Abstract
Free full text

Combining Radiotherapy and Cancer Immunotherapy: A Paradigm Shift
Abstract
The therapeutic application of ionizing radiation has been largely based on its cytocidal power combined with the ability to selectively target tumors. Radiotherapy effects on survival of cancer patients are generally interpreted as the consequence of improved local control of the tumor, directly decreasing systemic spread. Experimental data from multiple cancer models have provided sufficient evidence to propose a paradigm shift, whereby some of the effects of ionizing radiation are recognized as contributing to systemic antitumor immunity. Recent examples of objective responses achieved by adding radiotherapy to immunotherapy in metastatic cancer patients support this view. Therefore, the traditional palliative role of radiotherapy in metastatic disease is evolving into that of a powerful adjuvant for immunotherapy. This combination strategy adds to the current anticancer arsenal and offers opportunities to harness the immune system to extend survival, even among metastatic and heavily pretreated cancer patients. We briefly summarize key evidence supporting the role of radiotherapy as an immune adjuvant. A critical appraisal of the current status of knowledge must include potential immunosuppressive effects of radiation that can hamper its capacity to convert the irradiated tumor into an in situ, individualized vaccine. Moreover, we discuss some of the current challenges to translate this knowledge to the clinic as more trials testing radiation with different immunotherapies are proposed.
A clinical partnership of local tumor radiotherapy with immunotherapy is intriguing because radiotherapy is perceived as a generally immunosuppressive modality in the oncology community because of the well-known application of whole-body radiation to ablate the patient’s immune system in preparation for allogeneic transplant (1,2). Conversely, effects of local radiotherapy on tumors are rapidly emerging as opportunities to remodel and enhance immunity against cancer. This emerging role of radiotherapy has several immediate consequences. The first and most obvious to radiation biologists and oncologists is a need to revisit canonical forms of radiation-induced cell death from an immunological perspective. To this end we briefly describe the cross-talk between cancer and the immune system to set the stage to appreciate the effects of ionizing radiation. Second, the research available on the characteristics and kinetics of the specific molecular changes radiation elicits and on how they are sensed by the immune system, both in normal and cancer tissues, must be assessed. Understanding how the balance of these radiation-induced pathways contributes to enhancing vs overcoming tolerance to cancer is relevant to therapeutic applications. Third, the optimal regimens of radiotherapy, in terms of dose/fractionation and optimal sequencing for effective combination with available immunotherapies and establishment of optimal scheduling, must be defined. These three areas of research converge to build the platform of knowledge required for clinical translation.
Cancer and the Immune System: Evolving Cross-Talk
Each stage in the development and progression of cancer is the result of cross-talk between the tumor and the host’s immune system. This process is the subject of several excellent reviews (3–5), and the main points are summarized here. Tissue changes during neoplastic transformation are sensed by the innate immune system. Interferon γ, a key mediator of immunosurveillance against tumors (6,7) is produced by natural killer and γδ T cells to promote the cytotoxic activity of macrophages. The concerted action of these innate immune effectors leads to destruction of the incipient tumor, a process that has been termed elimination phase (8). The cytocidal activity of innate immune cells leads to the release of tumor-associated antigens for cross-presentation by dendritic cells (DCs). DCs take up and process the antigens into peptides that can be loaded into the major histocompatibility complex (MHC) class 1 and 2 molecules and recognized by CD8 and CD4 T cells, respectively. The confluence of proinflammatory cytokines produced by innate immune cells and danger signals generated by stressful death of the neoplastic cells activates tumor-specific T cells to perform antitumor activities (5). If the elimination of transformed cells is incomplete, some of the surviving cells generate escape mutants through genetic instability. This results in a state of equilibrium characterized by a balance between proliferation and killing of tumor cells by T cells (9). The equilibrium phase can maintain the tumor at a subclinical stage for a long time. However, the constant selective pressure of the immune system promotes the emergence of tumor cells that are highly resistant to immune rejection. In fact, resistance to immune rejection has been recognized as an essential requirement for tumors to become clinically detectable (10). This phase, defined as escape from immune control, continues to engage the immune system, which can still play a role in slowing down tumor progression. Clinical confirmation for this process has been reported (ie, in colorectal cancer the degree and quality of T-cell infiltration strongly predict for survival) (11–13). Importantly, although loss of the more immunogenic tumor antigens heralds the escape phase (14,15), neoantigens containing epitopes that can be recognized by T cells are constantly generated by the genomically unstable cancer cells (16). Therefore, interventions such as radiotherapy that promote the release of tumor neoantigens in an immunogenic way, together with strategies to overcome dominant immunosuppressive pathways, offer opportunities to recover effective immune reactivity.
