Abstract
Free full text

The mitochondrial KATP channel – fact or fiction?
Abstract
The mitochondrial ATP-dependent K+ channel (mitoKATP) is widely considered by many to play a central role in cardioprotection by ischemic and pharmacological preconditioning and by ischemic postconditioning. Nevertheless, several laboratories have questioned the existence of mitoKATP. This article summarizes the evidence for and against and addresses two key questions: How strong is the evidence for the presence of a KATP channel in mitochondria? Are the pharmacological agents used to modulate mitoKATP activity sufficiently specific to allow the role of these channels in cardioprotection to be established?
1. Introduction
The presence of electrophoretic pathways for K+ entry into mitochondria and electroneutral K+/H+ antiporter mechanisms for K+ efflux from mitochondria is well established, and together they play a critical role in the regulation of mitochondrial volume and function [1, 2]. Electrogenic proton ejection by the electron transport system (ETS) generates a very high electrical membrane potential (ΔΨ) that drives significant K+ influx by diffusive leak pathways [3] (see diagram in Fig. 1). If present, the mitochondrial ATP-sensitive K+ channel (mitoKATP) would constitute a parallel K+ influx pathway when open (Fig. 1). Its Vmax is estimated to be about the same as K+ influx through the leak pathway. This electrically coupled K+ for H+ exchange will alkalinize the matrix, causing phosphate to enter via the electroneutral Pi - H+ symporter. Net uptake of K+ salts will be accompanied by osmotically obligated water, resulting in matrix swelling. pH and volume regulation of the K+/H+ antiporter leads to increased K+ efflux by this pathway and achievement of a new steady state at slightly higher volume and matrix pH, with important effects on mitochondrial function [1, 2]. The major focus of this article is the mitoKATP channel, whose activation has been proposed to play a central role in ischemic preconditioning (see [4, 5]). One of us (Garlid) is a key proponent of this view whilst the other (Halestrap) is sceptical.
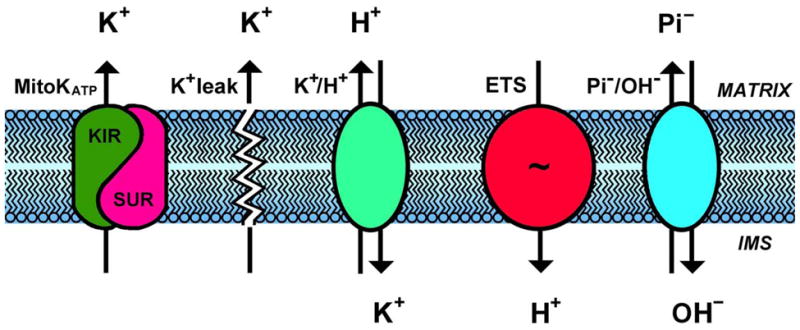
The mitochondrial K+ cycle consists of influx and efflux pathways for K+, H+, and anions. Electrogenic proton ejection by the electron transport system (“ETS”) generates an electrical membrane potential (ΔΨ) that drives K+ influx by diffusion (“K+ leak”) and via the mitochondrial ATP-sensitive K+ channel (mitoKATP). This electrically coupled K+ for H+ exchange will alkalinize the matrix, causing phosphate to enter via the electroneutral Pi/OH-antiporter. Net uptake of K+ salts will be accompanied by osmotically obligated water, resulting in matrix swelling. Excess matrix K+ is then ejected by the regulated K+/H+ antiporter, until a new steady state at higher matrix volume is achieved.
2. The case for (Garlid)
2.1 Development of the mitoKATP story
The reader is referred to reviews of mitochondrial K+ transport that detail the properties of mitoKATP [2, 6] and also address the issues raised here by Dr. Halestrap. Important contributions to this story that cannot be cited here for space reasons will be found in those papers. Following is a summary germane to the issue at hand.
In 1989, we observed that the inner membrane contains an ATP-dependent K+ channel, which we designated mitoKATP. During the next decade, we focused on the biophysical, biochemical, and pharmacological properties of mitoKATP reconstituted in lipid vesicles, measuring flux with the K+ probe PBFI. We introduced the light scattering technique to study mitoKATP-dependent K+ flux in isolated mitochondria.
