Abstract
Free full text

Histone Transfer Among Chaperones
Abstract
The eukaryotic processes of nucleosome assembly and disassembly govern chromatin dynamics, in which histones exchange in a highly regulated manner to promote genome accessibility for all DNA-dependent processes. This regulation is partly carried out by histone chaperones, which serve multifaceted roles in coordinating the interactions of histone proteins with modification enzymes, nucleosome remodelers, other histone chaperones, and nucleosomal DNA. The molecular details of the processes by which histone chaperones promote delivery of histones among their many functional partners are still largely undefined, but promise to offer insights into epigenome maintenance. Here we review recent findings on the histone chaperone interactions that guide the assembly of histones H3 and H4 into chromatin. This evidence supports the concepts of histone post-translational modifications and specific histone chaperone interactions as guiding principles for histone H3/H4 transactions during chromatin assembly.
Introduction
Histone chaperones are acidic proteins that shield the highly basic histone proteins from undesirable interactions within the cell, and guide correct histone transactions during nucleosome assembly and disassembly [1, 2]. The nucleosome is composed of 147 bp of DNA wrapped around a core of histone proteins, which contains two copies each of histone proteins H3, H4, H2A, and H2B [3, 4]. Histone chaperones, in addition to their protective cellular functions, promote productive histone interactions with other chaperones [5, 6], histone-modifying enzymes [7, 8], ATP-dependent nucleosome remodelers [9, 10], and ultimately DNA, where nucleosomes are formed. These exchanges require proper presentation of histones to the appropriate partners in defined and ordered pathways [5].
In this minireview, we focus on the histone chaperone interactions that guide the productive transfer of histones H3 and H4 with several of the histone chaperones involved in nucleosome assembly. Analysis of the stepwise processes of nucleosome assembly and disassembly highlights the importance of the H3/H4 chaperones [3, 4, 8]. The central 80 bp of the 147 bp of nucleosomal DNA wraps the (H3/H4)2 tetramer into a unit known as the tetrasome. Binding of an H2A/H2B dimer to each face of the tetrasome wraps the additional 67 bp of DNA to give rise to the complete nucleosome core particle [4]. The central positioning of histones H3/H4 in the nucleosome core particle has important implications, as H3/H4 must be deposited on the DNA prior to H2A/H2B and removed from DNA after H2A/H2B [11]. We review recent studies that have provided molecular insight into how histone chaperones present histones H3/H4 to downstream effectors. This evidence supports a common guiding principle that histone modifications modulate specific interactions between H3/H4 and multiple histone chaperones.
Transferring Histones Among Chaperones
From the moment that histones H3 and H4 are assembled into the co-folded H3/H4 dimer they are associated with histone chaperones (Figure 1). In a recent biochemical analysis of histone chaperone complexes formed from the newly synthesized H3 variant H3.1, four major complexes of H3.1 were found in the cytoplasm, of which three contain H3.1/H4 together [12]. The major complex of H3.1/H4 includes nuclear autoantigenic sperm protein (sNASP) and histone acetyltransferase1 (HAT1)/retinoblastoma associated binding protein 46 (RbAp46) [13], which is equivalent to the Hat1p/Hat2 complex in budding yeast (reviewed in [6, 14]). HAT1 is thought to acetylate the N-terminal tail of H4 at lysines 5 and 12, which mark newly synthesized H4 [14], but the precise function of these modifications remains unclear. H3.1/H4 also appears in a complex with the histone chaperone Anti-silencing function 1 (Asf1a or Asf1b) with Importin 4 for transport into the nucleus. H3/H4 acquires further post-translational modifications, such as acetylation of H3 lysine 56, which is dependent on the association with Asf1 [15], and H4 serine 47 phosphorylation [16]. The nuclear histones associate with chromatin assembly factor 1 (CAF-1) [17], Rtt106 (budding yeast) (described in [18]), or the histone regulator A (HIRA) (reviewed in [19]), Daxx and DEK proteins (reviewed in [20]), for subsequent nucleosome assembly of H3.1 and H3.3 through replication-dependent and -independent pathways, respectively [16, 18]. To gain a deeper understanding of these critical histone chaperone–histone complexes, we consider relevant structural and biochemical insights concerning their interactions.