Radiation Response of Tumors: The Role of the Immune System
Exposure to ionizing radiation of normal tissue and tumors has immediate and persisting consequences that span from modest inflammatory changes to distinct forms of programmed cell death. The dose and fractionation applied determines the degree and type of cell death in a tissue-specific fashion (17).
A role of T cells in the tumor response to ionizing radiation was first suggested in 1979 in experiments demonstrating reduced therapeutic efficacy in irradiated mice that lacked a normal T-cell repertoire (18). However, the relationship between radiation-induced tumor cell death and priming of antitumor T-cell responses was only recently elucidated. Several research milestones preceded this step. First, it was demonstrated that cell death is an efficient process to transfer antigens from tumor cells to DCs and that DCs are required to activate tumor-specific T cells (19,20). Moreover, during the past 5 years, a functional redefinition of cell death, based on its effects on immune cells (ie, tolerance or activation) has emerged. Molecular signals required to achieve an “immunogenic cell death” have been established (21,22). To date, they include: 1) cell surface translocation of calreticulin (an endoplasmic reticulum resident protein); 2) extracellular release of high-mobility group protein B1 (HMGB1, a nonhistone nuclear protein), and 3) release of ATP (the primary unit of cellular energy transfer) (23–26). Current evidence indicates that ionizing radiation and some, but not all, commonly used chemotherapy agents successfully induce each of these steps and culminate in immunogenic cell death. Additional or alternative signals and pathways remain an area of active investigation (22). Successful induction of immunogenic cell death also depends on characteristics intrinsic to tumor cells (27) and is modulated by the host’s genetic polymorphism in genes that encode key receptors. For instance, data suggest that patients carrying a Toll-like receptor 4 loss-of-function allele that cannot bind to HMGB1 have a worse outcome after chemotherapy and radiation (23). Likewise, expression of a loss-of-function allele of the purinergic receptor for ATP P2X (7) has been associated with poorer prognosis after treatment (25). However, the exact contribution of immunogenic cell death to the successful results of standard anticancer therapy observed in the clinic remains to be quantified.
With regards to ionizing radiation, additional unique and ubiquitous signals that act as proinflammatory modifiers of the tumor microenvironment have been demonstrated (28,29). For example, chemokines CXCL9, CXCL10, and CXCL16, which promote recruitment of effector CD8 and T-helper 1 CD4 T cells, were induced by radiation in different tumors (30–32). Proinflammatory cytokines induced by radiation include interleukin 1β, tumor necrosis factor α and type 1 and 2 interferons (30,33–36). In addition, tumor cells that receive sublethal doses of radiation undergo phenotypic changes that enhance their susceptibility to immune effectors (37–39). Enhanced expression of death receptors (40,41), MHC class 1 molecules (37,42,43), costimulatory molecules (44), adhesion molecules (45–47), and stress-induced ligands (48–50) on tumor cells exposed to radiation increased their recognition and killing by T cells in vitro and/or in vivo in several cancer models.
By coupling release and/or expression of new antigens with immune adjuvant-like effects, radiotherapy engages both the innate and adaptive arms of the immune system, with the potential to convert the irradiated cancer into an in situ vaccine that elicits tumor-specific T cells. Once vaccinated, the host is endowed with immune memory, a powerful weapon active against synchronous nonirradiated tumor sites and potentially against cancer cells that emerge from dormancy during the life of the host (51).
Such immune memory may explain radiotherapy’s repercussions on the final outcome of irradiated patients. Radiotherapy’s effect on local tumor control is associated with an effect on metastatic recurrence and eventual cancer survival. For instance, two meta-analyses of prospective, randomized trials in breast cancer demonstrated a direct contribution of adjuvant radiotherapy to patients’ long-term survival. The effect was independent of stage and extent of surgery (52,53). This increment in disease survival may derive from successful immunization against the primary tumor once the residual microscopic disease at the tumor bed and involved nodes is irradiated. In this scenario, the resulting immune memory would reject early systemic recurrences if the reactivated dormant cells can still be recognized by the immunized host (54). In this scenario, improved relapse-free survival observed in some patients could reflect a return to a phase of equilibrium between tumor and immune system of the host. Conversely, recurrence could be interpreted as successful escape from immune control (13).