Both techniques were used in our first paper on the pharmacology of mitoKATP [7]. In this paper, we compared reconstituted cardiac mitoKATP with reconstituted cardiac sarcolemmal KATP and showed that mitoKATP is about 2000 times more sensitive to diazoxide than sarcKATP. It was almost universally presumed at that time that sarcKATP was the mediator of cardioprotection by KATP channel openers (KCO), but we proposed that mitoKATP may be the pharmacological target [7]. Strong evidence for this hypothesis was published in the following year in collaboration with Gary Grover [8]. First, we showed that diazoxide protected the ischemic heart as well as cromakalim. Secondly, we showed that cromakalim shortened action potential duration (APD) through its action on sarcKATP, whereas diazoxide had no effect on APD. These straightforward experiments established the now widely-accepted view that mitoKATP is the key K+ channel involved in cardioprotection. A very important by-product of this finding was the renewal of interest in mitochondria as key players in cardioprotection and cell signalling.
At this point, we faced a conundrum: how is mitoKATP opened physiologically? A major difference between sarcolemmal and mitochondrial KATP channels is that ADP and acyl CoA esters activate sarcKATP, whereas they inhibit mitoKATP. While this explained why mitoKATP is closed under most conditions, it did not explain how it could be opened in the absence of KCO. We found that mitoKATP is activated by a mitochondrial PKCε that is intimately associated with mitoKATP [9]. For this study, we purified liver mitoKATP using detergent extraction of the inner membrane and several steps of ion exchange chromatography, leading to 200-fold purification of activity. After reconstitution into lipid vesicles, K+ flux analysis revealed that addition of the PKC activators 12-phorbol 13-myristate acetate (PMA), hydrogen peroxide, and the PKCε-specific peptide agonist, ΨεRACK, each reversed ATP inhibition of mitoKATP-dependent K+ flux. These effects were prevented by chelerythrine, by the PKCε-specific peptide antagonist, εV1-2, and by the mitoKATP inhibitor 5-hydroxydecanoate (5-HD). Note that the conclusions from these studies were based on both pharmacological and non-pharmacological (PKCε-specific peptides) data. In addition, the activating effect of PKC agonists was reversed by exogenous protein phosphatase 2A. Western blot analysis revealed the presence of PKCε in the reconstitutively active fraction. These results demonstrate persistent, functional association of mitochondrial PKCε and mitoKATP, and show that mitochondrial PKCε is tightly bound to the inner membrane.
More recently, we have shown that mitoKATP is opened via PKCε in isolated mitochondria by signalosomes isolated from perfused hearts treated with various cardioprotective regimes, including ischemic preconditioning, ischemic postconditioning, bradykinin, and ouabain. These new developments have been reviewed [10].
2.2 On the assay of mitoKATP activity by light scattering
The theoretical, experimental, and quantitative basis of the light scattering assay for ion transport in mitochondria were developed 20 years ago [11, 12]. Despite a robust body of evidence in support of its application to mitoKATP, its validity has been questioned by Halestrap and coworkers [13]. These issues were addressed in detail previously [14], and I will summarize just one key point: We used valinomycin to calibrate the response of mitochondria to diazoxide, cromakalim and 5-HD. We then measured the mitoKATP-mediated responses in four different assays - light scattering, matrix alkalinization, K+ influx into the matrix, and increases in matrix reactive oxygen species (ROS). All four assays yielded quantitatively identical results. Importantly, Wojtovich, et al. [15] showed recently that thallium flux through mitoKATP gave the same quantitative result as light scattering. Diazoxide addition to cells or perfused hearts has also been shown to cause mitochondrial swelling in situ [16–18].
Taken together, these results fully validate the light scattering assay of mitoKATP activity. Problems with reproducing the results lie in laboratory technique and have several sources. We have found that mitoKATP is rapidly inhibited during isolation by fatty acids that are continually released by phospholipases. Rapid mitochondrial isolation is perhaps the most critical factor in minimizing this problem [19]. Like most channels, mitoKATP undergoes rundown, and this can be reversed by adding PIP2 [15]. The light scattering assay of mitoKATP activity is successfully being applied in many laboratories around the world. Several scientists have visited our laboratory to master the technique. There is no question that light scattering provides an accurate account of mitoKATP-dependent K+ flux across the inner membrane.