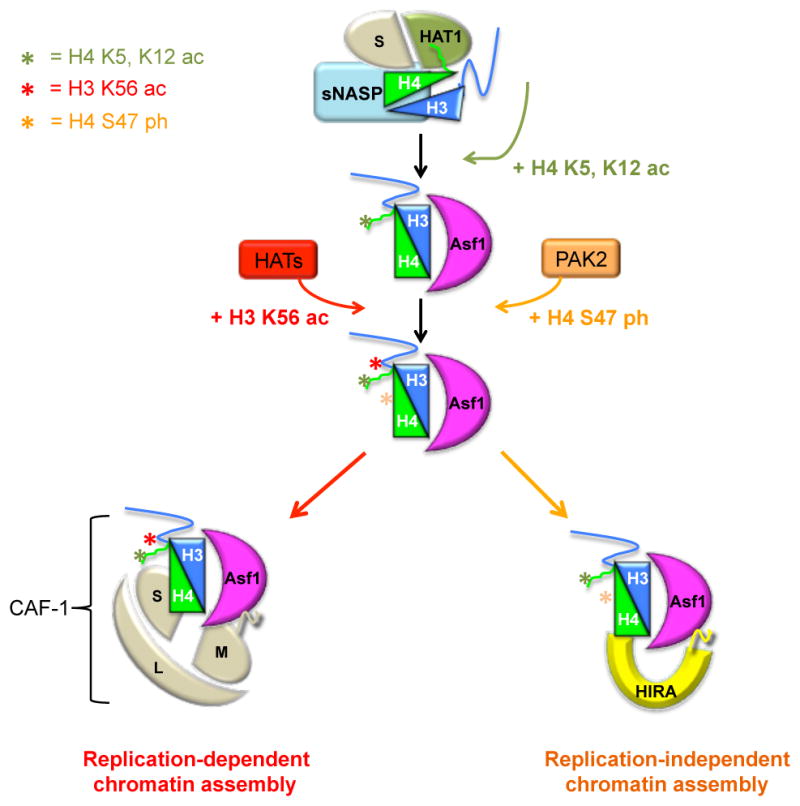
Newly synthesized histones H3/H4 (blue and green, respectively) are found in a complex along with sNASP (cyan), the small subunit of the CAF-1 complex (S), and HAT1 (olive). This complex promotes HAT1-mediated acetylation of K5 and K12 on the H4 N-terminal tail (green star). Association with Asf1 precedes the productive hand off of H3/H4 to the appropriate downstream chaperones, including CAF-1 (small, medium and large subunits are indicated by the letters S, M, and L in tan) and HIRA (yellow). The specificity behind these hand offs is partially driven by acquisition of specific histone post-translational modifications. Whereas acetylation of H3 K56 (red star) promotes H3/H4 accumulation on CAF-1 for replication-dependent chromatin assembly, phosphorylation of H4 S47 (orange star) drives HIRA-mediated replication-independent assembly.
New insights into RbAp46/RbAp48/Nurf55 association with H3 and H4
HAT1 is thought to acetylate the N-terminal tail of H4 at lysines 5 and 12 [14], while in a complex with RbAp46. The protein p55 (also known as Nurf55) is the single Drosophila melanogaster homolog of RbAp46 and RbAp48. The complex of RbAp46-HAT1 presents the N-terminal tail of H4 to HAT1 for site-specific acetylation through multimeric interactions. Crystallographic studies of human RbAp46 and p55/Nurf55 bound to H4 [21, 22], revealed how this WD-repeat protein (Figure 2A) interacts with the first alpha helix (aa 30 to 41) of H4 (Figure 2B). The edge of the disk-shaped WD beta-propeller forms the binding site for the H4 helix. This interaction would necessitate an unfolding of H4 from the H3/H4 dimer, but provides a means for the presentation of the H4 N-terminal tail to the HAT1 catalytic subunit. The H4-p55 interaction is relatively weak with a KD in the range of high nanomolar [22] to approximately 1 μM [21], which is similar to the affinity measured for the H3/H4-p55 complex [21]. p55/Nurf55 with amino acid substitutions that abolished binding to H4 was still able to bind the H3/H4 complex, which supported earlier results that p55 might also interact with H3 [23].