Similarly, the achievement of immunogenic cell death may explain the success of concurrent chemo-radiation. In many solid tumor types, concurrent use of chemotherapy agents with radiation is more effective than their sequential use. Both local control and systemic control are superior with concurrent treatment. Cancer cell immunogenicity might be more likely recovered by concurrent treatment through a mechanism called “repositioning,” a concept proposed by Zitvogel and colleagues (55,56). Radiation and chemical agents may complement each other in fulfilling the requirements for each of the three molecular signals of immunogenic cell death (23–26). We originally proposed an analogous hypothesis in relation to the extensive clinical evidence of inferiority of neoadjuvant cisplatin-based chemotherapy when compared with concurrent cisplatin and radiation in potentially curable cancers. As highlighted by Glynne-Jones and Hoskin (57,58), “[t]he small and meaningless reduction in size of the tumor” when cisplatin is used alone could represent the failure of the drug to induce an immunogenic cell death. We also suggested that a nonimmunogenic death of tumor cells might be promoting immune tolerance to the tumor (58). In view of the recent knowledge about immunogenic cell death, a concurrent use of radiation with cisplatin might complement the intrinsic inability of this drug to induce calreticulin translocation (an effect demonstrated for ionizing radiation), thus changing both quantity and quality of cell death, as supported by preliminary evidence (26,59). Another chemo-radiation combination that involves veliparid, a poly(ADP-ribose) polymerase inhibitor, works by promoting tumor immunogenicity through induction of antitumor T cells responsible for tumor inhibition in preclinical models of melanoma and pancreatic cancer (60).
Finally, the clinical observation that best demonstrates the induction of antitumor immunity by radiotherapy is the abscopal effect (ie, a tumor response in a metastasis outside the irradiated field, after treatment of another tumor site) (61–65). Although it has been reported in multiple tumor types over the years, abscopal effects are infrequent. Their uncommon occurrence reflects existing barriers to successful immunization by radiotherapy.
Why Are Abscopal Effects Uncommon?
Despite the fact that de novo immune responses to tumor-associated antigens occur in patients receiving radiotherapy, which indicates that at least some degree of immunization is detectable following treatment (66,67), radiotherapy per se is generally unable to subvert a patient’s immune tolerance toward the tumor.
Some explanations come from the experience derived from clinical testing of cancer vaccines. A monumental effort in developing effective cancer vaccines has generally failed, with objective tumor regressions in vaccination trials remaining elusive. An overview of 856 patients with metastatic solid cancers treated in multiple early-phase vaccination trials since 2004 showed a response in only 3.7%, based on standardized oncologic response criteria (68). However, a modest increase in survival has been achieved in selected vaccinated cancer patients (69), which demonstrates some ability of the immune system to delay tumor progression (11). The experience with cancer vaccines confirms the ample preclinical evidence of the many barriers in place to counteract the effector phase of immune rejection once tumors are established. Consistently, activation and expansion of T cells specific for the tumor-associated antigen present in the vaccine failed to correlate with tumor response in the human trials (70). In addition, the disappointing results stress the difficulty of selecting the best antigenic targets for any given tumor because it is unlikely that T cells recognizing only one or a few antigens will achieve a therapeutic impact. As mentioned before, tumors express a large number of neoantigens (16), but the antigens that are strongly immunogenic are usually already lost at the time of clinical presentation of the disease, “edited” out when tumors escape immune control (3,14,15). Consistently, the degree of expansion of vaccine-specific T cells generally failed to predict for responders and nonresponders after cancer vaccination. Rather, response depended upon the development of an antigenic “cascade” (ie, the expansion of T cell clones reactive against different tumor antigens than those used for vaccination) (71). Interestingly, radiation promotes antigenic cascade (72,73).
A series of necessary steps for T cells to reject a tumor are required (74,75), including the need to 1) effectively achieve homing within the tumor by extravasating from vessels and infiltrating the tumor microenvironment; 2) retain effector functions; and 3) form stable immunological synapses with their targets. In most tumors, obstacles are present at each of these steps (76,77). Although radiation can promote homing and extravasation of effector T cells at the tumor site and induce the expression of molecules that enhance the recognition of the tumor by T cells (30,32,37,40,42,48), these steps are generally insufficient, thus explaining, in part, the rarity of abscopal effects. A critical concentration of fully functional T cells primed against the tumor is required to achieve immune-mediated tumor rejection in experimental tumor models (78,79). A similarly critical threshold of concentration of CD8 T cells with cytolytic function is likely to be required to effectively inhibit tumor growth in the clinic.
Moreover, a highly suppressive tumor microenvironment that hinders effector T-cell function characterizes established cancers. Cancer cells release immunosuppressive cytokines, such as transforming growth factor β, and express surface receptors with inhibitory function for T cells, such as programmed death ligand 1 (80,81). In addition, myeloid cells populate the tumor microenvironment and promote tumor growth by suppressing T-cell functions. These include M2 macrophages, myeloid-derived suppressor cells, and immature DCs (82–85). CD4 T cells with regulatory function (Treg) also play an important role in inhibiting tumor rejection by direct contact with effector T cells and/or by secretion of immunosuppressive cytokines (86).