2.3 On the pharmacology of mitoKATP
We have studied a large number of KCO. Each of them opens mitoKATP to exactly the same extent as diazoxide. Moreover, the K+ flux via mitoKATP induced by PKCε activation, by PKG + cGMP, and by various signalosomes was identical to the K+ flux induced by diazoxide. KCO are hydrophobic, and all hydrophobic drugs will affect membrane enzymes. However, these secondary effects do not present a problem, because they are variable. In particular, many KCO are available that open mitoKATP and do not inhibit Complex II (succinate dehydrogenase). This simple pharmacological fact was ignored by those questioning mitoKATP.
Similar statements can be made about 5-HD. 5-HD is a fatty acid used largely for historic purposes in recognition of the work of Gross and coworkers [20]. They were the first to demonstrate that 5-HD and glibenclamide block the cardioprotection of IPC, and showed that 5-HD is an “ischemia-selective KATP antagonist” [20]. In fact, all fatty acids inhibit mitoKATP. 5-HD at 300 μM blocks IPC, but so does 5 μM linoleic acid (unpublished data). Arguments about 5-HD entering the beta oxidation pathway contain no information about how this would block mitoKATP. Moreover, this process is not fast enough - the swelling assay is nearly complete within one minute. Several mitoKATP blockers do not interact with the beta oxidation machinery, including glibenclamide, tetraphenylphosphonium, and a toxin under investigation that inhibits mitoKATP with an IC50 of 25 pM. Arguments about non-mitoKATP effects of 5-HD lack pharmacological perspective and in no way challenge the role of 5-HD as a mitoKATP antagonist.
2.4 On the relationship between mitoKATP and Complex II
We pointed out in 2001 that diazoxide is a partial inibitor of Complex II and also uncouples at high concentrations [21]. This, together with the results of Grimmsmann and Rustenbeck [22], triggered formation of a minor industry focussed on the non-mitoKATP effects of diazoxide [23–28]. The crucial error in the latter studies was the use of diazoxide concentrations much higher than required to open mitoKATP and certain to induce partial Complex II inhibition [21]. The primary focus of these studies was to evaluate the existence and relevance of mitoKATP, and this caused them to miss completely the scientifically interesting aspects of the relationship between mitoKATP and Complex II.
The important facts are (1) that several structurally distinct Complex II inhibitors were found to open mitoKATP and (2) that the concentrations required were orders of magnitude lower than those required to inhibit Complex II [21, 29–32]. Now combine these facts with our finding that both diazoxide and cromakalim open mitoKATP [7]. This is an unexpected result, because it is known that the beta cell KATP (SUR1/KIR6.1) is sensitive to diazoxide and insensitive to cromakalim and related drugs, whereas the cardiac KATP (SUR2A/KIR6.2) exhibits the reverse sensitivity. How is it that mitoKATP combines aspects of both these channels? Wojtovich, et al. [32] have provided a plausible answer by suggesting that Complex II is a important regulator of mitoKATP, such that inhibition of only 0.4% of Complex II causes mitoKATP opening. These findings, together with those of Ardehali, et al. [30], strongly suggest that the very low-abundance mitoKATP is embedded in the inner membrane in association with Complex II and that diazoxide opens mitoKATP by virtue of its interaction with Complex II. It is hoped that further studies will be undertaken to test this interesting and provocative hypothesis.
2.5 Conclusions
Denial of the existence of mitoKATP must take into account that the channel has been found in mitochondria from mammals, C. elegans, plants, trypanosomes, and multiple different tissues within organisms, strongly suggesting that mitoKATP is a fundamental property of mitochondria, and not an artifact. I also conclude that the light scattering assay of mitoKATP activity is sound when performed correctly and that the primary effects of diazoxide and 5-HD on the heart are due to their effects on mitoKATP. The molecular identity of mitoKATP is under active investigation by several laboratories, as is the interesting relationship between mitoKATP and Complex II.
3. Halestrap rebuttal
The majority of the issues raised by Dr Garlid are addressed in “The case against”, but I respond to three points below.