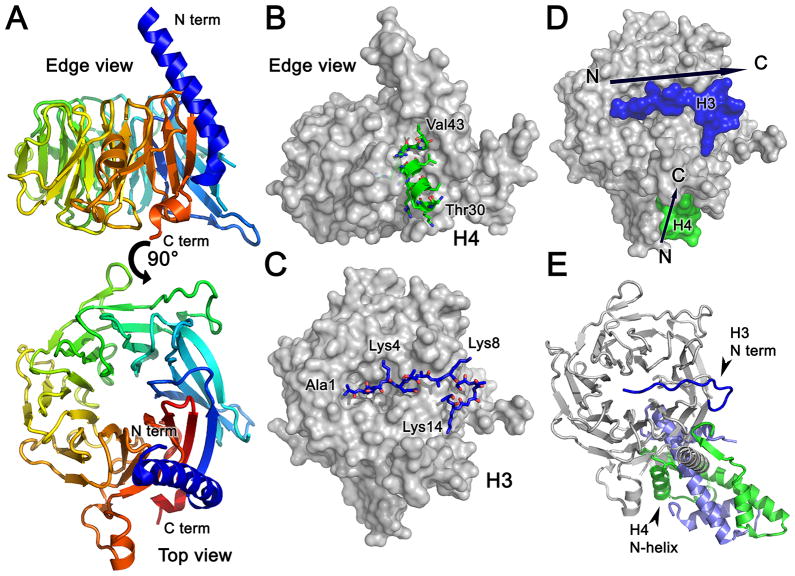
(A) Ribbon diagram showing the p55 structure with rainbow coloring (blue to red) from the N- to C-terminus. The “top” view in the lower panel was generated from the edge-on view in the upper panel by a 90° rotation about the horizontal axis of the page. (B) The p55-H4 complex from the edge-on view. The p55 subunit is presented as a surface rendering (grey) and the H4 N-terminal helix is shown in green, with oxygen atoms colored in red and nitrogen colored in blue. (C) The p55-H3 complex from the top view. The p55 subunit is presented as a surface rendering (grey) and the H3 N-terminal tail is shown in blue with oxygen atoms colored in red. (D) H3 and H4 can potentially bind to p55 simultaneously. The space-filling model of the superimposed p55-H3 and p55-H4 complexes is colored as in panels B and C. Arrows indicate the direction on the polypeptide chains, and these positions suggest that H3 and H4 could potentially bind to p55 at the same time. (E) Histone H4 cannot bind to p55 in the context of a correctly folded H3/H4 dimer. Ribbon diagram showing the superimposition of the p55-H3, p55-H4, and H3/H4 dimer complexes, colored as in panels B and C. This figure was generated using PDB IDs 2YBA (p55-H3) [25], 2XYI (p55-H4) [24], and 2HUE (H3/H4) [27].
To further define the molecular nature of the H3-p55 interaction, Nowak, et al. [24] and Schmitges, et al. [25] revealed how p55/Nurf55 specifically contacts the first 15 amino acids of the H3 N-terminal tail (Figure 2C). The potential binding site for H3 on the flat face of the p55/Nurf55 beta propeller was inferred from numerous mutagenesis and binding studies [24] and confirmed by the crystal structure of Nurf55 bound to the N-terminal tail of H3 (Figure 2C) [25]. Isothermal titration calorimetry experiments showed that Nurf55 binds preferentially to the unmodified H3 N-terminal peptide with a KD of 2 μM [24]. This affinity is much weaker than the affinity for the H4 peptide, approximately 35 nM, although measurements with intact H3/H4 complexes were not performed [24]. Taken together, these structural and binding studies showed that H3 binds to a different interface than H4, and it is possible that the two interactions could form simultaneously (Figure 2D), but H4 would have to at least partially unfold from the H3/H4 dimer in order to bind to p55 (Figure 2E). The binding results, however, do not support a positively cooperative binding model, as the affinity of p55/Nurf/RbAp46 for the H3/H4 complex [21] was found to be weaker than the binding to individual H3 or H4 peptides [22, 24]. This discrepancy may be due to the partial unfolding of H4 required for p55 binding. The multimeric nature of the p55 – H3/H4 interaction, instead, may provide specificity for the complex. Alternatively, as p55/Rbap46/48 is a member of multiple histone tail-modifying complexes – including HAT1, HDAC1/2, and PRC2 (reviewed in [26]) – binding to the H4 N-terminal helix and H3 N-terminal tail may differ in different contexts of histone recognition.
