Abstract
Aim
To identify the risk factors in children under five years of age for severe acute lower respiratory infections (ALRI), which are the leading cause of child mortality.Methods
We performed a systematic review of published literature available in the public domain. We conducted a quality assessment of all eligible studies according to GRADE criteria and performed a meta-analysis to report the odds ratios for all risk factors identified in these studies.Results
We identified 36 studies that investigated 19 risk factors for severe ALRI. Of these, 7 risk factors were significantly associated with severe ALRI in a consistent manner across studies, with the following meta-analysis estimates of odds ratios (with 95% confidence intervals): low birth weight 3.18 (1.02-9.90), lack of exclusive breastfeeding 2.34 (1.42-3.88), crowding - more than 7 persons per household 1.96 (1.53-2.52), exposure to indoor air pollution 1.57 (1.06-2.31), incomplete immunization 1.83 (1.32-2.52), undernutrition - weight-for-age less than 2 standard deviations 4.47 (2.10-9.49), and HIV infection 4.15 (2.57-9.74).Conclusion
This study highlights the role of the above seven risk factors in the development of severe pneumonia in under-five children. In addition, it emphasizes the need for further studies investigating other potential risk factors. Since these risk factors are potentially preventable, health policies targeted at reducing their prevalence provide a basis for decreasing the burden of childhood pneumonia.Free full text

Risk factors for severe acute lower respiratory infections in children – a systematic review and meta-analysis
Abstract
Aim
To identify the risk factors in children under five years of age for severe acute lower respiratory infections (ALRI), which are the leading cause of child mortality.
Methods
We performed a systematic review of published literature available in the public domain. We conducted a quality assessment of all eligible studies according to GRADE criteria and performed a meta-analysis to report the odds ratios for all risk factors identified in these studies.
Results
We identified 36 studies that investigated 19 risk factors for severe ALRI. Of these, 7 risk factors were significantly associated with severe ALRI in a consistent manner across studies, with the following meta-analysis estimates of odds ratios (with 95% confidence intervals): low birth weight 3.18 (1.02-9.90), lack of exclusive breastfeeding 2.34 (1.42-3.88), crowding – more than 7 persons per household 1.96 (1.53-2.52), exposure to indoor air pollution 1.57 (1.06-2.31), incomplete immunization 1.83 (1.32-2.52), undernutrition – weight-for-age less than 2 standard deviations 4.47 (2.10-9.49), and HIV infection 4.15 (2.57-9.74).
Conclusion
This study highlights the role of the above seven risk factors in the development of severe pneumonia in under-five children. In addition, it emphasizes the need for further studies investigating other potential risk factors. Since these risk factors are potentially preventable, health policies targeted at reducing their prevalence provide a basis for decreasing the burden of childhood pneumonia.
Pneumonia is one of the leading causes of morbidity and mortality globally in young children aged below five years (1). It is estimated that approximately 156 million cases of pneumonia occur annually in young children, resulting in approximately 1.4 million deaths (2,3). Over the past two decades, there were several attempts to investigate an association between various risk factors and pneumonia in young children, but no systematic reviews of published literature assessed the strength of association between the suspected risk factors and pneumonia. In this study, we aimed to assess the quality of available evidence and present summary estimates of the strength of association between the risk factors and severe pneumonia in children using meta-analysis.
Methods
We systematically reviewed all literature published from January 1, 1990 through March 31, 2012 to identify studies with data on risk factors for pediatric pneumonia. We searched a variety of databases-Medline (Ovid), Embase, CINAHL and Global Health Library using combinations of key search terms: pneumonia, low birth weight, undernutrition, breast feeding, crowding, smoking, indoor air pollution, immunization, HIV etc. (full search terms are available in Supplementary material). Hand searching of online journals was also performed by examining the reference lists for relevant articles. We did not apply any language or publication restrictions. Relevant full-text articles in foreign language were translated to English using Google translator.
We defined an episode of severe pneumonia in hospital setting as any child hospitalized overnight with an admission diagnosis of pneumonia or bronchiolitis. In community-based studies, the presence of lower chest wall indrawing in a child with cough and difficulty breathing with increased respiratory rate for age was used to define a case, using the same cut off values as in the WHO's case definition (4,5). We recognized that the eligible studies used varying case definitions for the putative risk factors. We therefore grouped the risk factor definitions into categories and analyzed the association between risk factor and outcome for each of these categories (Table 1). We classified the risk factors into three groups based on the consistency and strength of association with severe ALRI:
Table 1
Case definitions for the risk factors for severe acute lower respiratory infections (ALRI) used in the retained studies
| Risk factor | Definition |
|---|---|
| Low birth weight | Birth weight <2.5 kg irrespective of gestational age |
| Breastfeeding | 1) Lack of exclusive breastfeeding 2) No breastfeeding 3) Less than 4 mo exclusive breastfeeding |
| Crowding | 1)>7 people per household 2)>2 people sharing child’s bedroom 3)>2 people per room |
| Indoor air pollution | Use of biomass fuels for cooking or a description of indoor smoke |
| Undernutrition | 1) Weight for age <2 standard deviations (SD) 2) Height for age <2 SD 3) Weight for height <2 SD |
| Incomplete immunization | Lack of immunization for measles in a child aged >11 mo |
| HIV | Confirmed presence of HIV in a child |
| Passive smoking | 1) Smokers in house 2) Maternal smoking |
| Maternal education | No maternal education |
| Sex | Being male |
| Vitamin D deficiency | Presence of rickets |
| Preterm birth | Birth before 37 weeks gestation |
| Anemia | Mostly serum hemoglobin <11 g/dL |
| Zinc | Protection offered by zinc supplementation |
| Daycare center attendance | Attendance at daycare center |
| Birth interval | Birth interval <24 mo |
| Previous pregnancy | >3 previous pregnancies |
| Previous illness | Previous history of ALRI |
| Vitamin A deficiency | Serum retinol <0.7 µmol/L |
(i) those that consistently (ie, across all identified studies) demonstrated an association with severe ALRI, with a significant meta-estimate of the odds ratio, would be classified as “definite”;
(ii) those demonstrating an association in the majority (ie, in more than 50%) of studies, with a meta-estimate of the odds ratio that was not significant, would be classified as “likely;” and
(iii) those that were sporadically (ie, occasionally) reported as being associated with severe ALRI in some contexts were classified as “possible.” This classification is consistent with the one originally used by Rudan et al (2).