3.1 Methodology
It is not the ability of light scattering (LS) to measure changes in matrix volume that is disputed but whether LS can change independently of matrix volume. Dr Garlid assumes that any LS change, no matter how induced, reflects an equivalent matrix volume change. However, we have shown this is not the case, especially when changes in ANT conformation occur. This is why, unlike Dr Garlid, we routinely make parallel measurements of matrix volume with 3H2O [13, 33]. We also use split-beam spectrophotometry for LS measurements, with drug addition only to the sample cuvette. The reference cuvette then provides real time correction for the substantial drug-independent LS changes.
3.2 Specificity of pharmacology
Dr Garlid and colleagues reported that the K0.5 for mitoKATP opening is 0.5 μM for the reconstituted channel and 0.8 μM for isolated mitochondria [8] but about 30 μM for cardioprotection. Others have reported that 80 μM diazoxide is optimal for cardioprotection [34], and most studies use 50–100 μM. These concentrations cause oxidation of mitochondrial flavoprotein in both isolated myocytes [35] and perfused hearts [36].
3.3 Activation of mitoKATP by protein kinase C ε mediates preconditioning
We found that when purified free of sarcolemmal membranes (no monocarboxylate transporter 1), mitochondria from neither control nor IP hearts contained significant PKCε. Nor were changes in the mitochondrial phosphoproteome detected following IP [37]. Most published studies reporting PKCε translocation to mitochondria failed to quantify the sarcolemmal contamination of mitochondria in this manner.
4. The case against (Halestrap)
4.1 Evidence for the existence of the mitoKATP is unconvincing
In the eighties our research into the hormonal regulation of mitochondrial function in the liver led us to discover small changes in matrix volume mediated by enhance K+ uptake. These studies required the development of highly sensitive measurements of matrix volume that could detect changes as small as 5%. For this purpose we employed light scattering to monitor changes in real time and calibrated this with absolute measurements of matrix water using [14C]-sucrose (inner membrane impermeable) and 3H2O [1]. More recently, in response to the extensive data provided by Dr Garlid and others in support of a mitoKATP channel (see above) we went back to investigate the effects of a range of proposed openers and blockers of the mitoKATP on the matrix volume heart and liver measured using both light scattering and radioisotopic techniques.
We were unable to detect volume changes induced by either blockers such as glibencamide or 5-hydroxydecanoate, or by openers such as diazoxide, cromokalin, pinacidil or nicorandil whilst changes in volume could be detected with valinomycin at concentrations as low as 0.1 nM [13, 33]. Parallel titrations of light scattering changes, matrix volume and rates of respiration with increasing valinomycin concentrations allowed us to conclude that our techniques could detect changes in volume of <5% and of K+ flux of < 5 nmol.mg−1.min−1 [33]. However, in agreement with Garlid and colleagues, we were able to detect increases in light scattering induced by ATP or ADP that would be consistent with inhibition of a mitoKATP channel, but using 3H2O and [14C] sucrose we were unable to detect changes in matrix volume [13]. Furthermore, we could detect similar adenine-nucleotide induced changes in light scattering when mitochondria were incubated in K+-free media [33].
We accounted for these light scattering changes by invoking the well established effect of ATP and ADP to induce the “m” conformation of the ANT. It has been shown by several groups that when the ANT changes from the “c” to the “m” conformation upon addition of ADP or bongkrekic acid, the morphology of heart mitochondria changes from the orthodox to the condensed state and this is accompanied by an increase in light scattering [13, 38–40]. Our conclusion from these studies was that if a specific mitoKATP channel exists, its activity is extremely small and insufficient to cause appreciable changes in matrix volume. We are not alone in these conclusions (see [25, 28, 41]. It has been reported that mKATP activity is lost (“runs down”) quite rapidly in isolated mitochondria [15], but we routinely use mitochondria within 2 hours of preparation. Interestingly, we do observe that the ATP-inhibited decrease in light scattering of heart mitochondria suspended in respiratory medium is lost over time (Halestrap, unpublished data). However, as noted above, these light scattering changes were not associated with any changes in matrix volume as determined isotopically and were modulated by ligands of the ANT suggesting they reflect mitochondrial conformational changes rather than volume changes.