Noticeably, radiation has been shown to promote some of these suppressive mechanisms. For instance, radiation activates latent transforming growth factor β (87,88). There is also some evidence that radiation enhances the immunosuppressive and protumorigenic phenotype of macrophages (89,90). Recent data indicate that Treg cells are intrinsically more radio resistant than other T cells and are proportionally increased after radiotherapy (91,92). Although it remains to be determined whether Treg suppressive activity is partially impaired after irradiation (92,93), an increase in Treg cells has been observed in some cancer patients undergoing radiotherapy (66,94).
Overall, the ability of radiotherapy to induce an immune-mediated abscopal effect is likely to depend on its ability to sufficiently alter the preexisting immunosuppressive and tolerogenic tumor environment, with proimmunogenic effects prevailing over immunosuppressive effects (Figure 1). The exact nature of this balance is gradually being elucidated and will directly influence the selection of partnering immunotherapies. In preclinical models, promising results have already been obtained by combining radiotherapy with targeted interventions to break tolerance by either enhancing the function of antigen-presenting cells and/or activating T cells (Table 1). As discussed below, some of these strategies are finding their way to the clinic.
Table 1.
Combinations of immunotherapy and local radiotherapy tested in preclinical tumor models*
| Immunotherapy | Schedule of administration | Radiation effect relevant to the immune system | Detected immunomodulation | Reference(s) |
|---|---|---|---|---|
| Flt3-ligand | Postradiation | Release of tumor antigens | Induction of antitumor T cells | 97, 98 |
| Exogenous DCs, s.c. or i.v. | Postradiation | Recruitment of DCs and release of tumor antigens | Induction of antitumor T cells | 99 |
| Exogenous DCs, i.t. | Postradiation | Release of tumor antigens | Induction of antitumor T cells | 100, 101 |
| CpG, s.c. peritumorally, and i.t. | Pre- and postradiation | Release of tumor antigens | Induction of antitumor T cells | 102, 103 |
| Synthetic modified TLR-9 agonist, s.c. | Concomitant with and postradiation | Release of tumor antigens | Recruitment and activation of NKDCs | 104 |
| ECI301 (CCL3 variant), i.v. | Postradiation | Release of tumor antigens | Induction of antitumor T cells | 105 |
| Anti-CTLA-4 antibody, i.p. | Postradiation | Release of tumor antigens | Induction of ant-tumor T cells | 107, 108 |
| Induction of CXCL16 release | Improved recruitment of CCXR6+ effector CD8 T cells | 31 | ||
| Induction of NKG2D ligand expression on tumor cells | Stable interaction between NKG2D+ effector CD8 T and tumor cells | 50 | ||
| Anti-CD137 antibody, i.v or i.p. | Postradiation | Release of tumor antigens and/or MHC class 1 induction on tumor cells | Induction of antitumor T cells. | 109, 110 |
| Anti-CD137 and anti-PD-1 antibodies, i.p. | Concomitant with and postradiation | Release of tumor antigens | Induction of antitumor T cells | 111 |
| Adoptive T-cell transfer | Postradiation | Induction of Fas/CD95 on tumor cellsUpregulation of MHC class 1 on tumor cells | Improved killing of tumor cells by adoptively transferred effector CD8 T cells | 4042 |
| Vaccinia and avipox recombinants expressing CEA and T-cell costimula tory molecules | Pre- and postradiation | Induction of Fas/CD95 on tumor cells | Improved killing of tumor cells by vaccine-induced T cells, induction of antigenic cascade | 113 |
| Autologous tumor cell vaccine expressing GM-CSF | Postradiation | Upregulation of MHC class 1 on tumor cells | Improved killing of tumor cells by vaccine-induced T cells | 43 |
* CEA = carcinoembryonic antigen; CpG = C-G enriched, synthetic oligodeoxynucleotide; CTLA-4 = cytotoxic T lymphocyte–associated antigen 4; DC = dendritic cell; GM-CSF = granulocyte-macrophage colony-Ctimulating factor; i.p. = intraperitoneally; i.t. = intratumorally; i.v. = intravenously; MHC = major histocompatibility complex; NKDC = natural killer dendritic cell; PD-1 = programmed death 1; s.c. = subcutaneously; TLR = Toll-like receptor.