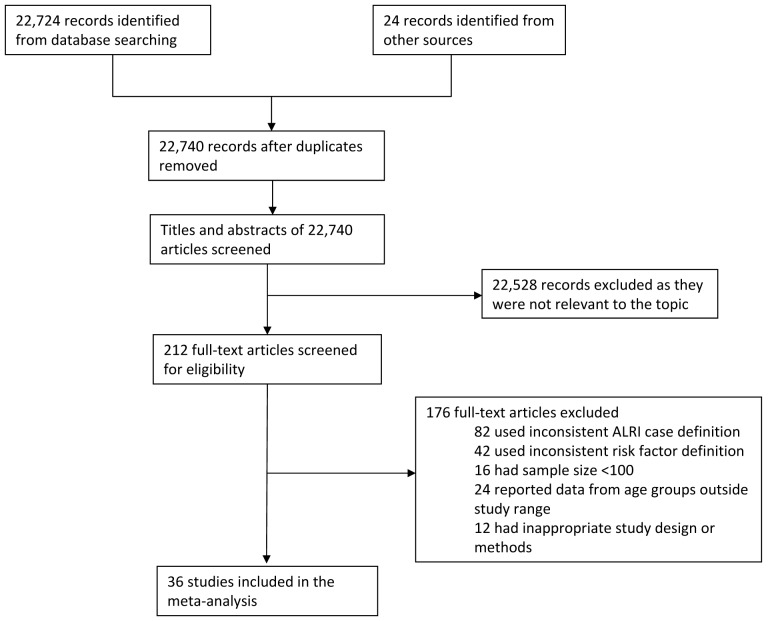
We included studies that reported severe pneumonia in children under five years of age (Table 2). Eligible study designs included randomized control trials (RCTs), observational studies (cohort, case-control, or cross-sectional) that assessed the relationship between severe pneumonia in children and any one of the putative risk factors. Studies were excluded if their sample size was less than 100 detected cases, if their case definitions did not meet our broad range of case definitions, or if the case definitions were not stated clearly and/or not consistently applied (Figure 1). Studies where health care workers went house to house to identify cases of pneumonia were considered as having active community-based case ascertainment. By contrast, studies where children with pneumonia presented to a health facility were considered as having passive hospital-based case ascertainment.
Table 2
Criteria for inclusion and exclusion of the reviewed studies
| Inclusion criteria | Exclusion criteria |
|---|---|
| • Studies on children hospitalized (overnight) with an admission diagnosis pneumonia and bronchiolitis odds ratio presence of lower chest wall indrawing in a child with cough and difficulty breathing with increased respiratory rate for age | • Case definitions not clearly stated or inconsistently applied |
| • Risk factors defined as stated in Table 1 | • Inappropriate control population – eg, children hospitalized for acute medical conditions (other respiratory conditions, diarrhea, meningitis, sepsis, etc.) |
| • Studies with sample size ≥100 | • Study designs – surveys or case series |
| • Studies in children aged below five years | • Methods for statistical analysis not clearly reported |
| • Study designs – randomized control trials or observational studies (case-control or cohort) | |
| • Studies reporting results using univariate or multivariate analysis |
The included studies used either multivariate or univariate analyses to report the association between the putative risk factors and the outcome, ie severe pneumonia. Since the multivariate design takes into account the interaction with other risk factors and potential confounders, we decided to report the results of the meta-analysis of these data separately. We decided that if there was significant heterogeneity in the data, ie, I2>80%, (corresponding to P <
< 0.005) (6), then we would report the meta-estimates from the random effects model (7). Importantly, we hypothesized that the effects of the risk factors were likely to be different in developing countries and industrialized countries. Because of this, we decided to report the results separately for developing (Table 3) and industrialized countries (Table 4). We extracted all relevant information from each retained study (Supplementary Table S2(supplementary Table 2)) and assessed the quality of included studies using a modified GRADE scoring system (Supplementary Tables S1(supplementary Table 1)) (8). Briefly, we assessed each article against the GRADE criteria and calculated the overall score for each article. We then calculated the cumulative score for each risk factor after accounting for the included studies (Supplementary Table S3(supplementary Table 3)). We used Stata 11.2 (StataCorp, College Station, TX, USA) for the meta-analysis (Figure 2; Supplementary Figure S1(supplementary Figure 1)).
