of mitotic pH3-labeled cells per mm2 of EGL in Boc−/− mice compared to Boc+/- mice (p<0.05) (Fig. 2H). Together, these in vivo data indicate that Boc plays a role in CGNP proliferation.
Since Boc modulates Shh signaling (Okada et al., 2006; Tenzen et al., 2006; Zhang et al., 2006), we next tested whether Boc mediates Shh-induced CGNP proliferation. We cultured CGNPs purified from Boc−/−, Boc+/- and Boc+/+ mice in the presence of varying concentrations of recombinant Shh (ShhN) (Fig. 2I). While Shh treatment induced the proliferation of WT CGNPs over 6 fold compared to unstimulated CGNPs, Shh stimulation increased Boc−/− CGNP proliferation only about 3 fold. Significant differences in the proliferation of Boc+/+, Boc+/- and Boc−/− CGNPs was observed at all concentrations of ShhN used (Fig. 2I), indicating that Boc promotes proliferation of CGNPs in a gene copy-number dependent manner. Together with our in vivo data, these results indicate that Boc−/− mice have a smaller cerebellum due to a decrease in Shh-dependent CGNP proliferation and that Boc acts cell-autonomously in CGNPs to regulate their proliferation.
Gas1 is important, but not absolutely required, for Shh-mediated CGNP proliferation
Whilst inactivation of Boc in CGNPs, which do not express Cdon, lead to a partial decrease in their proliferation, it did not abolish their response to Shh. Moreover, CGNP proliferation is not further decreased when Cdon is inactivated in Boc−/− mice (Fig. S1). These results are not consistent with a model where Boc and Cdon act like their Drosophila orthologues Ihog and Boi and are absolutely required for Hh signaling in vertebrates (Camp et al., 2010; Zheng et al., 2010). This raises the possibility that, unlike Drosophila, additional or different Shh binding molecules (other than Ptch1, Boc and Cdon) are required for vertebrate cells to respond to Shh.
Given that Gas1 binds Shh and modulates Shh signaling (Allen et al., 2007; Martinelli and Fan, 2007a, b; Seppala et al., 2007), we hypothesized that Gas1 may be this additional receptor. We first characterized the expression pattern of Gas1 in the developing cerebellum. Immunofluorescence stainings showed that Gas1 is restricted to the presumptive EGL of the cerebellar primordium at E14.5 and continues to be expressed in the EGL at E18.5 (Fig. 3A). At P6, like Boc, Gas1 localizes to Lim1+ cells in the EGL (Fig. 3B). Gas1 staining is most intense in the outer proliferative layer of the EGL (Lim1+, Pax6+, TAG1− cells) and was not detected in TAG1+ migratory granule neurons and in Calbindin+ PCs (Fig. 3C).
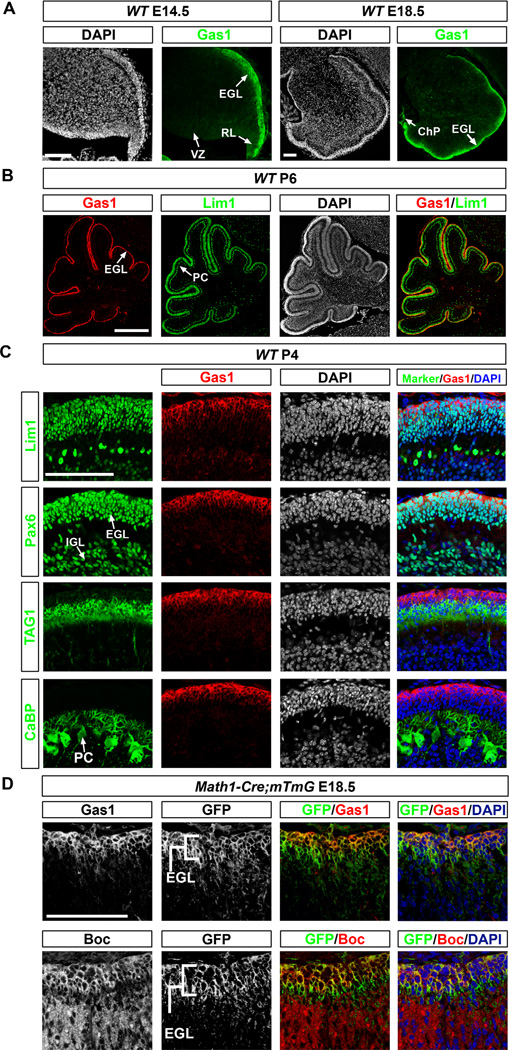
(A) Immunostaining of sagittal sections showing Gas1 expression in the RL and EGL of the developing cerebellum at E14.5 and E18.5. (B,C) P4-6 WT cerebellum sections immunostained for Gas1 (red) and various cerebellar cell markers (green). Gas1 is highly expressed by proliferating CGNPs (Lim1+ cells; Pax6+ in EGL). (D) Immunostaining of E18.5 cerebellum sections from Math1-Cre;mTmG mice show that Boc and Gas1 are expressed in Math1+,GFP+ CGNPs in the EGL. EGL, external germinal layer; IGL, internal granular layer; PC, Purkinje cells; RL, rhombic lip; VZ, ventricular zone; ChP, choroid plexus. Scale bars: (A, C and D) 100 µm, (B) 500 µm.
To determine whether Boc and Gas1 are co-expressed in CGNPs, we performed immunostainings on consecutive sections of cerebellum from Math1-Cre; mTmG E18.5 mice, where the CGNPs express GFP following Cre-mediated recombination. We used this strategy instead of double immunostainings as both anti-Boc and anti-Gas1 antibodies are produced in the same species. We found that both Boc and Gas1 co-localize with GFP+ cells, indicating that Gas1 and Boc are co-expressed in the same CGNPs (Fig. 3D).
Although the gross morphology of Gas1−/− cerebella appears normal, they are smaller in size compared to control cerebellum and have decreased proliferation in the outer EGL (Liu et al., 2001). While this phenotype is reminiscent of that of Boc−/− cerebella, no direct link has been made between the phenotype and the ability of Gas1−/− CGNPs to respond to Shh. To directly test this, we performed proliferation assays on purified CGNPs from Gas1−/− mice and control littermates. Our results show that Gas1 is essential for normal CGNP proliferation in response to Shh (Fig. 4C). Interestingly, the mutation of Gas1, similarly to the mutation of Boc, is not sufficient to abrogate the response of CGNPs to Shh.