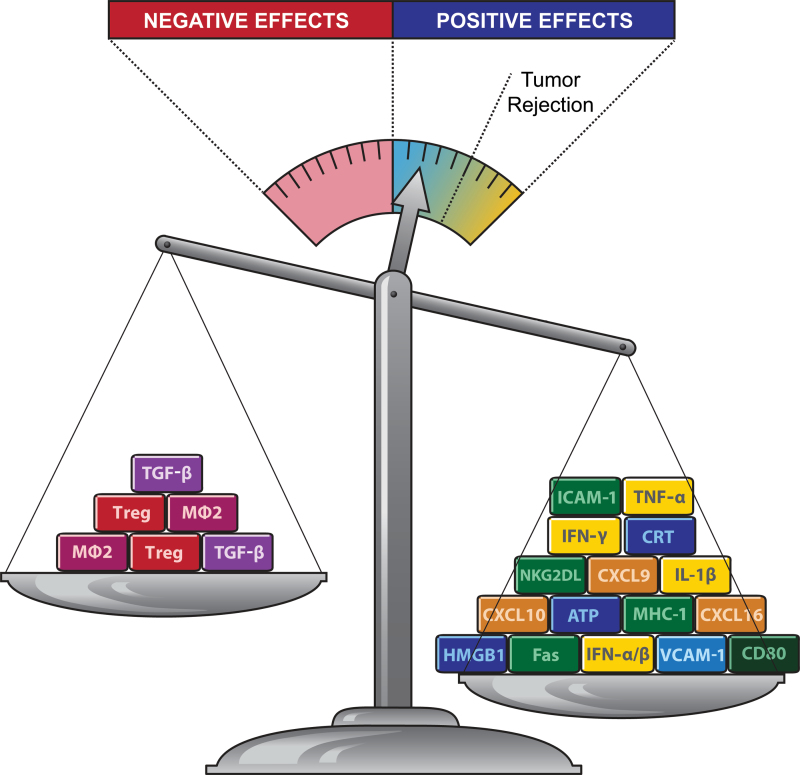
The balance between proimmunogenic and immunosuppressive effects of radiotherapy and tumor rejection. Radiation promotes the priming and effector phases of the antitumor immune response. Key molecular signals that promote priming of antitumor T cells by dendritic cells loaded with tumor antigens include exposure of calreticulin (CRT) and release of ATP and high-mobility group protein B1 (HMGB1). These signals are released by the tumor cells undergoing a radiation-induced immunogenic cell death and, together with interleukin 1β (IL-1β) lead to activation of tumor-specific T cells. Key molecular signals that promote the effector phase include the upregulation of chemokines CXCL9, CXCL10, and CXCL16, which attract activated T cells to the tumor. Tumor infiltration by T cells that produce interferon γ (IFN-γ) and tumor necrosis factor α (TNF-α) is facilitated by upregulation of vascular cellular adhesion molecule 1 (VCAM-1) on tumor endothelium. Radiation-induced upregulation of major histocompatibility complex class 1 (MHC-1), NKG2D ligands (NKG2DL), intercellular adhesion molecule 1 (ICAM-1), death receptor Fas, and costimulatory molecule CD80 on surviving tumor cells improves their recognition and killing by T cells. On the other hand, radiation activates immunosuppressive transforming growth factor β (TGF-β) cytokine and promotes accumulation of regulatory T cells (Treg) and protumorigenic M2 macrophages (MØ2). Data suggest that positive effects of radiation often predominate over negative ones but are insufficient to shift the balance of the immunosuppressive tumor microenvironment to achieve tumor rejection in the absence of targeted immunotherapy.
Characteristics and Kinetics of Successful Preclinical Combinations of Radiotherapy and Immunotherapy
Almost four decades ago, Milas et al. (95) explored in a preclinical model the concept of stimulating the immune system by administration of bacteria to improve the antitumor effects of local radiotherapy (95). In 2005, we proposed the concept of harnessing radiotherapy to help immunotherapy (96). Despite initial incredulity, during the following years, the number of preclinical studies that have reported successful results by combining local radiation and immunotherapy has steadily increased (Table 1). Although radiation has multiple effects that impact both the priming and effector phase of antitumor immune responses, from a practical point of view, it may be useful to discuss separately the studies designed to exploit one aspect over the other.
Based on the rationale that radiation generates an in situ vaccine at the tumor site, some studies have tested its combination with strategies to improve cross-priming of antitumor T cells. This was achieved by enhancing the number and function of DCs with the administration of DC growth factors or by injecting exogenously prepared DCs into or near the irradiated tumor (97–101). Administration of the DC growth factor Flt3-ligand to mice after they had received tumor radiotherapy showed the induction of antitumor T cells able to inhibit spontaneous metastases in a lung carcinoma model (97). Similarly, an abscopal effect was seen in a mouse model of breast cancer (98). In a mouse sarcoma model, previous tumor irradiation promoted the migration of exogenously prepared syngeneic DCs injected intravenously or subcutaneously near the tumor and promoted development of tumor-specific T cells and tumor regression (99). Intratumoral injection of DCs showed additive and synergistic antitumor effects in mouse models of melanoma and sarcoma, respectively (100), and the ability of this combination to induce effective antitumor immune responses was also reported by Kim et al. (101) in a fibrosarcoma model.