0.005) (6), then we would report the meta-estimates from the random effects model (7). Importantly, we hypothesized that the effects of the risk factors were likely to be different in developing countries and industrialized countries. Because of this, we decided to report the results separately for developing (Table 3) and industrialized countries (Table 4). We extracted all relevant information from each retained study (Supplementary Table S2(supplementary Table 2)) and assessed the quality of included studies using a modified GRADE scoring system (Supplementary Tables S1(supplementary Table 1)) (8). Briefly, we assessed each article against the GRADE criteria and calculated the overall score for each article. We then calculated the cumulative score for each risk factor after accounting for the included studies (Supplementary Table S3(supplementary Table 3)). We used Stata 11.2 (StataCorp, College Station, TX, USA) for the meta-analysis (Figure 2; Supplementary Figure S1(supplementary Figure 1)).
Table 3
Meta-estimates for risk factors for severe acute lower respiratory infections (ALRI) in children aged below 5 y in developing countries (meta-estimates for all risk factors except HIV are from multivariate analyses only)
| Risk factor | No. of studies included | Meta-estimate (95% CI) | P value from meta analysis | I2 for heterogeneity (%) |
|---|---|---|---|---|
| Low birth weight | 3 | 3.6 (0.8-16.3) | <0.005 | 96.4 |
| Breastfeeding | ||||
| lack of exclusive breastfeeding | 8 | 2.7 (1.7-4.4) | <0.005 | 87.5 |
| no breastfeeding | 4 | 2.8 (1.0-7.7) | <0.005 | 91.5 |
| <4 mo breastfeeding | 4 | 2.6 (1.6-4.4) | <0.005 | 79.0 |
| Crowding | ||||
| crowding (>7 persons/household) | 2 | 1.9 (1.5-2.5) | 0.829 | 0 |
| crowding (>2 persons sharing child’s bedroom) | 4 | 2.2 (1.8-2.7) | 0.475 | 0 |
| indoor air pollution | 5 | 1.6 (1.6-2.3) | <0.005 | 81.5 |
| Undernutrition | ||||
| weight for age <2 standard deviation (SD) | 6 | 4.5 (2.1-9.5) | <0.005 | 84.7 |
| height for age <2 SD | 2 | 4.8 (3.7-6.1) | 0.819 | 0 |
| weight for height <2 SD | 4 | 2.8 (1.8-4.3) | 0.051 | 61.4 |
| incomplete immunization | 5 | 1.8 (1.3-2.5) | 0.021 | 65.4 |
| HIV* | 2 | 4.6 (2.2-9.7) | <0.005 | 90.2 |
| Passive smoking | ||||
| smokers in house | 4 | 2.5 (0.8-8.0) | <0.005 | 97.9 |
| maternal smoking | 3 | 2.7 (1.0-7.8) | <0.005 | 84.4 |
| No maternal education | 5 | 1.9 (1.0-3.7) | 0.011 | 69.4 |
| Sex (being male) | 6 | 1.5 (1.0-2.3) | <0.005 | 89.9 |
| Vitamin D deficiency (rickets) | 3 | 7.3 (2.5-21.5) | 0.002 | 83.6 |
| Preterm births | 2 | 1.3 (0.8-2.1) | 0.582 | 0 |
| Zinc deficiency | 2 | 0.5 (0.3-0.9) | 0.250 | 24.4 |
| Day care attendance | 2 | 8.0 (3.6-17.7) | 0.188 | 42.2 |
| Birth interval <24 mo | 2 | 1.4 (0.9-2.2) | 0.765 | 0 |
| Birth order ≥3 (previous pregnancies >3) | 2 | 3.0 (2.0-4.6) | 0.788 | 0 |
| History of ALRI | 4 | 2.3 (1.8-3.1) | 0.372 | 4.2 |
Table 4
Meta-estimates for risk factors for severe acute lower respiratory infections (ALRI) in children aged below 5 y in industrialized countries (meta-estimates are from multivariate analyses only)
| Risk factor | No. of studies included | Meta-estimate (95% confidence interval) | P value from meta analysis | I2 for heterogeneity (%) |
|---|---|---|---|---|
| Breastfeeding | ||||
| Lack of exclusive breastfeeding | 2 | 1.3 (0.2-8.4) | <0.005 | 88.9 |
| No breast feeding | 2 | 1.3 (0.2-8.4) | <0.005 | 88.9 |
| Crowding | ||||
| Overcrowding (>2 people per room) | 2 | 2.3 (1.3-4.0) | 0.411 | 0 |
| Passive smoking | ||||
| Smokers in house | 2 | 2.1 (1.3-3.6) | 0.733 | 0 |
| Undernutrition | ||||
| Height for age <2 standard deviation (SD) | 2 | 1.3 (0.5-3.4) | <0.005 | 91.3 |
Received: March 9, 2013
Accepted: April 10, 2013
Results
Our initial literature search yielded 22 740 studies with suitable data (Figure 1). After screening of the titles and abstracts and a subsequent full text review against our eligibility criteria, we identified 36 studies with suitable data. Twenty seven studies reported odds ratios using multivariate analysis and 25 studies reported odds ratios using univariate analysis. Studies reporting the association between HIV and vitamin A deficiency and severe ALRI reported risk estimates using only univariate analysis. The quality of studies reporting the strength of association between risk factors (exposure to low birth weight, indoor air pollution, and lack of maternal education) and outcome (severe ALRI) was good (average cumulative modified GRADE score greater than or equal to 2). However, the quality of studies reporting the association between some other risk factors (incomplete immunization at one year and prematurity) was poor (average cumulative modified GRADE score less than 1) (Supplementary Table S3(supplementary Table 3)).