The reconstitution of mitoKATP activity from isolated mitochondrial preparations into proteoliposomes and phospholipids bilayers might be taken as definitive proof of the existence of the channel ([2, 42]. However, mitochondrial preparations are known to be heavily contaminated with plasma membranes unless carefully purified by density gradient centrifugation, and many published studies on the reconstituted mitoKATP did not appear to use such purification steps (see [43]). Thus there is an inherent risk of reconstituting the sarcolemmal KATP channels from these contaminating membranes rather than a real mitoKATP channel. Nevertheless, the pharmacological properties of the two are reported to be slightly different which might argue against this [6].
More recently a novel technique for detecting mitoKATP activity has been described that uses thallium (Tl+) as a surrogate for K+ and detects uptake fluorescently [15]. The pharmacological properties of the mKATP channel determined in this way match those determined by light scattering but the uptake equilibrates within seconds suggesting channel activity is very much faster than predicted by Garlid [21] which casts some doubt on exactly what this technique is measuring.
4.2 The pharmacological agents used to investigate the role of mitoKATP channels in ischemic preconditioning have additional actions that may explain their effects on preconditioning
In order to provide evidence for a key role of the mitoKATP in IP, the most convincing experiments would be to demonstrate a loss of ischemic preconditioning (IP) in mitoKATP knockout mice. However, the absence of a molecular identity for this channel precludes such an approach and the available evidence has come from the use of pharmacological agents that are reported to be specific for the mitoKATP over the well established sarcolemmal KATP channel. The most widely used of these agents are diazoxide as a specific mitoKATP opener and 5-hydroxydecanoate (5-HD) as a specific blocker. Data from Dr Garlid’s laboratory have suggested these to be specific for the mitoKATP channel when used at appropriate concentrations, although others have challenged this specificity (see [6, 25]). Thus there are published data showing that diazoxide can open and 5-HD block the sarcolemmal KATP channel under physiological conditions [35, 44–46]. These KATP channels are strongly expressed in the sarcolemma of the heart and have long been associated with cardioprotection [25, 47]. Indeed, hearts from the Kir 6.2 knockout mice were insensitive to either ischaemic or pharmacological preconditioning [45, 48, 49]. It was originally proposed that opening of these channels might hyperpolarize the cell leading to a shorter action potential duration (APD). This in turn would reduce calcium entry during ischaemia leading to protection of the heart from calcium overload (see [25]). This hypothesis is supported by the observation that mouse hearts whose KATP channel Kir 6.2 had been knocked out exhibited greater calcium overload and ischaemia/reperfusion injury than control hearts [50].
The ability of diazoxide to mimic IP is not disputed; it has been widely documented including in this laboratory [24]. However, there is disagreement over whether or not diazoxide exerts its effects through opening of the mitoKATP channel or through some alternative mechanism. In addition to its possible effects on the sarcolemmal KATP channel described above, diazoxide and other many putative mitoKATP channel openers have also been shown to have non-specific effects on mitochondria, including uncoupling and inhibition of components of the respiratory chain, especially succinate dehydrogenase (see [25, 33]). Since preconditioning can be induced by bona fide uncouplers, SDH inhibitors and other respiratory chain inhibitors, whether applied before or during ischemia (see [51, 52], this may provide another mechanism by which diazoxide and other mitoKATP channel openers exert their effects. This is especially true when high concentrations of such agents are used.
Another well established effect of diazoxide that might account for its ability to precondition is its ability to increase in mitochondrial ROS production, most probably via an effect on succinate dehydrogenase [27, 28, 36]. Since the presence of ROS-scavengers prevents the cardioprotective effects of a variety of preconditioning stimuli, it has been proposed that an increase in ROS production is a critical component of the signal transduction pathway of preconditioning (see [43]). Dr Garlid has proposed that this ROS production is secondary to mitoKATP channel opening leading to increased K+ entry into mitochondria and a small increase in matrix volume [53]. In unpublished studies we have investigated the effects of small (5–20%) increases in matrix volume mediated either by valinomycin (0.1–0.5 nM) or by changes in the osmolarity but have not observed any increase in ROS formation. Furthermore, others have shown that diazoxide-mediated ROS production by isolated mitochondria is independent of the presence of K+ in the medium [27, 28]. Taken together, these data do not provide strong support for the preconditioning effect of diazoxide being mediated by opening the mitoKATP. An effect of diazoxide on succinate dehydrogenase activity to increase ROS production that in turn signals preconditioning would seem to be an equally strong, if not stronger hypothesis [28].