In another approach, Toll-like receptor 9 agonist C-G enriched synthetic oligodeoxynucleotide (CpG) was used to mimic the signals derived from pathogens to induce type 1 interferons and resulted in strong activation of DCs and other innate immune cells in models of fibrosarcoma and lung carcinoma (102–104). In all of these examples, the combination treatment was far more effective than each treatment, radiation or immunotherapy, tested alone. The combination induced a systemically effective antitumor immunity that inhibited metastases. An abscopal effect mediated by T cells was also seen in fibrosarcoma and colon and lung carcinoma when radiation was combined with subsequent administration of ECI301, a synthetic variant of the chemokine CCL3 that improves intratumoral DC accumulation and subsequent priming of antitumor T cells (105).
Strategies aimed at improving the effector phase include T-cell activation with the addition of an antibody targeting the inhibitory checkpoint receptor CTLA-4 (cytotoxic T lymphocyte–associated antigen 4) given after radiotherapy. In syngeneic mice models of cancer, anti-CTLA-4 was effective as single agent only for relatively immunogenic tumors, but it required combination with vaccination for poorly immunogenic tumors that better mimic the clinical setting of cancer (106). With the irradiated tumor functioning as an in situ vaccine, the combination of radiotherapy and anti-CTLA-4 resulted in successful T-cell–mediated antitumor responses, whereas anti-CTLA-4 treatment by itself was ineffective. Inhibition of early lung metastases and responses of bulky tumors outside the radiation field were observed in mice with established poorly immunogenic mammary and colorectal carcinomas (107,108). Key molecular mechanisms of the synergy between radiation and anti-CTLA-4 include the induction in irradiated tumor cells of CXCL16 and the NKG2D ligand retinoic acid early inducible 1 (RAE-1). CXCL16 improved recruitment to the tumor of CXCR6+ effector CD8 T cells (31). The interaction between RAE-1 on tumor cells and NKG2D on effector CD8 T cells was required for arrest of the T cells and antitumor activity (50).
Initial evidence in lung and breast carcinoma and glioma suggests that antibodies that target other checkpoint receptors on T cells and/or costimulatory molecules such as CD137 can also be successfully combined with radiotherapy (109–111), which encourages further testing because the field of immunotherapy based on checkpoint blockade is gaining momentum (112).
Other studies have tested radiotherapy in combination with vaccines or adoptive T-cell transfer. For instance, radiation-induced upregulation of Fas/CD95 in tumor cells synergized with vaccination and adoptive T-cell transfer by improving T-cell killing of colon carcinoma cells (40,113). Notably, vaccination was effective when given before radiation, with boosts following radiation (113). Reits et al. showed that radiation induced the upregulation of MHC class 1 expression, enhancing tumor cell recognition by cytotoxic T cells in vitro. In vivo radiation synergized with adoptive cytotoxic T cells transfer, which caused colon carcinoma regression (42). Similarly, whole brain radiotherapy enhanced MHC class 1 expression in a murine model of invading glioma. In that model, brain radiotherapy before peripheral vaccination with an autologous tumor cell vaccine transduced to secrete granulocyte-macrophage colony-stimulating factor (GM-CSF) resulted in extended survival of the irradiated mice (43).
Translation of Successful Preclinical Combinations to the Clinic
Some of these successful preclinical combinations have shown promise once translated to the clinic. For instance, strategies aimed at improving DC numbers and/or functionality have inspired two clinical trials. Our group tested in the clinic the combination of subcutaneous GM-CSF with local radiotherapy in patients with metastatic solid tumors. In this protocol, radiotherapy was given over 2 weeks, with subcutaneous GM-CSF introduced during the second week and maintained for 14 consecutive days. An abscopal response, defined as a response in any lesion outside the radiotherapy field, was detected in 30% of the patients (Figure 2) (51). Another example comes from a phase I trial of intratumoral injection of autologous DCs in patients with advanced hepatocellular carcinoma 2 days after a single fraction of radiotherapy. A partial response was reported in two of 14 patients (114). In a different study, DCs were injected into sarcomas during fractionated radiotherapy given as neoadjuvant treatment. At surgery, the tumors showed infiltration by T cells, with tumor-specific immune responses demonstrated in nine of 17 patients. Remarkably, at 1-year follow-up, 12 of 17 patients were free from progression of their cancer (115).