Asf1-mediated histone chaperone transactions
On their way to the nucleus, histones H3/H4 associate with the histone chaperone Asf1, which serves a pivotal role in specifying the fate of newly synthesized H3/H4 (Figure 1) (reviewed in [8]). Structures of yeast and human Asf1 bound to a heterodimer of H3/H4 revealed the interactions of Asf1 with both H3 and H4 [27, 28] that promote the formation of a high affinity complex (KD approximately 2.5 nM) (Figure 3) [29]. Asf1 binds to H3/H4 more tightly than other H3/H4 histone chaperones studied to date [29], but the affinity is slightly weaker than the affinity of H3/H4 for DNA [30]. For Asf1 to have a direct role in histone exchange from DNA, other factors would be required for histone removal from the DNA. Potentially, unknown specific post-translational modifications may facilitate histone transfer to Asf1 by weakening histone-DNA contacts. This high affinity is also consistent with the function of Asf1 as a histone “sink” sequestering H3/H4 for processes requiring histones, as most nuclear H3/H4 that is not associated with nucleosomal DNA is associated with Asf1 [31, 32]. Next, while still bound to Asf1, histones H3.1/H4 and H3.3/H4 partition to different downstream chaperones, including CAF-1 and HIRA, which guide H3/H4 to different fates (Figure 1).
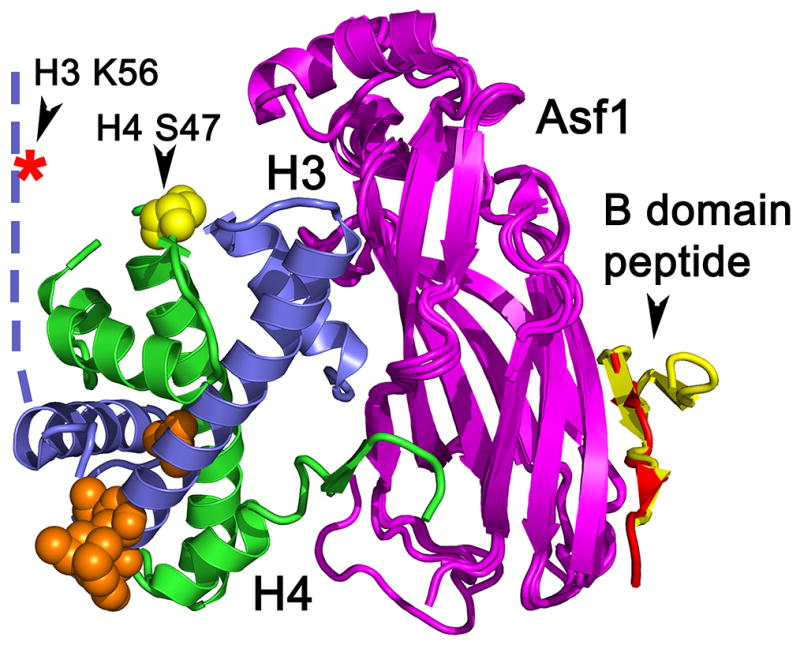
Ribbon diagram showing the complex of Asf1 (magenta) bound to the dimer of histones H3 (blue) and H4 (green) (PDB ID 2HUE), superimposed onto the Asf1 (magenta) complex with the B-domain peptide from HIRA (yellow) (PDB ID 2I32) [37] and CAF-1 middle-sized subunit (red) (PDB ID 2Z3F) [38]. Phosphorylation of serine 47 (yellow) of histone H4 is relevant to the HIRA assembly pathway. The acetylation of lysine 56 (indicated by a red star) is relevant to the CAF-1 and Rtt106 assembly pathways. Residues in the core domains that differ between the histone H3 variants, H3.1 and H3.3 (aa 31, 87, 89, 90, and 96), are shown in orange because of their involvement in both pathways.