740 studies with suitable data (Figure 1). After screening of the titles and abstracts and a subsequent full text review against our eligibility criteria, we identified 36 studies with suitable data. Twenty seven studies reported odds ratios using multivariate analysis and 25 studies reported odds ratios using univariate analysis. Studies reporting the association between HIV and vitamin A deficiency and severe ALRI reported risk estimates using only univariate analysis. The quality of studies reporting the strength of association between risk factors (exposure to low birth weight, indoor air pollution, and lack of maternal education) and outcome (severe ALRI) was good (average cumulative modified GRADE score greater than or equal to 2). However, the quality of studies reporting the association between some other risk factors (incomplete immunization at one year and prematurity) was poor (average cumulative modified GRADE score less than 1) (Supplementary Table S3(supplementary Table 3)).
Definite risk factors
The following risk factors were consistently associated with severe ALRI:
Low birth weight. Four hospital-based studies (9-12) (three from developing region) reported an association between low birth weight and severe ALRI using multivariate analysis. The overall and developing region risk-ratio meta-estimate were 3.2 (95% CI 1.0 to 9.9) and 3.6 (95% CI 0.8 to 16.2), respectively (Figure 2; Table 3). Three additional studies (13-15), one from developing region, reported odds ratios for low birth weight and severe ALRI using univariate analysis. When data from these studies was combined with the data from studies using multivariate analysis, the odds ratio meta-estimate was 3.2 (95% CI 1.0 to 10.0) and 1.8 (95% CI 1.3 to 2.7) for developing and industrialized regions, respectively.
Indoor air pollution exposure. Five studies (10,16-19), all from developing region, reported an association between exposure to indoor air pollution (use of solid and biomass fuels) and severe ALRI using a multivariate analysis. The overall odds ratio meta-estimate was 1.6 (95% CI 1.1 to 2.3) (Figure 2; Table 3). Three additional studies (13,20,21) reported the association using a univariate analysis. The inclusion of these studies did not alter the odds ratio meta-estimate.
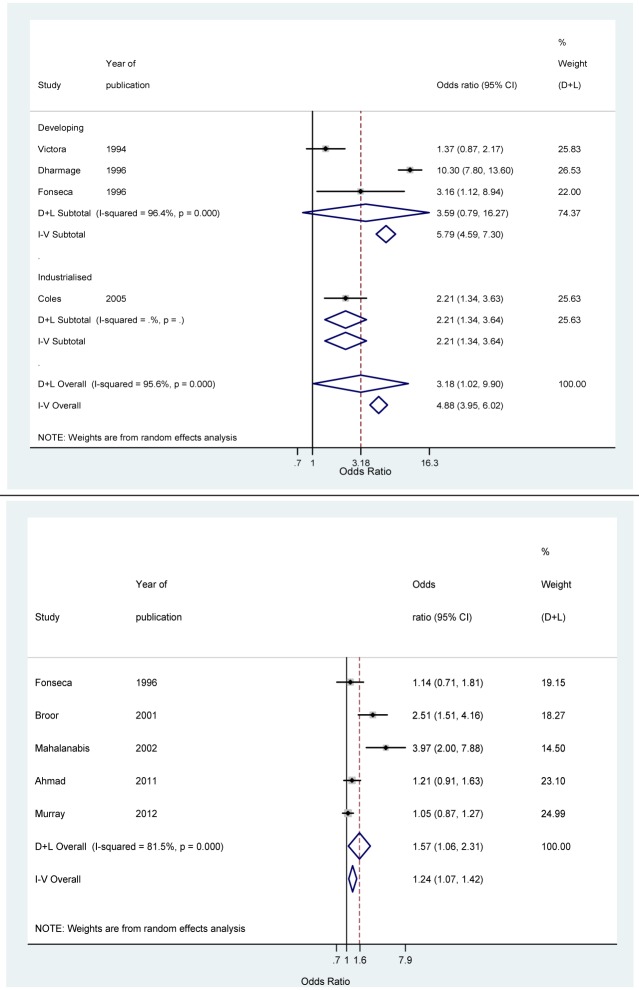
Breastfeeding. Ten hospital-based studies (9-11,14,16,19,22-25), 8 from developing countries, reported an association between lack of exclusive breastfeeding (defined as only breast milk in the first four months of life) and ALRI using a multivariate analysis. The odds ratio meta-estimate for developing and industrialized regions were 2.7 (95% CI 1.7 to 4.4) and 1.3 (95% CI 0.2 to 8.4), respectively (Figure 3; Table 3; Table 4). Six additional studies (12,13,15,26-28) (4 from developing countries) reported the association between lack of exclusive breastfeeding and severe ALRI using a univariate analysis. The addition of those studies did not have any substantial effect on the odds ratio meta-estimate. Six studies (9,10,14,19,22,25), 4 from developing regions, reported the association between no breastfeeding and severe ALRI. These studies showed an odds ratio meta-estimate similar to lack of exclusive breastfeeding. Four studies (11,16,23,24) from developing region reported an association between partial breastfeeding (less than four months of breast feeding) and severe ALRI. The odds ratio meta-estimate was again similar to that for lack of exclusive breastfeeding. Four studies (13,15,27,28), 3 from developing and 1 from industrialized countries, reported odds ratios using univariate analysis. Inclusion of those studies did not alter the odds ratios.
Forrest plots from meta-analysis odds ratio estimates for the following risk factors: lack of exclusive breastfeeding (top) and incomplete immunization (bottom). D+L indicates meta-estimates from random effects model; I-V from fixed effects model.