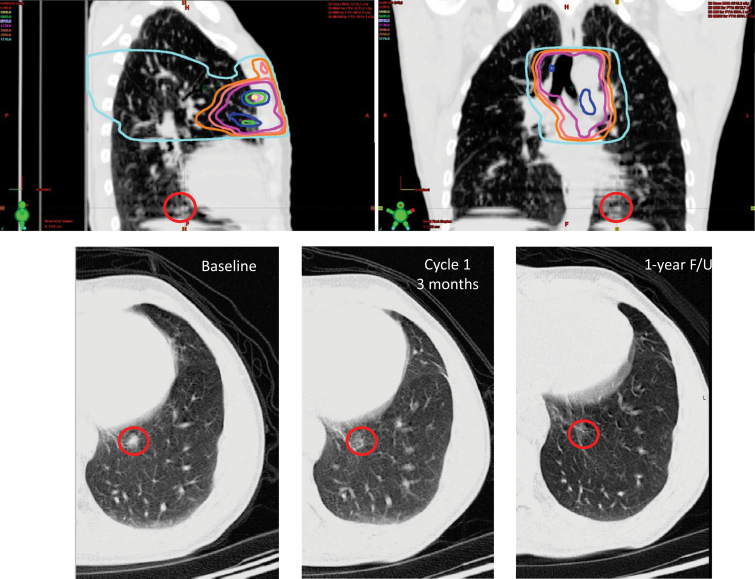
A case of an abscopal response in a patient with metastatic, poorly differentiated thymic carcinoma treated in a trial of granulocyte-macrophage colony-stimulating factor (GM-CSF) and radiation (51). Top panels, computed tomography cuts with dose overlays of radiotherapy to the mediastinum. At the base of the left lung, outside the radiation field, a biopsy-proven metastasis is visualized (red circle). Bottom panels, this lesion regressed after radiotherapy and GM-CSF. Although the patient eventually progressed elsewhere in the body, this area never recurred, and the patient is alive with stable disease 38 months later. F/U = follow-up.
Intratumoral injection of CpG in 15 patients with low-grade B-cell lymphoma receiving radiotherapy to the same tumor site resulted in abscopal responses in nonirradiated tumor sites and development of tumor-reactive CD8 T cells. One complete clinical response and three partial responses were seen (116).
The combination of radiotherapy with ipilimumab, the anti-CTLA-4 antibody approved by the US Food and Drug Administration for treatment of metastatic melanoma patients, has been tested in a phase I/II trial in patients with metastatic castrate-resistant prostate cancer. A single radiation dose of 8 Gy was given before start of ipilimumab treatment and showed the same toxicity profile seen with ipilimumab alone and similar prostate-specific antigen responses (117). Randomized phase III trials are underway to further test the possible benefits of this combination. Importantly, a recent case of an abscopal effect of radiotherapy after clinical progression on ipilimumab in a melanoma patient (118) mimics the synergy demonstrated in preclinical studies of local radiation and anti-CTLA-4 (107,108). In this case report, parallel immunological changes suggested antitumor responses. Notably, radiation was given to this patient in three fractions of 9.5 Gy, a regimen comparable with the radiation regimen (8GyX3) that showed optimal synergy with anti-CTLA-4 therapy preclinically (108).
In a phase II trial, Gulley et al. tested a recombinant poxviral-based vaccine that expressed prostate-specific antigen in combination with standard definitive radiotherapy in patients with localized prostate cancer (72). The first vaccination was performed before radiation, and booster vaccinations were given monthly. The goal of this randomized study was to test feasibility of vaccination in the setting of radiotherapy. The vaccine was given with local GM-CSF and low-dose systemic interleukin 2. T-cell responses to prostate-specific antigen were seen in 13 of 17 patients completing the vaccinations, with evidence of generation of new responses to prostate-associated antigens (antigenic cascade) in patients who received the combination treatment.
Predictably, the optimal sequencing of radiotherapy depends on the type of immunotherapy tested. Clinical protocols testing novel combinations in different tumor systems are necessary to define the best time of radiotherapy in the context of the immunotherapy tested.
Optimal Radiation Regimens With Specific Immunotherapies
The common access to radiotherapy facilities and the large existing experience with the effects of ionizing radiation in cancer and normal tissues facilitate the prospective study of this combination. However, the optimal radiation regimens to be used to harness the proimmunogenic effects of radiation remain to be defined. It is also unclear whether standard radiation doses and fractionations for a given tumor type should be modified if radiation is to be used to convert the tumor into an in situ vaccine (119,120).
For instance, in combination with anti-CTLA-4, we compared different radiation regimens in two carcinoma models growing in syngeneic mice. Marked differences in induction of tumor-specific T cells and of an abscopal effect were found (108). Each regimen had similar ability to inhibit the growth of the irradiated tumor when radiation was used alone. The addition of anti-CTLA-4, however, caused complete regression of the majority of irradiated tumors and an abscopal effect in mice receiving a hypofractionated regimen (8 Gyx3) but not in mice treated with a single dose of 20 Gy. An additional fractionated regimen tested, 6 Gyx5, showed intermediate results, which indicates that a specific therapeutic window may exist for the optimal use of radiotherapy as an immune adjuvant. These results pertain to the combination of radiation and CTLA-4 blockade; whether they can be translated to combinations with other immunotherapy strategies remains to be verified.