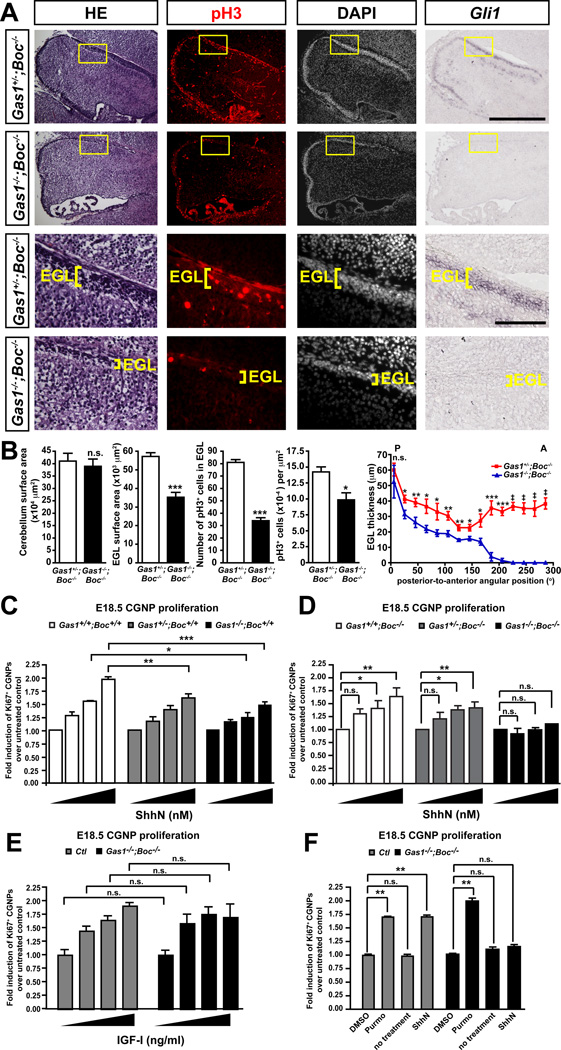
(A) Haematoxylin-eosin staining on sagittal sections of E18.5 cerebellum revealing a thinner EGL in Gas1−/−;Boc−/− cerebella than control. Anti-pH3 immunostaining of sagittal sections of Gas1+/-;Boc−/− and Gas1−/−;Boc−/− cerebella counterstained with DAPI. RNA in situ hybridization showing the loss of expression of the Shh transcriptional target Gli1 in Gas1−/−;Boc−/− cerebella at E18.5. (B) Quantification of: cerebellum surface area, EGL surface area, pH3+ cells in EGL, pH3+ cells per µm2, and EGL thickness along the postero-anterior axis, n=4 animals/group. (C) CGNPs purified from Gas1+/+;Boc+/+ (n=3), Gas1+/-;Boc+/+ (n=4) and Gas1−/−;Boc+/+ (n=4) mice cerebella at E18.5 were cultured with 0, 3, 10, 30 nM ShhN. Proliferating cells were visualized by immunostaining with an anti-Ki67 antibody. Data is represented as fold CGNP proliferation over untreated control (C, D and E) or DMSO control (F). (D) Similar to (C) but CGNPs were purified from Gas1+/+;Boc−/− (n=3), Gas1+/-;Boc−/− (n=3) and Gas1−/−;Boc−/− (n=3) mice cerebella at E18.5. (E) CGNPs were purified from control (Ctl; Gas1+/+;Boc−/− and Gas1+/-;Boc−/−) (n=3) and Gas1−/−;Boc−/− (n=3) mice cerebella at E18.5 and treated with 0, 20, 50 or 100 ng/ml of IGF-I. (F) Similar to (E) but CGNPs were treated with either DMSO, 0.150 µM purmorphamine, or 30 nM ShhN. p values measured from Student’s t-test (B), two-way ANOVA (C, E), and ANOVA (D, F). EGL, external germinal layer. Scale bars: top two rows=500 µm, bottom two rows=100 µm.
Shh-dependent proliferation is completely lost in Gas1−/−;Boc−/− CGNPs
To determine whether Boc and Gas1 might have partially redundant functions in Shh-dependent CGNP proliferation, we examined the cerebellum of E18.5 Gas1−/−;Boc−/− embryos, since these animals die at birth. Hematoxylin-eosin staining of Gas1−/−;Boc−/− cerebella revealed a significant loss of the EGL compared to controls (Fig. 4A). Although Gas1+/-;Boc−/− and Gas1−/−;Boc−/− cerebella showed no significant difference in the cross-sectional area of the whole cerebellum, the overall area of Gas1−/−;Boc−/− EGL was reduced by about 30% compared to controls (p<0.001) (Fig. 4B). Quantitation of the EGL along the postero-anterior axis showed that the difference in EGL thickness is greatest towards the anterior pole of the cerebellum (Fig. 4B and S2D). Marker analysis showed that Lim1 and Pax6 were properly expressed in the EGL of Gas1−/−;Boc−/− embryos compared to controls (Fig. S2A,B), thus, CGNPs are specified and localize normally. Furthermore, Cdon expression was not changed in the absence of Gas1 and Boc (Fig. S2C). However, the proliferation of Gas1−/−;Boc−/− CGNPs was severely decreased compared to Gas1+/-;Boc−/− CGNPs (p<0.001) (Fig. 4B). Moreover, the number of pH3+ cells per µm2 of EGL surface area was lower in Gas1−/−;Boc−/− than Gas1+/-;Boc−/− animals (p<0.05) (Fig. 4B), demonstrating that the decrease in pH3+ cells in the EGL is not simply due to a total decrease in EGL area. These results indicate that Gas1 and Boc account for a large part of CGNP proliferation at this stage in vivo.
In addition to Shh, Insulin Growth Factor (IGF) and Notch signaling also promote CGNP proliferation (Corcoran et al., 2008; Solecki et al., 2001). Residual CGNP proliferation is observed in other mutant cerebella that lack Shh signaling (Corrales et al., 2004), thus, the proliferation observed in the EGL of Gas1−/−;Boc−/− cerebellum is probably independent of Shh signaling. To test whether Gas1−/−;Boc−/− cells have completely lost Shh responsiveness, we cultured CGNPs purified from E18.5 Gas1+/+;Boc−/− and Gas1−/−;Boc−/− cerebella with various ShhN concentrations. We found that while Gas1+/+;Boc−/− CGNPs proliferate in vitro in response to Shh, Gas1−/−;Boc−/− CGNPs show no enhanced proliferation in response to Shh (Fig. 4D). Importantly, the proliferative response of Gas1−/−;Boc−/− CGNPs to IGF-I, another factor able to stimulate CGNP proliferation, remained similar to that of control cells (Fig. 4E). Furthermore, treatment with purmorphamine, a Smo agonist, induced the proliferation of Gas1−/−;Boc−/− CGNPs (p<0.01) (Fig. 4F), indicating that Boc and Gas1 function upstream of Smo. Together, our data indicates that the presence of either Gas1 or Boc is absolutely required for Shh to promote CGNP proliferation. Given that Shh signaling in the cerebellum begins only at E17.5 and that Shh signaling plays an even more important role in CGNP proliferation after birth than at E18.5 (Corrales et al., 2004; Flora et al., 2009; Lewis et al., 2004), we anticipate that the EGL of Gas1−/−;Boc−/− mice would be much more severely reduced post-natally. However, because Gas1−/−;Boc−/− mice die at birth, conditional alleles will be required to directly test this.
To test whether the lack of a proliferative response of Gas1−/−;Boc−/− CGNPs to Shh in vitro is consistent with loss of Shh signaling in vivo, we examined the expression of Gli1, a Shh transcriptional target (Corrales et al., 2004), by RNA in situ hybridization. While control cerebella had intense Gli1 signal in the EGL, Gli1 expression was not detected in Gas1−/−;Boc−/− cerebella (Fig. 4A), confirming the inactivation of Shh signaling in Gas1−/−;Boc−/− cerebella.
Boc and Gas1 interact with Ptch1 and form distinct receptor complexes
We next investigated the molecular mechanism by which Boc and Gas1 act and, more specifically, whether they associate with Ptch1 to constitute the Shh receptor complex. We found that Boc and Gas1 can each co-immunoprecipitate with Ptch1, indicating that Boc and Gas1 can physically interact with Ptch1 (Fig. 5A). Importantly, these interactions are specific to Ptch1, as both Dispatched-1 (Disp1) and Smo, two multi-span transmembrane proteins also involved in Shh signaling, failed to interact with either Boc or Gas1 (Fig. S3). Furthermore, the addition of Shh did not modify the ability of Ptch1 to interact with Boc, suggesting that their interaction is constitutive (Fig. 5B).