Incomplete immunization. We defined incomplete immunization as lack of immunization for measles at the end of the first year of life since bronchopneumonia is a common complication in children with measles, and has a high case fatality rate (29-31). Five studies (10,16,19,20,32), all from developing region, reported an association between incomplete immunization and severe ALRI. The overall odds ratio meta-estimate was 1.8 (95% CI 1.3 to 2.5). Three additional studies (13,27,33) from developing region also reported this association. Inclusion of these studies did not alter the odds ratio meta-estimate. Two studies from industrialized region (15,34) reported the association between incomplete immunization and severe ALRI using a univariate analysis. The odds ratio meta-estimate, however, did not report a significant association between the risk factor and the outcome. (Figure 3; Table 3; Table 4)
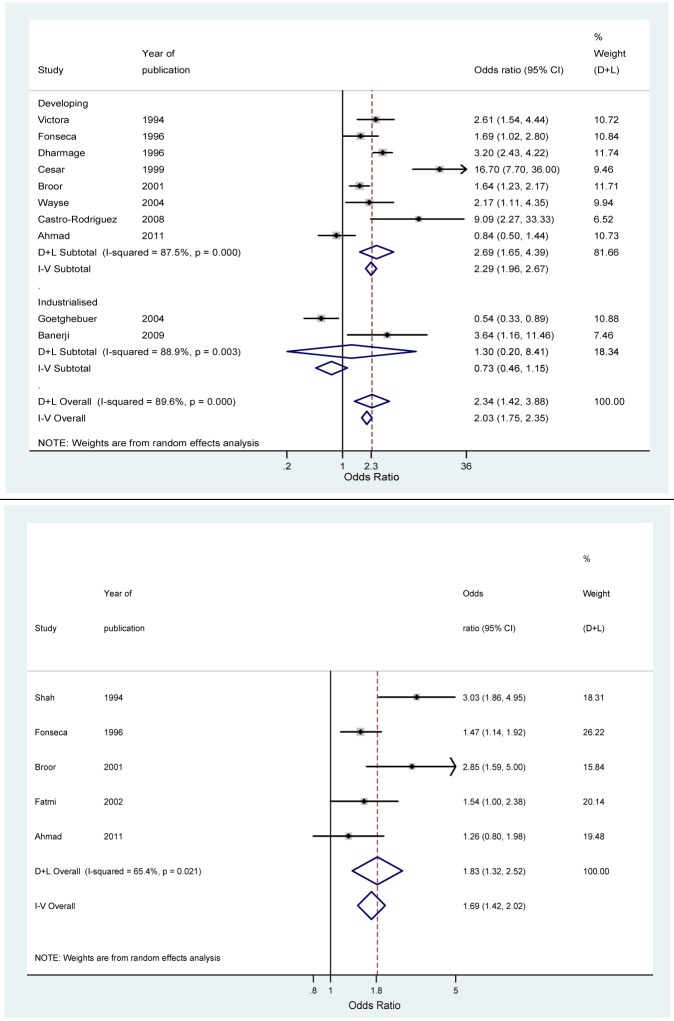
Crowding. Three studies (9,10,25) (two from developing countries) reported an association between crowding (>7 persons per household) and severe ALRI using a multivariate analysis. The odds ratio meta-estimate for the developing region was 1.9 (95% CI 1.5 to 2.5) (Figure 4; Table 3). Three additional studies (13,16,32) from developing region reported an association between crowding (>7 persons per household) and severe ALRI using a univariate analysis. Inclusion of these studies did not alter the odds ratio meta-estimate. Four studies (11,20,28,35), all from developing regions, defined crowding as more than 2 persons sharing a child’s bedroom. These studies, using a multivariate analysis, reported an odds ratio meta-estimate of 2.2 (95% CI 1.8 to 2.7) (Figure 4; Table 3). Two additional studies (9,14), one from developing region, reported the association using univariate analysis. Again, addition of this study did not alter the odds ratio meta-estimate.
Forrest plots from meta-analysis odds ratio estimates for the following risk factors: crowding (top) and undernutrition (bottom). D+L indicates meta-estimates from random effects model; I-V from fixed effects model.
Seven additional studies (9,11,13,15,17,27,34), 5 from developing region, reported an association between crowding (>2 persons per room) and severe ALRI using a multivariate or univariate analysis. The overall odds ratio meta-estimate for the developing region was similar to those using other case definitions, with odds ratio meta-estimate 2.0 (95% CI 1.2 to 3.2). The odds ratio meta-estimate for the two studies from industrialized region was similar, with odds ratio meta-estimate 2.3 (95% CI 1.3 to 4.0).