It is likely that a threshold exists in terms of the size of the radiation fraction necessary to induce an optimal immune response. Shaue et al. (120) studied the impact of dose size on immune response in a syngeneic murine model of melanoma. Although B16-OVA tumor by itself generated little tumor immunity, doses of 7.5 Gy and greater, but not 5 Gy, were immunostimulatory (120).
Finally, other immune response modifiers (121,122) may be revealed as better partners of radiotherapy to immunize cancer patients. For example, blocking transforming growth factor β in concert with radiotherapy can, in principle, offset its immunosuppressive effects while also counteracting DNA damage repair, angiogenesis, and metastasis (123). This comprehensive strategy is currently being tested in preclinical models and in clinical trials.
Conclusions
A new role for radiotherapy as a valuable partner of cancer immunotherapy is emerging. Preclinical evidence was recently confirmed by clinical objective responses reported in patients with different types of cancers at advanced stage of disease. The optimal immunotherapy to combine with radiotherapy remains to be defined. However, initial responses in the clinic have occurred with diverse immunotherapy approaches, which supports a general role of radiotherapy as a valid adjuvant.
Dose and fractionation are likely to be key variables in determining the effects of ionizing radiation on the immune system of the patients and/or in determining the success of radiotherapy when combined with different forms of immunotherapy. Similarly, the correct sequencing of radiotherapy and immunotherapy depends on the type of immunotherapy chosen.
In any event, radiation effects on the immune system have uncovered a novel application of this modality beyond that of a therapy that merely aims to accomplish local control of tumors, a paradigm shift from its current use in cancer. More than a century after the discovery of radium, ionizing radiation continues to surprise by revealing additional clinical effects and consequences.
Funding
This work was supported by the National Institutes of Health (R01 CA113851; to SD, R01 CA161891 to SCF); the USA Department of Defense Breast Cancer Research Program (BC100481 to SCF and SD); and the Chemotherapy Foundation (to SD).
References
Articles from JNCI Journal of the National Cancer Institute are provided here courtesy of Oxford University Press
Full text links
Read article at publisher's site: https://doi.org/10.1093/jnci/djs629
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3576324?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1093/jnci/djs629
Article citations
Pre-operative stereotactic radiosurgery and peri-operative dexamethasone for resectable brain metastases: a two-arm pilot study evaluating clinical outcomes and immunological correlates.
Nat Commun, 15(1):8854, 14 Oct 2024
Cited by: 0 articles | PMID: 39402027 | PMCID: PMC11473782
Radiation drives tertiary lymphoid structures to reshape TME for synergized antitumour immunity.
Expert Rev Mol Med, 26:e30, 23 Oct 2024
Cited by: 0 articles | PMID: 39438247 | PMCID: PMC11505612
Review Free full text in Europe PMC
Prospects of Synergy: Local Interventions and CAR T Cell Therapy in Solid Tumors.
BioDrugs, 38(5):611-637, 30 Jul 2024
Cited by: 0 articles | PMID: 39080180 | PMCID: PMC11358237
Review Free full text in Europe PMC
Targeting Hepatic Cancer Stem Cells (CSCs) and Related Drug Resistance by Small Interfering RNA (siRNA).
Cell Biochem Biophys, 82(4):3031-3051, 26 Jul 2024
Cited by: 1 article | PMID: 39060914
Review
Use of Radiation Therapy for Ataxia-Telangiectasia Mutated (ATM)-Mutation Metastatic Renal Cell Carcinoma: A Case Report.
Cureus, 16(7):e64781, 17 Jul 2024
Cited by: 0 articles | PMID: 39156348 | PMCID: PMC11329860
Go to all (583) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Combining radiotherapy and immunotherapy: a revived partnership.
Int J Radiat Oncol Biol Phys, 63(3):655-666, 01 Nov 2005
Cited by: 219 articles | PMID: 16199306 | PMCID: PMC1489884
Review Free full text in Europe PMC
[Abscopal effect: Myth or reality?]
Cancer Radiother, 25(6-7):533-536, 28 Aug 2021
Cited by: 2 articles | PMID: 34462213
Review
Synergizing radiation therapy and immunotherapy for curing incurable cancers. Opportunities and challenges.
Oncology (Williston Park), 22(9):1064-70; discussion 1075, 1080-1, 1084, 01 Aug 2008
Cited by: 68 articles | PMID: 18777956 | PMCID: PMC3474236
Review Free full text in Europe PMC
Immune checkpoint inhibitors with radiotherapy and locoregional treatment: synergism and potential clinical implications.
Curr Opin Oncol, 27(6):445-451, 01 Nov 2015
Cited by: 24 articles | PMID: 26447875
Review
Funding
Funders who supported this work.
NCI NIH HHS (2)
Grant ID: R01 CA113851
Grant ID: R01 CA161891
 and
and 