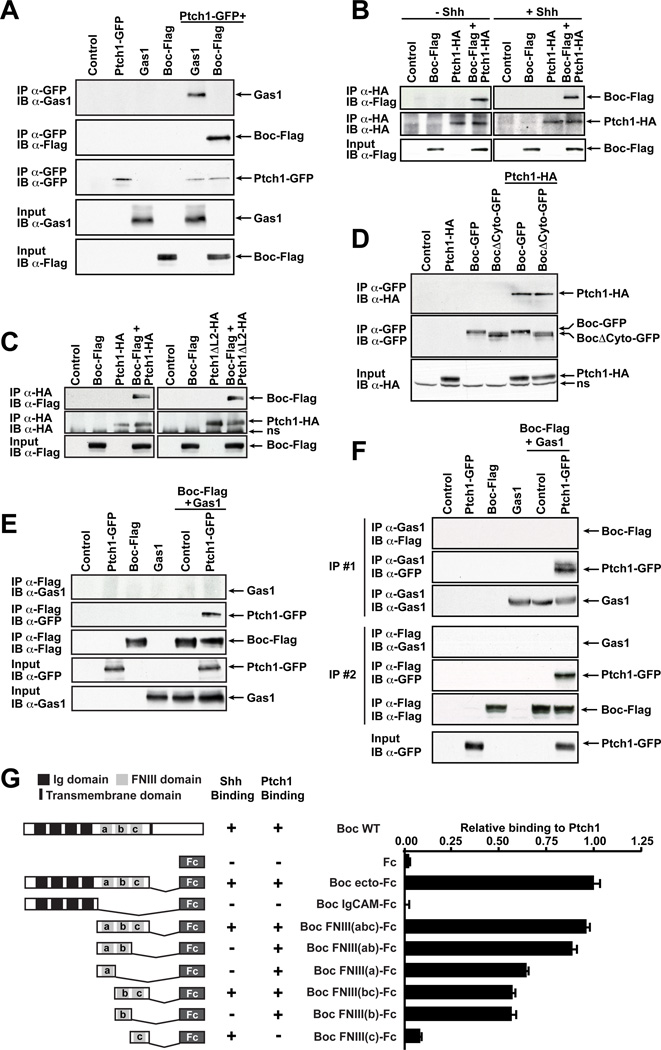
(A) Boc and Gas1 interact with Ptch1. COS7 cells were transfected with the indicated constructs and lysates were immunoprecipitated (IP) with an anti-GFP antibody and immunoblotted (IB) with anti-Gas1, anti-Flag or anti-GFP antibodies. (B) Boc interacts with Ptch1 in a constitutive manner. COS7 cells expressing Ptch1-HA and Boc-Flag were treated with ShhN and subjected to anti-HA IP and either anti-Flag or anti-HA IB. (C) The Ptch1 L2 region is not required for the Boc-Ptch1 interaction. Anti-HA IP was perfomed on COS7 lysates expressing Boc-Flag and Ptch1-HA or Ptch1ΔL2-followed by anti-Flag or anti-HA IB. (D) The Boc cytoplasmic tail is not required for the Boc-Ptch1 interaction. COS7 cells expressing Ptch1-HA and Boc-GFP or BocΔcyto-GFP were subjected to anti-GFP IP and either anti-HA or anti-GFP IB. (E, F) Ptch1 forms receptor complexes with either Boc or Gas1 but not both. Boc-Flag was co-transfected with Gas1 with or without Ptch1-GFP in COS7 cells. (E) Lysates were IP with anti-Flag antibodies, followed by anti-Gas1 or anti-GFP IB. (F) Lysates were first immunoprecipated (IP #1) with anti-Gas1 antibodies. Supernatants from IP#1 were subjected to a second immunoprecipitation (IP #2) with anti-Flag antibodies. Both anti-Gas1 (IP #1) and anti-Flag (IP #2) immunoprecipitates were IB with anti-Gas1, anti-Flag and anti-GFP antibodies. See Fig. S4 for a schematic of this experiment. (A–F) Protein expression inputs were verified by IB with the indicated antibodies. ns, non-specific. (G, left) Diagram of WT Boc, Boc-Fc and Boc-Fc mutant proteins. (G, right) The Boc-Ptch1 interaction is mediated by the Boc FNIII(ab) domains. COS7 cells expressing Ptch1-GFP were incubated with conditioned mediated containing Boc-Fc proteins. Bound proteins were labeled with HRP-conjugated anti-Fc antibody and peroxidase activity measured.
Mapping studies showed that the second large extracellular loop of Ptch1 (L2), which is necessary for binding to Shh (Marigo et al., 1996), was not required for the interaction with Boc. Ptch1ΔL2-HA, a Ptch1 construct where L2 is deleted, interacted with Boc to an extent similar to full length Ptch1-HA (Fig. 5C). This is consistent with the binding of Shh to Ptch1 not being necessary for Ptch1 to interact with Boc. We next mapped the domain(s) of Boc mediating its interaction with Ptch1. BocΔCyto-GFP, a mutant lacking the cytoplasmic domain of Boc, interacted with Ptch1 as strongly as full-length Boc-GFP (Fig. 5D), indicating that the cytoplasmic domain is not required for its association with Ptch1.
To further characterize the region of Boc that interacts with Ptch1, we performed binding assays with various derivatives of Boc-Fc fusion proteins encompassing the Boc extracellular domain and cells expressing Ptch1-GFP. Deletion analysis of the Boc extracellular domain revealed that removal of the FNIIIc domain (mutant Boc FNIII(ab)), shown to be required and sufficient for Shh binding (Okada et al., 2006), only marginally affected Ptch1 binding, while truncation of both the FNIIIa and FNIIIb domains (mutant FNIII(c)) abolished it almost entirely (Fig. 5G). Boc-Fc constructs containing either the FNIIIa or FNIIIb domains alone bound to Ptch1 at levels that were about 60% of that of Boc ecto-Fc. Together our data indicate that the Boc FNIIIa and FNIIIb domains are required and sufficient to mediate its interaction with Ptch1. In addition, the Boc FNIIIc domain, which is necessary for Shh binding, is not required for the Boc-Ptch1 interaction, further supporting a Shh-independent interaction between Boc and Ptch1.
We next tested whether Boc interacts with Gas1 and did not detect an interaction between Boc and Gas1 either in the absence or presence of Ptch1 (Fig. 5E, top panel, lanes 5–6), despite detecting a strong interaction between Boc and Ptch1 (Fig. 5E, middle panel, lane 6). These experiments suggest that Boc/Ptch1 complexes do not contain detectable amounts of Gas1 and that Boc, Ptch1 and Gas1 are unlikely to form a tripartite complex.
To further confirm these results, we performed the complementary experiment and looked for the presence of Boc in Gas1/Ptch1 complexes. Lysates of cells transfected with Ptch1-GFP, Boc-Flag and Gas1 were first immunoprecipitated with anti-Gas1 antibodies and, despite detecting a strong interaction between Gas1 and Ptch1 (Fig. 5F, IP#1 middle panel, lane 6), we did not detect an interaction between Boc and Gas1 in the absence nor presence of Ptch1 (Fig. 5F, top panel lanes 5–6). To confirm that Boc is indeed able to interact with Ptch1 in these lysates and test whether both Boc/Ptch1 and Gas1/Ptch1 complexes are present in the same cell lysates, we recovered the supernatants from the anti-Gas1 immunoprecipitation (IP#1) and subjected them to a second immunoprecipitation, this time with anti-Flag antibodies to immunnoprecipitate Boc (Fig. 5F; see Fig. S4 for a schematic). We found that the Ptch1-GFP remaining in the supernatant efficiently co-immunoprecipitated with Boc (Fig. 5F, IP#2 middle panels, lane 6). Together, our data indicates that while Boc and Gas1 can both interact with Ptch1, it is unlikely that Boc, Gas1 and Ptch1 form a tripartite complex. Moreover, these results suggest that the Boc-Ptch1 and the Gas1-Ptch1 complexes are distinct molecular entities.