To engage in DNA replication-dependent nucleosome assembly pathways, Asf1-H3.1/H4 associates with the histone chaperone, chromatin assembly factor 1 (CAF-1) [31, 33]. CAF-1 is a three-subunit complex composed of a large subunit, p150 in humans (p90 in yeast, and p180 in Drosophila), a small subunit RbAp48 (p55 in Drosophila and Msl1p in yeast), and a middle-sized subunit p60 in yeast and humans (p105 in Drosophila). Two major nuclear CAF-1 and H3/H4-containing complexes [32] have been identified, but interestingly, the larger-sized complex lacks Asf1 [32]. In addition, earlier studies found that the largest subunit of CAF-1 can dimerize, giving a complex that lacked the middle sized CAF-1 subunit [34]. In the DNA replication-independent assembly pathway, the chaperones HIRA [35] and Daxx [32, 36] sequester Asf1-H3.3/H4 complexes. Biochemical analyses do not agree on the composition of these complexes or the precise roles of these chaperones in nucleosome assembly [32, 36]. Similar to CAF-1, the larger-sized nuclear HIRA complex also lacks Asf1. Each complex likely has different interactions with histones, as the binding of Asf1 would be predicted to maintain the H3.1/H4 or H3.3/H4 proteins in the dimer rather than the tetramer form. Together, these results suggest that major reorganizations of histone chaperone-histone interactions occur that may facilitate the formation of H3/H4 tetramers, but the molecular nature of these is still undefined.
Histone hand off
Given the high affinity of Asf1 for H3/H4, how Asf1 “hands off” H3/H4 to CAF-1 and HIRA is an open question in understanding histone transactions. A possible mechanism for the exchange of H3/H4 between Asf1 and CAF-1 centers on the binding sites for the B-domain regions of CAF-1 or HIRA, which are located on the opposite face of Asf1 in relation to where H3/H4 are bound (Figure 3) [37, 38]. The identical binding sites of CAF-1 and HIRA on the edge of the Asf1 beta-sheet sandwich contribute to the mutually exclusive nature of replication-dependent and replication-independent nucleosome assembly. Since this face of Asf1 is opposite of the H3/H4 binding site, CAF-1 or HIRA can potentially bind simultaneously to an intact Asf1-H3/H4 complex. Indeed, Asf1 is found in HIRA and CAF-1 complexes along with H3/H4. However, the affinity of the Asf1-p60 peptide complex is only approximately 2 μM, which places a limit on the overall stability of the large complex, unless the additional, direct interactions between the chaperones and the histones contribute significantly to an overall higher affinity (Figure 3). For example, CAF-1 binds to Asf1 directly through the middle-sized subunit, as well as to the H4 N-terminal helix and the N-terminal tail of H3 through the smallest subunit (Figures 2 and and3).3). The combinatorial nature of the interactions in this multimeric structure provides a mechanism to produce a specific and stable complex. In addition, the affinity of CAF-1 for histones may be influenced by recognition of specific histone modifications.
Along this line, recent studies have identified histone post-translational modifications that play significant roles in modulating histone associations with specific histone chaperones. These include H3 K79 methylation [39], H3 K56ac, which promotes association with CAF-1 and Rtt106, [18], and H4 S47ph, which promotes association with HIRA [16]. The best-studied modification is acetylation of H3 K56, which occurs most frequently on newly synthesized histones during S-phase [39, 40]. H3 K56ac is important for both normal S-phase progression and DNA damage repair in replicating cells, thereby ensuring genomic stability [40–45]. It enhances accumulation of H3/H4 on CAF-1, and promotes CAF-1-dependent nucleosome assembly in vivo and in vitro [18]. Most recently, the Zhang lab established that phosphorylation of H4 S47 enhances the interaction of H3/H4 for HIRA but not CAF-1 [16] (Figure 1). Moreover, shRNA depletion of the kinase PAK2 decreased both H4 S47ph levels and H3.3 occupancy of chromatin, suggesting that PAK2 is the kinase responsible for this modification. Together with H3 K56ac, this study on H4 S47ph provides intriguing further evidence of a histone code governing nucleosome assembly and disassembly. However, the mechanism by which these modifications direct histone transfer from Asf1 to CAF-1 versus HIRA, and the molecular manner in which specific chaperones recognize specific histone post-translational modifications, remains unknown. These and possibly unknown modifications might increase binding to histones in a combinatorial manner, or even weaken the Asf1-H3/H4 interaction.