The situation is no better when data obtained using the proposed mitoKATP channel blocker 5-HD are considered. First, not all groups observe an attenuating effect of 5-HD on cardioprotection mediated by IP or diazoxide, especially when hemodynamic performance of the heart is used to assess recovery after ischaemia/reperfusion [24, 54]. Second, measurement of the matrix volume of mitochondria isolated from 5-HD treated hearts showed 5-HD to increase rather than decrease the volume, the opposite of might be expected for a mitoKATP channel blocker [24]. Third, the specificity of 5-HD for the mitoKATP channel over the sarcolemmal KATP channel is disputed (see [25]) and the drug could be exerting effects via inhibition of the latter whose knockout has been shown to prevent IP as discussed above. Fourthly, 5-HD can be activated to its CoA derivative and then further metabolised through the oxidation pathway. As such it can act as a poor respiratory substrate under substrate-deprived conditions while at the same time inhibiting oxidation of normal fatty acids (see [24, 25]). Such inhibition occurs because in the penultimate step of oxidation 3,5-dihydroxydecanoyl-CoA competes strongly with the normal physiological 3-hydroxyacyl-CoA for oxidation by l-3-hydroxyacyl-CoA dehydrogenase, but its oxidation has much slower kinetics [24, 55]. In the perfused heart application of 5-HD decreases flavoprotein fluorescence, consistent with its use as a respiratory substrate [36].
4.3 Conclusions
Our inability to detect mitoKATP channel activity in isolated heart mitochondria and the non-specific effects of the drugs used to study it lead us to conclude that the mitoKATP channel is not involved in preconditioning. Rather, the effects of agents such as diazoxide to mimic preconditioning may be best explained by their ability to inhibit succinate dehydrogenase, and possibly other components of the respiratory chain, leading to increase ROS production which then signals preconditioning via protein kinase C activation. Downstream of PKC activation opening of the mitochondrial permeability transition pore at reperfusion is inhibited, most probably mediated by decreased oxidative stress (see [43]).
5. Garlid rebuttal
5.1 Methodology
Considerable and unavoidable error resides in the dual labeling protocols to measure matrix water, as described by Dr Halestrap. During an investigation of the structure of water in mitochondria [56], we carried out extensive studies using these techniques, and a brief look at that paper will illustrate the painstaking care needed for accurate measurements. MitoKATP opening may lead to a volume increase as low as 5%. Our error analysis indicates that it is not possible to achieve this precision using the dual labeling technique with small amounts of mitochondria.
Dr Halestrap’s laboratory routinely uses mitochondria within 2 hours of preparation. We see no mitoKATP activity 2 hours after isolation. Following is a technique that we use for training new laboratory personnel and that generally leads to success. We did not use it in published work, so it is presented here for the first time. When the final pellet has been resuspended to about 20 mg/ml, add 10 μM bromoenol lactone (BEL) and incubate for 1 min. Then bring up the suspension to 30 ml with sucrose containing 0.5% BSA, to remove fatty acids, and reisolate.
5.2 Conformational change
The putative morphological change occuring on addition of ATP or ADP is an artifact. It arises from the fact that most laboratories, including our own, add ATP as a 1 M solution. When added before the mitochondria, or infused slowly, ATP has no effect on light scattering (K+-free medium). When added after mitochondria, there is an immediate, significant drop in absorbance. This effect is seen when any polyanion is added to mitochondria from a high-concentration stock. It is most likely due to aggregation in the vicinity of the bolus of polyanion.
5.3 Mitochondrial PKCε
There are two objections to the comment that heart mitochondria contain no “significant” PKCε. First, mitochondrial PKCε has been reported by numerous laboratories, beginning with Baines, et al. [57]. Second, what is meant by “significant”? There is clearly a small PKCε band in the Western blots reported by Halestrap, et al. [43], consistent with what we observed prior to purification [9]. The molar amounts of mitoKATP and MPT are vanishingly small, and so too, presumably, are the molar amounts of their associated PKCεs. There is clearly adequate PKCε to do the job.