Binding of Shh to Ptch1 is not sufficient to activate Shh signaling
Our results indicate that Boc and Gas1 are required for Shh-mediated CGNP proliferation and that they form independent complexes with Ptch1. While Boc and Gas1 are essential components of these receptor complexes, they could function as partners of Ptch1, but not necessarily as receptors that bind to Shh. To determine whether the binding of Shh to Gas1 and/or Boc (and Cdon) is required for a Shh response, we generated a mutant Shh protein unable to bind Boc/Cdon/Gas1 but retaining the ability to bind Ptch1. If this mutant Shh molecule with altered specificity no longer activates signaling, it would suggest that Shh binding to Boc/Cdon/Gas1 is required for pathway activation. Conversely, if this mutant form of Shh activates the pathway, it would support a model where binding to Ptch1 alone is sufficient for Shh signaling.
The amino acids responsible for mediating the interaction between Shh and Boc/Cdon have been identified from co-crystal structures of Shh and the third FNIII domain of Cdon and Boc (Fig. 6A,B) (Kavran et al., 2010; McLellan et al., 2008). Although similar structural data is unavailable for Shh in complex with Ptch1, mutagenesis of Shh surface amino acids has identified residues required and residues dispensable for the binding of Shh to Ptch1 (Fig. 6C) (Bosanac et al., 2009). Since Shh E90 is a contact amino acid between Shh and Cdon/Boc (McLellan et al., 2008) that is not required for binding to Ptch1 (Fig. 6A,C) (Bosanac et al., 2009), we predicted that a mutation at this site might affect binding to Boc and Cdon, but not to Ptch1. In contrast, Shh R154 is a contact amino acid between Shh and Boc/Cdon (McLellan et al., 2008) that is also required for Ptch1 binding (Fig. 6B,C) (Bosanac et al., 2009); thus, a R154 mutation is expected to affect binding of Shh to Boc, Cdon and Ptch1.
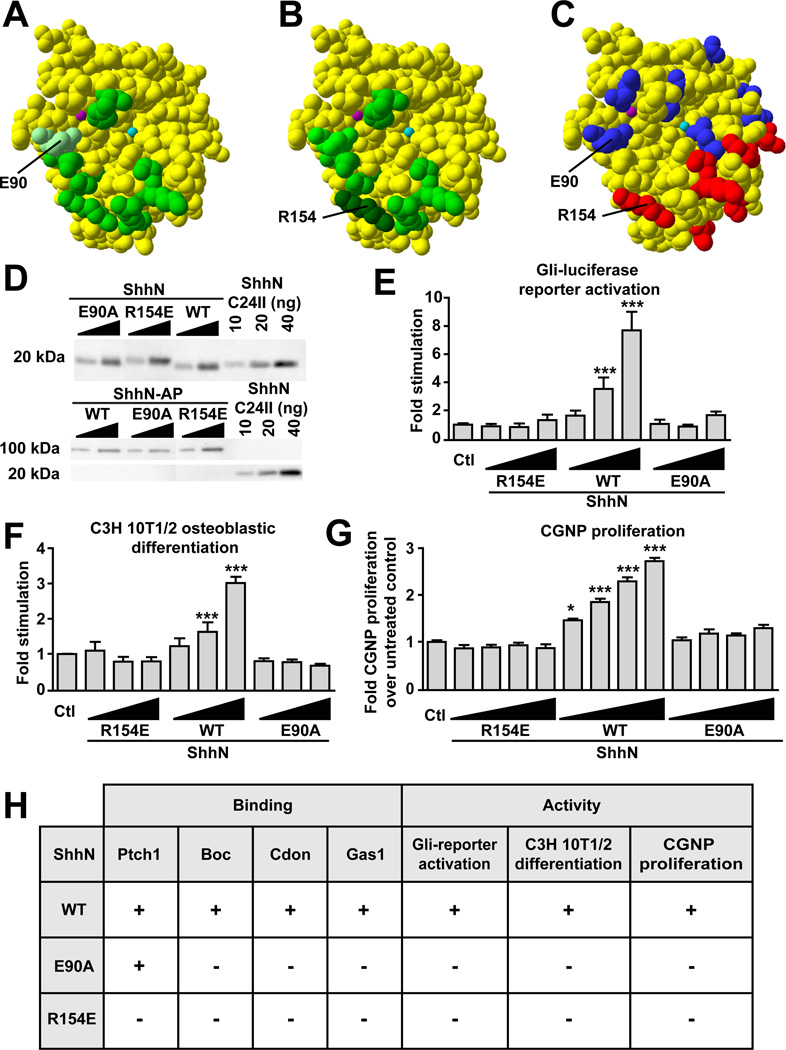
(A, B, C) Structural representation of ShhN. Residues colored in light, medium and dark green represent amino acids contacting Boc/Cdon FNIII(c) domain. E90 is represented in light green (A). R154 is represented in dark green (B). (C) Amino acids important for Ptch1 binding are colored in red while residues dispensible for the Ptch1-Shh interaction are illustrated in blue. (D) Anti-ShhN immunoblots showing the expression of purified ShhN WT, E90A, and R154E in comparison to ShhN C24II (top panel). Anti-ShhN immunoblot showing conditioned medium (CM) containing ShhN-AP WT, E90A, and R154E in comparison to ShhN C24II (bottom panel). (E) Stimulation of Gli-luciferase reporter activity in C3H 10T1/2 cells treated with 17, 50 and 150 nM ShhN WT, E90A or R154E mutants. (F) Differentiation of C3H 10T1/2 cells treated with 12.5, 25 and 50 nM ShhN WT, E90A or R154E mutants. (E–F) Each condition was performed in duplicate, n=3. (G) Induction in rat P4 CGNP proliferation upon treatment with 6.25, 12.5, 25 and 50 nM of ShhN WT, E90A or R154E mutants. Proliferation was measured by 3H-thymidine incorporation. Each condition was performed in triplicate, n=3. (E–G) Two-way ANOVA comparing the activity of ShhN E90A to ShhN WT was performed. (H) Table summarizing binding of ShhN mutants to Ptch1, Boc, Cdon and Gas1 and their functional activity.