Histone chaperone mediated presentation of histones to modification enzymes
Histone chaperones do not merely have a passive role in the histone modification process, but in some cases direct modifications to their histone cargo. This was first observed for the chaperone Hat2, which promotes the histone acetylation activity of Hat1 in the Hat1/Hat2 complex by a mechanism that is not understood [13]. In yeast, both Asf1 and the nucleosome assembly protein 1 (Nap1) related histone chaperone Vps75 associate with and enhance the histone acetyltransferase (HAT) activity of Rtt109 [41, 46, 47]. Specifically, Asf1 enhances Rtt109-mediated acetylation of H3 K56, whereas Vps75 enhances Rtt109 acetylation activity towards H3 K9 and K27 [47], suggesting that different chaperones present histones in different ways. In support, recent biophysical analyses of Vps75 and Nap1 indicate binding to a tetramer of (H3/H4)2 [48], in contrast to the H3/H4 dimer binding of Asf1 [27, 28]. A clever approach, using covalently linked H3/H4 tetramers, showed that Asf1 can only bind uncrosslinked H3/H4 dimers, but Vps75 and Nap1 bind most effectively to crosslinked (H3/H4)2 tetramers [48]. To ascertain that the H3/H4 molecules are canonical tetramers and not two dimers, PELDOR measurements find that spin-spin coupling between H4R45C/H3Q125C-mutated H3/H4 molecules bound to Vps75 correspond to a distance indicative of (H3/H4)2 tetramer formation. These experiments unequivocally demonstrated that Nap1 and Vps75 bind to a canonical (H3/H4)2 tetramer. Moreover, the Vps75-(H3/H4)2 complex potentiated Rtt109 acetylation of H3 more efficiently than the Vps75-H3/H4 dimer.
How does histone chaperone presentation of histones influence the activity of modification enzymes? Some insight comes from kinetics studies, which found that the Vps75-Rtt109 interaction increases catalytic turnover of substrate [49]. The crystal structure of the complex determined at a resolution of 4.25 Å revealed two Vps75 molecules bound to a single Rtt109 enzyme, but the histone binding cleft was too small to accommodate the H3/H4 tetramer. However, Su and coworkers used 15N, 13C-labeled H3 hydrogen/deuterium exchange coupled to NMR [50] to identify the N-terminal tail of H3 as the site of decreased H/D exchange in the presence of Vps75-Rtt109. This suggested that the catalytic cleft binds to the H3 N-terminus, keeping the (H3/H4)2 tetramer intact. Interestingly, the crystal structure of the Vps752-Rtt109 complex, at a higher resolution of 2.7Å, revealed a conformational change that explains how Vps75 enhances the catalytic activity of Rtt109 [50]. A disordered region in the unbound form of Vps75 (130–175) associates with Rtt109 to generate a binding pocket for the H3 N-terminus, which is the site of the Rtt109-catalyzed acetylation. To expand on how specific chaperones may influence specific epigenetic marks, similar studies with Asf1-Rtt109 would be valuable. Different modes of presentation of the histone to the enzyme would help explain why different lysines are acetylated by Rtt109 when the histones are presented by either Vps75 or Asf1. Together, these results established a mode of histone chaperone-histone binding that in essence alters the presentation of histones to a downstream effector, Rtt109, and influences its specificity and histone acetylation activity.