6. Overall conclusion
The two authors share the conviction that transport of K+ across the inner mitochondrial membrane is an important and tightly regulated process that determines mitochondrial matrix volume and influences mitochondrial function. They also agree that mitochondria play a critical role in reperfusion injury and as a target for cardioprotection. The points of difference relate to how the two processes are connected. More specifically there is disagreement on the strength of evidence for an active mitoKATP channel and the specificity of the openers and blockers of this channel in relation to their effects on cardioprotection. The problem with the arguments against mitoKATP is that they focus only on nonspecific effects of one particular compound or they require a different claim of an artifact for each of the many different techniques and methods used to support the existence of mitoKATP. The problem with the argument for mitoKATP is that there is a shortage of potent compounds to activate or inhibit the channel that also do not affect the sarcolemmal channels. A major problem is that the molecular identity of mitoKATP has not yet been determined. Fortunately, several excellent laboratories are working toward this goal, and a positive result will finally remove the uncertainty over whether or not the mitoKATP channel exists. It will then become possible to use genetic ablation and over-expression techniques rather than the less specific pharmacological interventions to establish what role the mitoKATP channel plays in preconditioning.
Acknowledgments
Dr Garlid’s research was supported in part by grants HL067842 and HL36573 from the National Heart, Lung and Blood Institute. Work in Dr Halestrap’s laboratory has been supported for many years by The British Heart Foundation.
References
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.yjmcc.2011.12.011
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3617982?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Article citations
Redox Regulation of Mitochondrial Potassium Channels Activity.
Antioxidants (Basel), 13(4):434, 03 Apr 2024
Cited by: 1 article | PMID: 38671882 | PMCID: PMC11047711
Review Free full text in Europe PMC
Mitochondrial Cation Signalling in the Control of Inflammatory Processes.
Int J Mol Sci, 24(23):16724, 24 Nov 2023
Cited by: 2 articles | PMID: 38069047 | PMCID: PMC10706693
Review Free full text in Europe PMC
Immunohistochemical, pharmacovigilance, and omics analyses reveal the involvement of ATP-sensitive K+ channel subunits in cancers: role in drug-disease interactions.
Front Pharmacol, 14:1115543, 25 Apr 2023
Cited by: 4 articles | PMID: 37180726 | PMCID: PMC10167295
Ischemic Tolerance-A Way to Reduce the Extent of Ischemia-Reperfusion Damage.
Cells, 12(6):884, 13 Mar 2023
Cited by: 2 articles | PMID: 36980225 | PMCID: PMC10047660
Review Free full text in Europe PMC
Kir6.1 and SUR2B in Cantú syndrome.
Am J Physiol Cell Physiol, 323(3):C920-C935, 25 Jul 2022
Cited by: 13 articles | PMID: 35876283 | PMCID: PMC9467476
Review Free full text in Europe PMC
Go to all (51) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Essential role of mitochondrial Ca2+-activated and ATP-sensitive K+ channels in sildenafil-induced late cardioprotection.
J Mol Cell Cardiol, 44(1):105-113, 16 Oct 2007
Cited by: 52 articles | PMID: 18021798
MCC-134, a single pharmacophore, opens surface ATP-sensitive potassium channels, blocks mitochondrial ATP-sensitive potassium channels, and suppresses preconditioning.
Circulation, 107(8):1183-1188, 01 Mar 2003
Cited by: 21 articles | PMID: 12615799 | PMCID: PMC3680097
Selective pharmacological agents implicate mitochondrial but not sarcolemmal K(ATP) channels in ischemic cardioprotection.
Circulation, 101(20):2418-2423, 01 May 2000
Cited by: 148 articles | PMID: 10821820
The Slo(w) path to identifying the mitochondrial channels responsible for ischemic protection.
Biochem J, 474(12):2067-2094, 09 Jun 2017
Cited by: 23 articles | PMID: 28600454 | PMCID: PMC5568769
Review Free full text in Europe PMC
Funding
Funders who supported this work.
British Heart Foundation
NHLBI NIH HHS (4)
Grant ID: P01 HL036573
Grant ID: HL067842
Grant ID: HL36573
Grant ID: R01 HL067842