We introduced mutations of these residues into alkaline-phosphatase (AP)–tagged ShhN (ShhN-AP) and tested their binding to Boc, Cdon, Gas1, and Ptch1 (Fig. 6D,H and Table S1). Consistent with our structural predictions, ShhN-AP R154E was unable to bind to Boc, Cdon and Ptch1. Also in agreement with our predictions, ShhN-AP E90A did not bind to Boc and Cdon, but retained the ability to bind Ptch1, with a dissociation constant not signficantly different (p>0.05) from that of WT ShhN (Fig. 6H and Table S1). We also assessed the binding of our Shh mutants to Gas1 and found that they behaved similarly towards Gas1 as they did with Boc and Cdon: ShhN-AP E90A and R154E were both unable to bind Gas1. Thus, according to the binding characteristics of our Shh mutants, some common amino acids may mediate the interaction of Shh with Gas1, Boc, and Cdon, a finding consistent with previous reports (Kavran et al., 2010; McLellan et al., 2008)
We next examined the effect of the E90A and R154E mutations on Shh signal transduction. We introduced these mutations into untagged ShhN and recombinant proteins were purified (Fig. 6D). To measure the signaling activity of the ShhN E90A and R154E mutants, we performed transcription reporter assays using cells stably transfected with a Gli-luciferase reporter plasmid. While WT ShhN activated Shh-mediated transcription in a concentration-dependent manner, ShhN E90A and R154E mutants were unable to do so (Fig. 6E). We next tested the ability of our Shh mutants to promote the osteoblastic differentiation of C3H 10T½ cells and neither ShhN E90A nor R154E were able to induce alkaline phosphatase expression, a marker of differentiation (Fig. 6F). Finally, we assayed the ability of these altered-specificity Shh ligands to induce CGNP proliferation. We found that while WT ShhN activated Shh-mediated proliferation in a dose-dependent manner, both ShhN E90A and R154E mutants were unable to induce proliferation (Fig. 6G). Together, these data show that ShhN E90A, which interacts with Ptch1 but not with Boc, Cdon, and Gas1, fails to induce Shh signaling and Shh-dependent cellular responses. This indicates that binding of Shh to Ptch1 alone is not sufficient to activate Shh signaling, suggesting that binding to Boc, Cdon, or Gas1 cell surface proteins is absolutely required for Shh-dependent signal transduction to occur.
DISCUSSION
In this study, we used three complementary approaches to establish the receptor requirements for Shh signaling in vertebrates. In one approach, we generated an altered-specificity Shh ligand (ShhN E90A) that cannot bind Boc, Cdon, and Gas1, but still binds Ptch1. This ligand was unable to induce Shh-dependent signaling, Shh-dependent osteoblastic differentiation, and CGNP proliferation, indicating that binding to Ptch1 alone is not sufficient to induce Shh signaling. In a second approach, we used gene inactivation of Boc and/or Gas1, and examined the ability of CGNPs, which already do not express Cdon, to respond to Shh. While inactivation of either Boc or Gas1 caused a partial decrease in the ability of CGNPs to respond to Shh, the inactivation of both completely abolished the response. Thus, Boc and Gas1 are absolutely required for CGNPs to respond to Shh, consistent with our finding that a Shh mutant unable to bind Boc and Gas1 cannot activate Shh signaling in CGNPs. Further supporting the idea that Boc and Gas1 are Shh receptors functioning upstream of Smo, we showed that a Smo agonist rescued the proliferation defect of Gas1;Boc mutant CGNPs. Thirdly, we used a biochemical approach to show that Boc and Gas1 interact with Ptch1. The interaction between Boc and Ptch1 appears to be constitutive as it is not modulated by Shh and it occurs independently of the Shh-binding domains in Boc and Ptch1. We also observed that Boc/Ptch1 complexes do not contain detectable amounts of Gas1 (and that Gas1/Ptch1 complexes do not contain detectable amounts of Boc), suggesting that Boc/Ptch1 and Gas1/Ptch1 complexes are distinct entities.
Together, these results lead us to propose the following model. Boc, Cdon, and Gas1 interact with Ptch1 to form Boc/Ptch1, Cdon/Ptch1 or Gas1/Ptch1 complexes (Fig. 7), with the Boc/Ptch1 interaction being mediated by the FNIIIa and FNIIIb domains of Boc. The combination of receptor complexes present in a cell would depend on the expression of Boc, Cdon, and Gas1. In the presence of Shh, the ligand binds to Boc/Ptch1, Cdon/Ptch1, and Gas1/Ptch1 receptor complexes, leading to the de-repression of Smo, which in turn activates a series of signal transduction events that result in Gli-mediated transcription. In the case of Boc and Cdon, the interaction with Shh is direct and is mediated by the third FNIII (FNIIIc) domain of Boc and Cdon (McLellan et al., 2008; Okada et al., 2006; Tenzen et al., 2006; Yao et al., 2006).
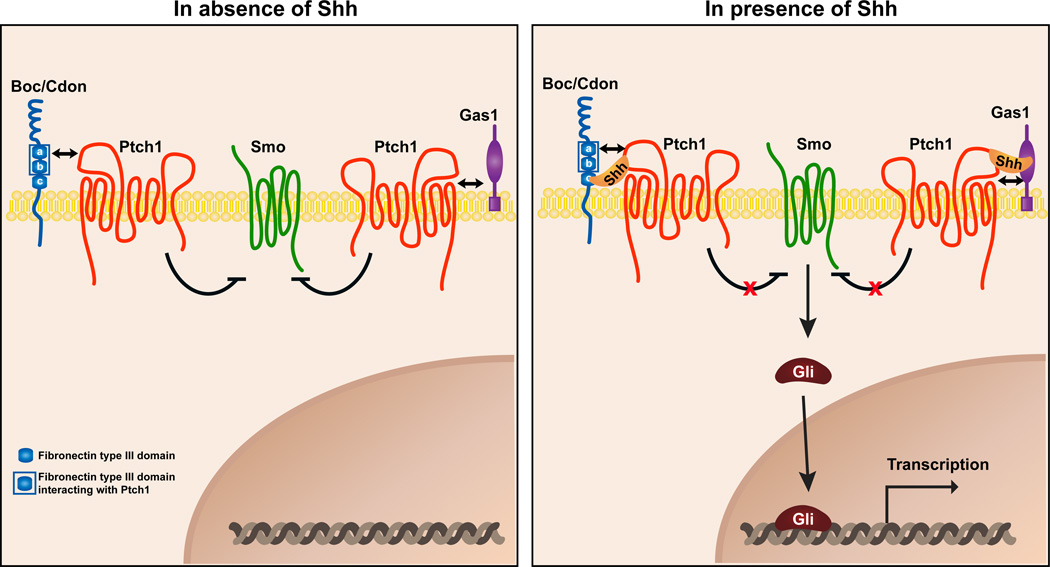
(Left) In the absence of Shh, Boc, Cdon and Gas1 interact with Ptch1 to form Boc/Ptch1, Cdon/Ptch1 or Gas1/Ptch1 complexes. The Boc/Ptch1 interaction is mediated by the FNIIIa and FNIIIb domains of Boc. (Right) Shh binding to Boc/Ptch1, Cdon/Ptch1, and Gas1/Ptch1 receptor complexes leads to the de-repression of Smo, which in turn activates signal transduction events that result in Gli-mediated transcription. In the case of Boc and Cdon, the interaction with Shh is direct and is mediated by the FNIIIc domain of Boc and Cdon. Unlike Ihog and Boi which promote the relocalization of Ptc to the surface of Drosophila cells, Boc and Gas1 do not seem to relocalize Ptch1 to the surface of mammalian cells (Fig. S5A-C).
Gas1 is an essential vertebrate Shh binding protein
Previous studies have shown that forced expression of Boc, Cdon, or Gas1 can potentiate Shh signaling and that inactivation of Cdon or Gas1 can decrease Shh signaling in the neural tube (Allen et al., 2007; Martinelli and Fan, 2007a; Tenzen et al., 2006). Although these studies suggested a modulatory role for Boc, Cdon, and Gas1 in Shh signaling, whether Ptch1 alone is sufficient in the absence of any of these receptors remained an open question. Our study, together with a companion study by Allen et al. showing that embryos mutant for Boc, Cdon, and Gas1 completely lack Shh signaling in the neural tube, demonstrate that in vertebrates these receptors do not function solely as auxiliary Shh receptors but are absolutely required for Shh signaling in vivo.