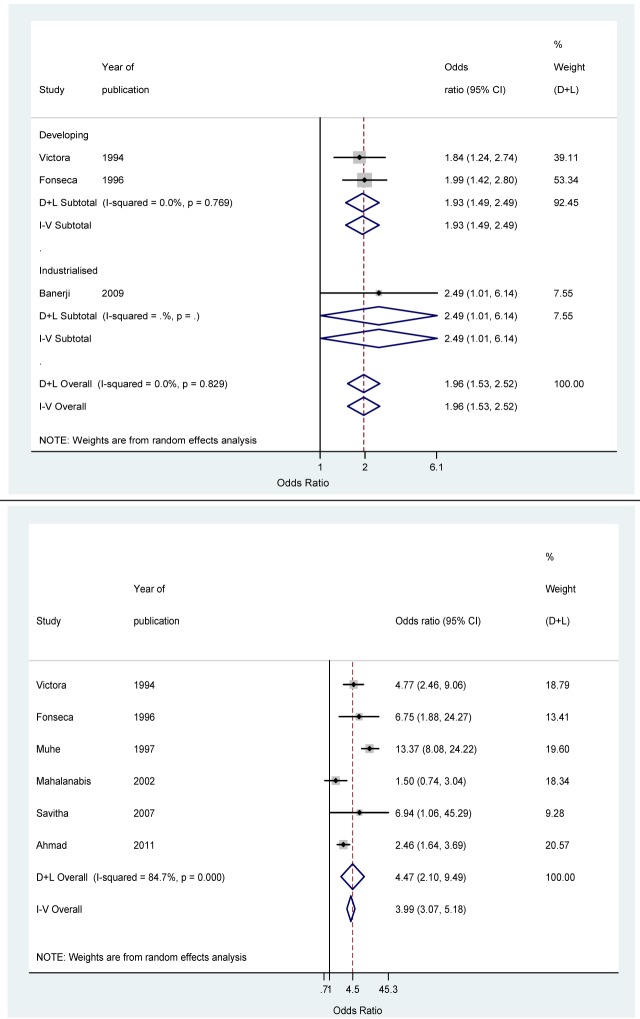
Undernutrition. Undernutrition has been variously defined in the literature: underweight (weight for age <2 standard deviations [SD]), stunted (height for age <2 SD), and wasted (weight for height <2 SD). Six studies (9,10,17,19,27,36), all from developing regions, reported an association between being underweight and risk for severe ALRI using a multivariate analysis. The overall odds ratio meta-estimate was 4.5 (95% CI 2.1 to 9.5). Four additional studies (12,15,23,32), 2 from developing region, reported this association using a univariate analysis. Inclusion of these studies decreased the overall odds ratio estimate by about 30%, with odds ratio meta-estimate 3.2 (95% CI 1.6 to 6.4). There was no significant association between being underweight and the risk of severe ALRI in industrialized countries, with odds ratio meta-estimate 1.2 (95% CI 0.6 to 2.5). Four studies (10-12,15), two from developing region, reported an association between stunting and severe ALRI using a multivariate analysis. Three additional studies (9,23,32), all from developing regions, reported this association using a univariate analysis. The overall odds ratio meta-estimate (for studies using univariate and multivariate analysis) for the developing regions was 2.6 (95% CI 1.5 to 4.6). In the case of the two studies from industrialized region using multivariate analysis, the overall odds ratio meta-estimate was similar to the risk for being underweight, with odds ratio meta-estimate 1.3 (95% CI 0.5 to 3.4).
Four studies (10,16,32,36), all from developing countries, reported an association between wasting and severe ALRI. The overall odds ratio meta-estimate from these four studies was 2.8 (95% CI 1.8 to 4.3). Six additional studies (9,11,12,15,33,37), 4 from developing region, reported this association using a univariate analysis. Inclusion of these studies did not substantially alter the overall odds ratio meta-estimate for developing countries. The two studies from developing countries again did not report a significant association. (Figure 4; Table 3).
HIV infection. Only two studies (38,39) reported an association between HIV and severe ALRI. Both studies were from sub-Saharan Africa and used univariate analysis. The overall odds ratio meta-estimate was 4.6 (95% CI 2.2 to 9.7). (Supplementary Figure S1(supplementary Figure 1)).
Likely risk factors
The following risk factors where associated with severe ALRI in a majority of included studies:
Passive smoking. We observed an inconsistent association between the presence of smokers in the house and severe ALRI in the studies included in this review. While 4 studies (11,20,40,41) (three from developing region) reported an association between the presence of smokers in the house and severe ALRI using a multivariate analysis, 2 (15,34) failed to do so. Overall, the odds ratio meta-estimate (from studies using a multivariate analysis) did not report a significant association between the risk factor and the outcome, with odds ratio meta-estimate 2.4 (95% CI 1.0 to 5.8). The evidence of an association between the presence of smokers in the house and severe ALRI was again inconsistent when studies using a univariate analysis were considered. While only 2 studies (14,33) (one from developing region) demonstrated an association, 3 studies (9,13,32), all from developing region, did not do so. Inclusion of these univariate studies did not substantially alter the overall odds ratio meta-estimate. Three studies (25,42,43), all from the industrialized region, reported an association between maternal smoking and severe ALRI using a multivariate analysis. The overall odds ratio meta-estimate was 2.7 (95% CI 1.0 to 7.8). However, another study (14) from industrialized region, did not report such an association when the data were analyzed using a univariate analysis. Inclusion of this study did not substantially alter the overall odds ratio meta-estimate.
Lack of maternal education The association between the lack of maternal education and severe ALRI was inconsistent. While one study (28) from developing region reported an association using a multivariate analysis, 5 studies (10,12,17,19,22), 1 from developing region, did not do so. The overall odds ratio meta-estimate was 1.6 (95% CI 1.0 to 2.6). When studies using a univariate analysis were considered, only 3 of the 4 studies (18,27,32) (all from developing region) reported an association between the absence of maternal education and severe ALRI. Inclusion of these univariate studies did not substantially alter the overall odds ratio meta-estimate.
Sex. The association between male sex and severe ALRI was inconsistent. Only 3 of the 6 studies (10,11,20,22,28), all from developing region, reported an association between male sex and severe ALRI using a multivariate analysis. Overall, the odds ratio meta-estimate was 1.5 (95% CI 1.0 to 2.3). Only 3 of 7 studies (9,14,17,18,32,37), 6 from developing region, reported such an association using a univariate analysis. Inclusion of these univariate studies did not substantially alter the odds ratio meta-estimate.