Conclusions
The dynamic nature of chromatin calls for histone-interacting proteins to serve versatile roles in modulating the many complexes that regulate histone metabolism. Histone chaperones demonstrate such versatility in regulating nucleosome assembly and disassembly. Recent insights offer both structural and epigenetic considerations for the regulation of histone transfer in chromatin dynamics, by going beyond how histone chaperones recognize histones, to how they correctly position them for subsequent pathways. Several structural models of complexes that involve histone chaperones have been solved in the last half-decade, and these provide tantalizing suggestions of how histones are handled by their chaperones. Future research lies in elaborating these hypotheses, and clarifying how histones are shuttled among their many interacting partners. Already, specific post-translational modifications (H3 K56ac, H4 S47ph) have been demonstrated to influence the specificity of these interactions, and their subsequent nucleosome assembly activities. Determining the molecular mechanisms by which these modifications direct histone fate will advance our understanding of chromatin assembly. Furthermore, discoveries of similarly functioning modifications may provide further support for a histone code that regulates direct binding to chaperones.
Acknowledgments
Due to limitations on the number of references, we were unable to directly cite all of the relevant literature, and many relevant references can be found within the cited original research papers and reviews. We are grateful to Sarah Roemer, Jean Scorgie, and Mark Parthun for their helpful suggestions and critical reading of the manuscript. We acknowledge support from NIH GM079154 to MEAC.
References
Full text links
Read article at publisher's site: https://doi.org/10.1042/bst20110737
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3494481?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Article citations
Glutamylation of Npm2 and Nap1 acidic disordered regions increases DNA mimicry and histone chaperone efficiency.
iScience, 27(4):109458, 08 Mar 2024
Cited by: 1 article | PMID: 38571760 | PMCID: PMC10987829
Distinct histone H3-H4 binding modes of sNASP reveal the basis for cooperation and competition of histone chaperones.
Genes Dev, 35(23-24):1610-1624, 24 Nov 2021
Cited by: 8 articles | PMID: 34819355 | PMCID: PMC8653785
The histone chaperone Nrp1 is required for chromatin stability and nuclear division in Tetrahymena thermophila.
Epigenetics Chromatin, 14(1):34, 23 Jul 2021
Cited by: 8 articles | PMID: 34301312 | PMCID: PMC8299592
Mechanistic insights into histone deposition and nucleosome assembly by the chromatin assembly factor-1.
Nucleic Acids Res, 46(19):9907-9917, 01 Nov 2018
Cited by: 49 articles | PMID: 30239791 | PMCID: PMC6212844
Review Free full text in Europe PMC
Dynamic intramolecular regulation of the histone chaperone nucleoplasmin controls histone binding and release.
Nat Commun, 8(1):2215, 20 Dec 2017
Cited by: 12 articles | PMID: 29263320 | PMCID: PMC5738438
Go to all (19) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Protein structures in PDBe (5)
-
(2 citations)
PDBe - 2HUEView structure
-
(1 citation)
PDBe - 2I32View structure
-
(1 citation)
PDBe - 2Z3FView structure
-
(1 citation)
PDBe - 2YBAView structure
-
(1 citation)
PDBe - 2XYIView structure
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Fly Fishing for Histones: Catch and Release by Histone Chaperone Intrinsically Disordered Regions and Acidic Stretches.
J Mol Biol, 429(16):2401-2426, 10 Jun 2017
Cited by: 41 articles | PMID: 28610839 | PMCID: PMC5544577
Review Free full text in Europe PMC
DNAJC9 integrates heat shock molecular chaperones into the histone chaperone network.
Mol Cell, 81(12):2533-2548.e9, 14 Apr 2021
Cited by: 23 articles | PMID: 33857403 | PMCID: PMC8221569
The histone chaperones Nap1 and Vps75 bind histones H3 and H4 in a tetrameric conformation.
Mol Cell, 41(4):398-408, 01 Feb 2011
Cited by: 59 articles | PMID: 21329878 | PMCID: PMC3093613
The histone shuffle: histone chaperones in an energetic dance.
Trends Biochem Sci, 35(9):476-489, 03 May 2010
Cited by: 122 articles | PMID: 20444609 | PMCID: PMC4004086
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NIGMS NIH HHS (2)
Grant ID: GM079154
Grant ID: R01 GM079154