In Drosophila, Ihog and Boi, the orthologs of Boc and Cdon, are absolutely required for Hh signaling (Camp et al., 2010; Zheng et al., 2010). If Hh receptor requirements were entirely conserved, it would have been expected that vertebrate cells lacking Boc and Cdon would not be able to respond to Shh. However, the inactivation of Boc in CGNPs (which do not express Cdon) leads to a partial decrease in their proliferative response to Shh. Moreover, inactivation of Cdon in Boc−/− cerebella did not further decrease CGNP proliferation compared to inactivation of Boc alone (Fig. S1). Hence, unlike Drosophila which absolutely require either Ihog or Boi for Hh signaling, vertebrate cells still respond to Shh in absence of Boc and Cdon. These results highlight a fundamental difference between Drosophila and vertebrate Hh signaling and raised two possible mechanisms for Hh reception in vertebrates: Either Ptch1 is sufficient for Shh signaling or, unlike Drosophila, additional Shh binding molecules enable vertebrate cells to respond to Shh. Our data, together with results from Allen et al. (Allen et al., submitted), support a model where Gas1 acts with Boc and Cdon as essential Shh receptors. We showed that the combined inactivation of Boc, together with Gas1, completely abolishes the ability of CGNPs to respond to Shh, and that a mutant Shh ligand which binds Ptch1 (but not Boc, Cdon nor Gas1) is insufficient to activate Shh signaling. Thus, Gas1 – which is not present in the Drosophila genome – is an essential vertebrate Shh binding protein.
Other differences also exist between Hh signaling in invertebrates and vertebrates. The crystal structure of Hh in complex with Ihog, and Shh in complex with Cdon show that while Shh interacts with the third FNIII domain of Cdon and Boc (McLellan et al., 2008; Okada et al., 2006; Tenzen et al., 2006), Hh interacts with the non-orthologous first FNIII domain of Ihog and Boi (McLellan et al., 2006; Yao et al., 2006). In addition, the mode of Hh binding by the FNIII domains is not conserved between Drosophila and vertebrates (McLellan et al., 2008).
Previous studies in Drosophila S2 cells have shown that expression of Ihog results in a dramatic relocalization of Ptc to the cell suface (Zheng et al., 2010). Surprisingly, neither Boc nor Gas1 expression resulted in an increased relocalization of Ptch1 to the surface in at least two mammalian cell types (Fig. S5A,B). Surface biotinylation experiments also led to the same conclusion (Fig. S5C). While it is possible that tagging Ptch1 with GFP may interfere with its relocalization, we think that this is unlikely to occur, as Ptch1-GFP appears properly targeted to the cell surface given that we were able to detect an interaction between Boc and Ptch1-GFP in our cell surface binding assays (Fig. 5G). Thus, in contrast to what was observed for Ihog and Ptc, Boc and Gas1 do not appear to play a major role in relocalizing Ptch1 to the cell surface. Taken together, these results highlight important differences between Drosophila and vertebrate Hh signal reception.
Another fundamental difference is the requirement for the primary cilium for vertebrate Hh signaling (Eggenschwiler and Anderson, 2007). While Boc and Gas1 do not relocalize Ptch1 to the cell surface, it will be interesting to determine whether they contribute to its localization at the primary cilium (Corbit et al., 2005; Rohatgi et al., 2007).
Another interesting subject for future investigation is whether posttranslational modifications and multimerization of Shh (Dessaud et al., 2008) affect its interaction with the receptor complexes described in this study.
Are there specialized functions for Boc, Cdon, and Gas1?
Although Boc and Gas1 seemed to act redundantly in our CGNP proliferation experiments, it is possible that, in other cellular contexts, Boc, Gas1, or Cdon might impart additional non-redundant functions to the Shh signaling pathway. For example, Boc is required for Shh-mediated axon guidance (Fabre et al., 2010; Okada et al., 2006). Shh guides axons through a Src-family kinase (SFKs) dependent and Gli-independent pathway, where SFKs couple Shh signaling to cytoskeletal changes that elicit axon turning (Charron et al., 2003; Yam et al., 2009). It is possible that axon guidance by Shh is a specific function of Boc, since inactivation of Cdon did not disrupt axon guidance (Fabre et al., 2010; Okada et al., 2006). Thus, it is likely that different Shh receptor complexes, besides eliciting a canonical Shh signal transduction cascade, also impart additional functions. We speculate that the acquisition of novel functions for the vertebrate Shh signaling pathway during evolution might have paralleled the appearance of additional Shh binding proteins, such as Gas1.
A role for Boc, Cdon and Gas1 receptors in Hh signaling pathway-dependent pathologies
Many lines of evidence suggest that disruption of Hh proteins binding to Boc, Cdon, and Gas1 have pathological outcomes in humans. It is noteworthy that mutations of Shh amino acids important for binding to Boc, Cdon, and Gas1 have been identified in holoproencephaly (HPE) and in brachydactyly (McLellan et al., 2008). Moreover, mutations in GAS1 have been identified in HPE patients (Ribeiro et al., 2010) and, in mouse, inactivation of Cdon and Gas1 lead to HPE (Seppala et al., 2007; Zhang et al., 2006). In addition, while Boc−/− mice do not display HPE, inactivation of Boc in a Cdon mutant background enhances the severity of the HPE phenotype in a dosage-dependent manner (Zhang et al., 2010). Thus, the identification and molecular understanding of the exact components and interactions found in the vertebrate Shh receptor complexes might help our understanding of pathologies associated with defective Shh signaling. Additionally, because we show that, in addition to cell fate specification, these complexes are also involved in the control of cellular proliferation, our results provide new avenues for the treatment of Hh pathway-dependent cancers (Scales and de Sauvage, 2009).
EXPERIMENTAL PROCEDURES
See Supplementary material for additional procedures.
Mice
Boc and Cdon (Okada et al., 2006; Zhang et al., 2010) and Gas1 (Martinelli and Fan, 2007a) mice were described previously.
Plasmids and reagents
Recombinant ShhN C24II and IGF-I were from R&D Systems. pEGFP-mCdon, pEGFP-mBoc and pCA-gap-EGFP were previously described (Okada et al., 2006). pEGFP-mPtch1, mGli1 and pcDNA3-mGas1 were kindly provided by C.C. Hui and V. Wallace. pEGFP-Smo was kindly provided by P. Beachy.(Okada et al., 2006)
Histology and immunohistochemistry
β-gal activity detection and immunochemistry on sections were performed according to protocols described previously (Charron et al., 2003; Fabre et al., 2010; Okada et al., 2006). Antibody dilutions: rabbit anti-mouse Lim1 (1:500, T. Jessell lab), rabbit anti-mouse Pax6 (1:100, Chemicon), rabbit anti-mouse Calbindin (1:200, Chemicon), goat anti-mouse TAG1 (1:400), rabbit anti-GFP (1:1000, Invitrogen), goat anti-mouse Boc (1:100, R&D), goat anti-mouse Cdon (1:250, R&D), goat anti-mouse Gas1 (1:200, R&D). Prior to performing immunostainings with anti-Boc antibody, sections were subjected to antigen retrieval for 1hr at 98°C in a sodium citrate buffer (10 mM sodium citrate, 0.05% Tween 20, pH 6.0), cooled at RT for 20 minutes and washed extensively with PBS.