Vitamin D deficiency. Vitamin D deficiency was defined broadly as the presence of clinical rickets in 4 of the 5 studies that investigated an association between the risk factor and severe ALRI (the one remaining study defined this as Vit D3<22.5 nmol/L). Three studies (19,23,36), all from developing regions, demonstrated an association between the presence of rickets and severe ALRI using a multivariate analysis. The odds ratio meta-estimate was 7.3 (95% CI 2.5 to 21.5). Two studies (27,34) (one from developing region) demonstrated a similar association using a univariate analysis. Inclusion of these studies did not substantially affect the odds ratio meta-estimate.
Preterm birth. Two studies (20,22), both from developing region, did not demonstrate an association between preterm birth and severe ALRI using a multivariate analysis. The overall odds ratio meta-estimate was not significant, with odds ratio 1.3 (95% CI 0.8 to 2.1). However, three studies (13,14,33) (2 from developing region) demonstrated such an association when using a univariate analysis. Inclusion of these studies significantly altered the odds ratio meta-estimate to 1.9 (95% CI 1.3 to 2.8).
Anemia. Anemia was inconsistently defined in the 5 studies. Only 2 studies defined this using the measurement of hemoglobin (Hb) in blood (but used different cut-off values of Hb to define anemia). Two studies (12,44), one from developing region, demonstrated an association between anemia and severe ALRI using a multivariate analysis. The overall odds ratio meta-estimate was 3.9 (95% CI 2.4 to 6.3). Two of the 3 studies (23,27,37) (all from developing countries), also demonstrated an association between anemia and severe ALRI using a univariate analysis. Inclusion of these univariate studies did not significantly alter the odds ratio meta-estimate.
Zinc deficiency. Two studies (45,46), both from developing region, reported an inverse association between zinc supplementation and severe ALRI using a multivariate analysis, with odds ratio meta-estimate 0.5 (95% CI 0.3 to 0.9). However, one study (47) from developing region did not report a protective effect of zinc supplementation on severe ALRI using a univariate analysis. Inclusion of this study did not substantially alter the odds ratio meta-estimate.
Possible risk factors
The following risk factors were sporadically associated with severe ALRI in the identified studies:
Day care. Two studies (9,10) from developing region reported an association between daycare attendance and severe ALRI using a multivariate analysis. The overall odds ratio meta-estimate was 8.0 (95% CI 3.6 to 17.7). However, another study (15) from industrialized region did not report such an association when using a univariate analysis. Inclusion of this study significantly altered the odds ratio meta-estimate to 3.7 (95% CI 0.7 to 20.2).
Birth interval. Two studies (10,22), both from developing regions, did not report an association between birth interval (<24 months) and severe ALRI using a multivariate analysis, with odds ratio meta-estimate 1.4 (95% CI 0.9 to 2.2). Two more studies (9,11) from developing region also failed to observe such an association when using a univariate analysis. Inclusion of these studies did not significantly alter the odds ratio meta-estimate.
Birth order. Two studies (10,22), both from developing region, reported an association between three or more previous pregnancies and severe ALRI using a multivariate analysis. The overall odds ratio meta-estimate was 3.0 (95% CI 2.0 to 4.6). However, another study (9) (again from developing regions) using a univariate analysis did not report such an association. Addition of this study did not substantially alter the odds ratio meta-estimate, which amounted to 2.1 (95% CI 1.2 to 4.4).
Previous history of ALRI. Four of the 5 studies (9,10,15,28,32) included in this review reported an association between the risk factor (history of ALRI) and outcome (severe ALRI) using a multivariate analysis. The overall odds ratio meta-estimate did not demonstrate a significant association, with odds ratio meta-estimate 1.7 (95% CI 0.8 to 3.4).
Vitamin A deficiency. Only one study, from industrialized region (12), reported an association between vitamin A deficiency (serum retinol <20 µg/dL) and severe ALRI. However this association was not significant, with OR 1.01 (95% CI 0.47 to 2.16).
Discussion
Our study is the first comprehensive attempt to systematically assess the effect of a multitude of possible risk factors on severe ALRI in children aged less than five years. We identified, in total, 19 risk factors, which had been reported to be associated with severe ALRI in the published literature. We observed a consistent significant association between 7 risk factors (low-birth-weight, undernutrition, indoor air pollution, incomplete immunization at one year, HIV, breastfeeding, and crowding) and severe ALRI (definite risk factors). We also observed that 7 risk factors (parental smoking, lack of maternal education, vitamin D deficiency, male sex, preterm births, anemia, and zinc deficiency) had an inconsistent association with severe ALRI that was not significant (likely risk factors). We further observed that 5 risk factors (daycare, birth interval, birth order, previous history of ALRI, and vitamin A deficiency) were sporadically reported to be associated with severe ALRI (possible risk factors).
There was considerable variation in the quality of eligible studies included in this review (Supplementary Table S3(supplementary Table 3)). We used a modified GRADE scoring (8) to assess the quality of included studies. The modified grade score varied from 0.9 (in the case of preterm births) to 4 in the case of daycare attendance. However, we recognize that this score only partially reflects the quality of the included studies. The included studies used variable case definitions. While we grouped together studies using similar case definitions, in some cases there was a substantial variability. For example, in the case of low birthweight, while all studies used a case definition of birthweight less than 2500 g, Victora et al (9) used a cut-off of <2000 g to define low-birth-weight. Similarly, there was a considerable difference in the age groups of included participants across studies. While 19 studies reported outcomes for 0-59 months age range, 17 studies only included a narrow age range (eg, 0-11 months or 0-23 months). Since we pooled the data from different age ranges (but using the same case definitions), we may have over-estimated the odds ratio meta-estimates if a substantial number of the included studies were for a lower age range.