Isolation of CGNPs and in vitro proliferation assays
CGNPs were isolated from either E18.5 or P4 mouse or rat cerebella using a modified protocol previously described (Wechsler-Reya and Scott, 1999). Briefly, isolated cerebella were cut in small pieces and treated with 0.25% trypsin and DNaseI. Following trituration, single cell suspensions were centrifuged through a 30% to 65% Percoll step gradient. Cells harvested at the 30% interphase were resuspended in Neurobasal supplemented with B27, 0.5 mM L-Glutamine and Pen/Strep and plated in 96-well plates pre-coated with 100 µg/ml poly-D-Lysine. For CGNP proliferation assays with IGF-I, cells were resuspended in Neurobasal supplemented with 0.06% D-glucose, 100 µg/ml apo-transferrin, 16 µg/ml putrescine, 30 nM sodium selenite, 20 ng/ml progesterone and 1 mg/ml BSA. For 3H-thymidine incorporation assays, cells were seeded at 4×105 cells/well in a 96-well plate in triplicate and treated with ShhN C24II for 48h. CGNPs were pulsed with 1 µCi/ml 3H-thymidine (PerkinElmer) for the last 12h. Incorporation was measured using the Filtermate harvester (PerkinElmer) and TopCount NXT beta counter (Packard). Alternatively, CGNPs were seeded at 2.5×104 cells/well of a 96 well/plate and cultured as described above. Following 48h in culture, cells were fixed with 4% PFA, blocked with 10% PHT and immunostained with mouse anti-Ki67 antibody (1:100, Becton-Dickinson).
Statistical Methods
Unless otherwise noted, all data are expressed as mean ± SEM. The statistical tests used to measure differences are indicated in the appropriate legends. Statistical significances are indicated as follows: *p < 0.05; **p < 0.01; ***p < 0.001; n.s., not significant.
ACKNOWLEDGEMENTS
We are grateful to M. Cayouette, C. Jolicoeur, A. Kania, P. Fabre, E. Diaz, K. Tsanov and J.J. Guo for providing advice and/or reagents, S. McConnell and A. Okada for Boc and Cdon mice, and D. Leahy for providing the Shh-Cdon crystallographic data prior to publication. We thank J. Barthe, J. Cardin and F. Bourque for expert technical assistance. We thank D. van Meyel, P.T. Yam, M. Cayouette and A. Kania for critical comments on the manuscript. This work was supported by a grant from the Canadian Cancer Society Research Institute (CCSRI). BCW is supported by NIH grant DA-004443 to P. Schiller. APM is supported by NIH grant NS-033642. LI and ML are supported by Fonds de Recherche en Santé du Québec (FRSQ) post-doctoral training awards. FM is a Research Fellow of The Terry Fox Foundation. FC was a Canadian Institutes of Health Research (CIHR) New Investigator and is now an FRSQ Research Scientist.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Allen BL, Tenzen T, McMahon AP. The Hedgehog-binding proteins Gas1 and Cdo cooperate to positively regulate Shh signaling during mouse development. Genes Dev. 2007;21:1244–1257. [Europe PMC free article] [Abstract] [Google Scholar]
- Bosanac I, Maun HR, Scales SJ, Wen X, Lingel A, Bazan JF, de Sauvage FJ, Hymowitz SG, Lazarus RA. The structure of SHH in complex with HHIP reveals a recognition role for the Shh pseudo active site in signaling. Nat Struct Mol Biol. 2009;16:691–697. [Abstract] [Google Scholar]
- Camp D, Currie K, Labbe A, van Meyel DJ, Charron F. Ihog and Boi are essential for Hedgehog signaling in Drosophila. Neural Dev. 2010;5:28. [Europe PMC free article] [Abstract] [Google Scholar]
- Charron F, Stein E, Jeong J, McMahon AP, Tessier-Lavigne M. The morphogen sonic hedgehog is an axonal chemoattractant that collaborates with netrin-1 in midline axon guidance. Cell. 2003;113:11–23. [Abstract] [Google Scholar]
- Charron F, Tessier-Lavigne M. Novel brain wiring functions for classical morphogens: a role as graded positional cues in axon guidance. Development. 2005;132:2251–2262. [Abstract] [Google Scholar]
- Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437:1018–1021. [Abstract] [Google Scholar]
- Corcoran RB, Bachar Raveh T, Barakat MT, Lee EY, Scott MP. Insulin-like growth factor 2 is required for progression to advanced medulloblastoma in patched1 heterozygous mice. Cancer Res. 2008;68:8788–8795. [Europe PMC free article] [Abstract] [Google Scholar]
- Corrales JD, Rocco GL, Blaess S, Guo Q, Joyner AL. Spatial pattern of sonic hedgehog signaling through Gli genes during cerebellum development. Development. 2004;131:5581–5590. [Abstract] [Google Scholar]
- Dahmane N, Ruiz i Altaba A. Sonic hedgehog regulates the growth and patterning of the cerebellum. Development. 1999;126:3089–3100. [Abstract] [Google Scholar]
- Dessaud E, McMahon AP, Briscoe J. Pattern formation in the vertebrate neural tube: a sonic hedgehog morphogen-regulated transcriptional network. Development. 2008;135:2489–2503. [Abstract] [Google Scholar]
- Eggenschwiler JT, Anderson KV. Cilia and developmental signaling. Annu Rev Cell Dev Biol. 2007;23:345–373. [Europe PMC free article] [Abstract] [Google Scholar]
- Fabre PJ, Shimogori T, Charron F. Segregation of ipsilateral retinal ganglion cell axons at the optic chiasm requires the Shh receptor Boc. J Neurosci. 2010;30:266–275. [Abstract] [Google Scholar]
- Flora A, Klisch TJ, Schuster G, Zoghbi HY. Deletion of Atoh1 disrupts Sonic Hedgehog signaling in the developing cerebellum and prevents medulloblastoma. Science. 2009;326:1424–1427. [Europe PMC free article] [Abstract] [Google Scholar]
- Ingham PW, Placzek M. Orchestrating ontogenesis: variations on a theme by sonic hedgehog. Nat Rev Genet. 2006;7:841–850. [Abstract] [Google Scholar]
- Jiang J, Hui CC. Hedgehog signaling in development and cancer. Dev Cell. 2008;15:801–812. [Abstract] [Google Scholar]
- Kavran JM, Ward MD, Oladosu OO, Mulepati S, Leahy DJ. All Mammalian Hedgehog Proteins Interact with Cell Adhesion Molecule, Down-regulated by Oncogenes (CDO) and Brother of CDO (BOC) in a Conserved Manner. J Biol Chem. 2010;285:24584–24590. [Europe PMC free article] [Abstract] [Google Scholar]
- Kenney AM, Rowitch DH. Sonic hedgehog promotes G(1) cyclin expression and sustained cell cycle progression in mammalian neuronal precursors. Mol Cell Biol. 2000;20:9055–9067. [Europe PMC free article] [Abstract] [Google Scholar]
- Lewis PM, Gritli-Linde A, Smeyne R, Kottmann A, McMahon AP. Sonic hedgehog signaling is required for expansion of granule neuron precursors and patterning of the mouse cerebellum. Dev Biol. 2004;270:393–410. [Abstract] [Google Scholar]
- Liu Y, May NR, Fan CM. Growth arrest specific gene 1 is a positive growth regulator for the cerebellum. Dev Biol. 2001;236:30–45. [Abstract] [Google Scholar]
- Marigo V, Davey RA, Zuo Y, Cunningham JM, Tabin CJ. Biochemical evidence that patched is the Hedgehog receptor. Nature. 1996;384:176–179. [Abstract] [Google Scholar]
- Martinelli DC, Fan CM. Gas1 extends the range of Hedgehog action by facilitating its signaling. Genes Dev. 2007a;21:1231–1243. [Europe PMC free article] [Abstract] [Google Scholar]
- Martinelli DC, Fan CM. The role of Gas1 in embryonic development and its implications for human disease. Cell Cycle. 2007b;6:2650–2655. [Abstract] [Google Scholar]
- McLellan JS, Yao S, Zheng X, Geisbrecht BV, Ghirlando R, Beachy PA, Leahy DJ. Structure of a heparin-dependent complex of Hedgehog and Ihog. Proc Natl Acad Sci USA. 2006;103:17208–17213. [Europe PMC free article] [Abstract] [Google Scholar]
- McLellan JS, Zheng X, Hauk G, Ghirlando R, Beachy PA, Leahy DJ. The mode of Hedgehog binding to Ihog homologues is not conserved across different phyla. Nature. 2008;455:979–983. [Europe PMC free article] [Abstract] [Google Scholar]
- Okada A, Charron F, Morin S, Shin DS, Wong K, Fabre PJ, Tessier-Lavigne M, McConnell SK. Boc is a receptor for sonic hedgehog in the guidance of commissural axons. Nature. 2006;444:369–373. [Abstract] [Google Scholar]
- Ribeiro LA, Quiezi RG, Nascimento A, Bertolacini CP, Richieri-Costa A. Holoprosencephaly and holoprosencephaly-like phenotype and GAS1 DNA sequence changes: Report of four Brazilian patients. Am J Med Genet A. 2010;152 A:1688–1694. [Abstract] [Google Scholar]
- Rohatgi R, Milenkovic L, Scott MP. Patched1 regulates hedgehog signaling at the primary cilium. Science. 2007;317:372–376. [Abstract] [Google Scholar]
- Roussel MF, Hatten ME. Cerebellum development and medulloblastoma. Curr Top Dev Biol. 2011;94:235–282. [Europe PMC free article] [Abstract] [Google Scholar]
- Scales SJ, de Sauvage FJ. Mechanisms of Hedgehog pathway activation in cancer and implications for therapy. Trends Pharmacol Sci. 2009;30:303–312. [Abstract] [Google Scholar]
- Seppala M, Depew MJ, Martinelli DC, Fan CM, Sharpe PT, Cobourne MT. Gas1 is a modifier for holoprosencephaly and genetically interacts with sonic hedgehog. J Clin Invest. 2007;117:1575–1584. [Abstract] [Google Scholar]
- Solecki DJ, Liu XL, Tomoda T, Fang Y, Hatten ME. Activated Notch2 signaling inhibits differentiation of cerebellar granule neuron precursors by maintaining proliferation. Neuron. 2001;31:557–568. [Abstract] [Google Scholar]
- Tenzen T, Allen BL, Cole F, Kang JS, Krauss RS, McMahon AP. The cell surface membrane proteins Cdo and Boc are components and targets of the Hedgehog signaling pathway and feedback network in mice. Dev Cell. 2006;10:647–656. [Abstract] [Google Scholar]
- Wallace VA. Purkinje-cell-derived Sonic hedgehog regulates granule neuron precursor cell proliferation in the developing mouse cerebellum. Curr Biol. 1999;9:445–448. [Abstract] [Google Scholar]
- Wechsler-Reya RJ, Scott MP. Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog. Neuron. 1999;22:103–114. [Abstract] [Google Scholar]
- Yam PT, Langlois SD, Morin S, Charron F. Sonic hedgehog guides axons through a noncanonical, Src-family-kinase-dependent signaling pathway. Neuron. 2009;62:349–362. [Abstract] [Google Scholar]
- Yao S, Lum L, Beachy P. The ihog cell-surface proteins bind Hedgehog and mediate pathway activation. Cell. 2006;125:343–357. [Abstract] [Google Scholar]
- Zhang W, Hong M, Bae GU, Kang JS, Krauss RS. Boc modifies the holoprosencephaly spectrum of Cdo mutant mice. Dis Model Mech. 2010 [Europe PMC free article] [Abstract] [Google Scholar]
- Zhang W, Kang JS, Cole F, Yi MJ, Krauss RS. Cdo functions at multiple points in the Sonic Hedgehog pathway, and Cdo-deficient mice accurately model human holoprosencephaly. Dev Cell. 2006;10:657–665. [Abstract] [Google Scholar]
- Zheng X, Mann RK, Sever N, Beachy PA. Genetic and biochemical definition of the Hedgehog receptor. Genes Dev. 2010;24:57–71. [Europe PMC free article] [Abstract] [Google Scholar]
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.devcel.2011.04.017
Read article for free, from open access legal sources, via Unpaywall:
http://www.cell.com/article/S1534580711001717/pdf
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1016/j.devcel.2011.04.017
Article citations
β-arrestins Are Scaffolding Proteins Required for Shh-Mediated Axon Guidance.
J Neurosci, 44(30):e0261242024, 24 Jul 2024
Cited by: 0 articles | PMID: 38886055
Distinct expression patterns of Hedgehog signaling components in mouse gustatory system during postnatal tongue development and adult homeostasis.
PLoS One, 19(6):e0294835, 07 Jun 2024
Cited by: 0 articles | PMID: 38848388
Dual and opposing roles for the kinesin-2 motor, KIF17, in Hedgehog-dependent cerebellar development.
Sci Adv, 10(17):eade1650, 26 Apr 2024
Cited by: 1 article | PMID: 38669326 | PMCID: PMC11051677
Suppression of apoptosis impairs phalangeal joint formation in the pathogenesis of brachydactyly type A1.
Nat Commun, 15(1):2229, 12 Mar 2024
Cited by: 1 article | PMID: 38472182 | PMCID: PMC10933404
Mutations in cdon and boc affect trunk neural crest cell migration and slow-twitch muscle development in zebrafish.
Development, 150(14):dev201304, 13 Jul 2023
Cited by: 1 article | PMID: 37390228 | PMCID: PMC10357035
Go to all (164) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Overlapping roles and collective requirement for the coreceptors GAS1, CDO, and BOC in SHH pathway function.
Dev Cell, 20(6):775-787, 01 Jun 2011
Cited by: 186 articles | PMID: 21664576 | PMCID: PMC3121104
The hedgehog co-receptor BOC differentially regulates SHH signaling during craniofacial development.
Development, 147(23):dev189076, 14 Dec 2020
Cited by: 21 articles | PMID: 33060130 | PMCID: PMC7758635
Temporal and spatial expression patterns of Hedgehog receptors in the developing inner and middle ear.
Int J Dev Biol, 61(8-9):557-563, 01 Jan 2017
Cited by: 2 articles | PMID: 29139542
Cdon and Boc: Two transmembrane proteins implicated in cell-cell communication.
Int J Biochem Cell Biol, 44(5):698-702, 03 Feb 2012
Cited by: 27 articles | PMID: 22326621
Review
Funding
Funders who supported this work.
CIHR
Canadian Cancer Society (1)
Grant ID: 018200
Fonds de Recherche du Québec - Santé
NIDA NIH HHS (3)
Grant ID: R37 DA004443
Grant ID: DA-004443
Grant ID: R01 DA004443
NINDS NIH HHS (3)
Grant ID: R01 NS033642
Grant ID: R37 NS033642
Grant ID: NS-033642
National Institutes of Health (2)
Grant ID: DA-004443
Grant ID: NS-033642