Although we only included the studies with a sample size ≥100, there was considerable variability in the sample size of the included studies- ranging from 146 (26) to 350 648 (43). This is reflected in the wide confidence intervals for some of the reported odds ratio estimates included in the meta-analysis. The included studies were significantly heterogeneous.
648 (43). This is reflected in the wide confidence intervals for some of the reported odds ratio estimates included in the meta-analysis. The included studies were significantly heterogeneous.
Twenty eight of the 36 included studies obtained data on the presence of risk factors only from interviewing mothers of the eligible subjects. Eight studies (26,34,36-39,43,44) used either records/laboratory diagnosis alone or in combination with maternal history for data collection. This introduced several biases in the studies, such as recall bias, interviewer bias, and misclassification bias. We also observed that there was considerable variation in the adjustment for potential confounders in the included studies. While 2 studies did not adjust for potential confounders (20,33), 9 studies did (15,16,19,25,37,39,40,42,43). The majority of the identified risk factors appeared to be related to poverty and hence significant collinearity is likely to exist among them. Only sixteen (9-11,13,15,17,18,22,28,35,39,40,42,43,46,47) of the 36 included studies adjusted for poverty as a confounder.
Hospitalization for acute lower respiratory infections in young children poses a substantial burden on health services, especially in developing countries. In spite of this, the evidence regarding the risk factors for this major burden of disease is in many cases sparse, of variable quality, and not generalizable. However, since the majority of these risk factors are potentially preventable, governments should consider what action can be taken to decrease the prevalence of these risk factors. This, along with increasing coverage of vaccines for Streptococcus pneumoniae and Haemophilus influenzae type B, should substantially decrease the burden of childhood pneumonia in developing countries. Moreover, the odds ratio meta-estimates reported in this review should be useful for modeling the global, regional, and national estimates of severe ALRI if the national/regional prevalence of these risk factors are known (2). Future studies should investigate the role of poverty, HIV, and other risk factors currently classified “likely” or “possible” risk factors for ALRI, and should attempt to obtain more precise estimates of risk for “definite” risk factors by studying larger samples in diverse settings and by more careful measurement and analysis of possible confounding factors.
Acknowledgments
Funding received from Bill and Melinda Gates Foundation.
Ethical approval Not required.
Declaration of authorship The study was initiated by HN, IR, and HC. The study was designed by HN, IR, HC, and SJ. SJ, KHM, DP, and RF reviewed literature, collected and coded the data. HN performed data analysis. SJ, KHM, DP, and IR performed interpretation of the results. HN and HC supervised the study and participated in the explanation and discussion of the results. The manuscript was drafted by SJ and KHM under the mentorship of HN, while DP, RF, and IR reviewed and revised the initial version. All authors read and approved the final manuscript.
Competing interests All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
Additional Material
References
Articles from Croatian Medical Journal are provided here courtesy of Medicinska Naklada
Full text links
Read article at publisher's site: https://doi.org/10.3325/cmj.2013.54.110
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3641871/pdf/CroatMedJ_54_0110.pdf
Citations & impact
Impact metrics
Article citations
Prediction of acute respiratory infections using machine learning techniques in Amhara Region, Ethiopia.
Sci Rep, 14(1):27968, 14 Nov 2024
Cited by: 0 articles | PMID: 39543232 | PMCID: PMC11564824
An analysis of cause-specific under-5 mortality in Bangladesh using the demographic and health survey 2011 and 2017-2018.
BMC Pediatr, 24(1):572, 09 Sep 2024
Cited by: 0 articles | PMID: 39251961 | PMCID: PMC11386380
Factors associated with severe pneumonia among children <5 years, Kasese District, Uganda: a case-control study, January-April 2023.
Pneumonia (Nathan), 16(1):13, 25 Jul 2024
Cited by: 0 articles | PMID: 39049136 | PMCID: PMC11270805
Quantifying the increased risk of illness in malnourished children: a global meta-analysis and propensity score matching approach.
Glob Health Res Policy, 9(1):29, 31 Jul 2024
Cited by: 0 articles | PMID: 39085934 | PMCID: PMC11290152
Prenatal and early life exposure to air pollution and the risk of severe lower respiratory tract infections during early childhood: the Espoo Cohort Study.
Occup Environ Med, 81(4):209-216, 28 Apr 2024
Cited by: 0 articles | PMID: 38604660 | PMCID: PMC11103339
Go to all (119) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Risk factors for respiratory syncytial virus associated with acute lower respiratory infection in children under five years: Systematic review and meta-analysis.
J Glob Health, 5(2):020416, 01 Dec 2015
Cited by: 145 articles | PMID: 26682048 | PMCID: PMC4676580
Review Free full text in Europe PMC
Effects of short-term exposure to air pollution on hospital admissions of young children for acute lower respiratory infections in Ho Chi Minh City, Vietnam.
Res Rep Health Eff Inst, (169):5-72; discussion 73-83, 01 Jun 2012
Cited by: 24 articles | PMID: 22849236
Risk factors for mortality from acute lower respiratory infections (ALRI) in children under five years of age in low and middle-income countries: a systematic review and meta-analysis of observational studies.
PLoS One, 10(1):e0116380, 30 Jan 2015
Cited by: 115 articles | PMID: 25635911 | PMCID: PMC4312071
Review Free full text in Europe PMC
Risk factors for mortality from acute lower respiratory tract infections in young Gambian children.
Int J Epidemiol, 22(6):1174-1182, 01 Dec 1993
Cited by: 38 articles | PMID: 8144302