Abstract
Free full text

Unfolding dynamics of the mucin SEA domain probed by force spectroscopy suggest that it acts as a cell protective device
Abstract
MUC1 and other membrane-associated mucins harbor long, up to a micrometer, extended highly glycosylated mucin domains and SEA domains situated on their extracellular parts. These mucins line luminal tracts and organs, and are anchored to the apical cell membrane by a transmembrane domain. The SEA domain is a highly conserved domain that undergoes a molecular strain-dependent autocatalytic cleavage during folding in the endoplasmic reticulum, a process required for apical plasma membrane expression. So far no specific function has been designated for the SEA domain. Here, we constructed a recombinant protein consisting of three SEA domains in tandem and used force spectroscopy to assess the dissociation force required to unfold individual, folded SEA domains. Force-distance curves revealed three peaks, each representing unfolding of a single SEA domain. Fitting the observed unfolding events to a worm-like chain model yielded an average contour length of 32 nm per SEA domain. Analysis of forces applied on the recombinant protein revealed an average unfolding force of 168 pN for each SEA domain at a loading rate of 25 nNs−1. Thus, the SEA domain may act as a breaking point that can dissociate before the plasma membrane is breached when mechanical forces are applied to cell surfaces.
Introduction
The epithelial cells of the gastrointestinal and respiratory tracts are exposed to large mechanical forces. These forces have to be absorbed and managed without destruction of the epithelial integrity. Mucosal surfaces are covered by lubricating mucus that has the ability to dampen mechanical stress. However, also the epithelial cells need ways to protect their apical membranes. The highly glycosylated mucins, covering the epithelial surfaces of luminal organs, play a major role in the protection of epithelia [1]. Mucins are characterized by a PTS domain, which comprises serine-, threonine-, and proline-rich, often repeated, sequences that are decorated with a high number of O-linked glycans standing for up to 80% of the mucin mass [2]. The gel-forming mucins give mucus its lubricant property at the same time as they protect the epithelium by forming a matrix where bacteria are trapped. The second group of mucins is anchored in the apical membrane of epithelial cells by a single-pass transmembrane domain. Here, these membrane mucins can protrude up to 1.5 µm into the lumen, thereby constituting a major building block of the glycocalyx [3]. The membrane mucins include the MUC1, MUC3, MUC4, MUC12, MUC13, MUC15, MUC16, MUC17, and MUC20 mucins. These membrane mucins share common features. In addition to an N-terminal signal sequence followed by a long PTS domain that provides a combinatorial library of O-glycans to form a mucin domain, thus enabling membrane-tethered mucins to act as binding sites for bacterial adhesins, these membrane mucins have a transmembrane domain and a C-terminal cytoplasmic tail [4].
MUC1 is ubiquitously expressed on surfaces of epithelial cells lining the mammary gland and respiratory, gastrointestinal, and reproductive tracts as well as on hematopoietic cells [3, 5, 6]. The human MUC1 gene is comprised of 7 exons that undergo alternative splicing generating a number of protein products of which some lack the membrane anchor (MUC1/SEC), others the extracellular mucin domain and some have a different CT (MUC1-CT80 and MUC1-CT58) [7–9]. The MUC1 mucin is abundant in lungs and stomach but less abundant in the intestine where MUC3, MUC12, MUC13, and MUC17 are abundant, but less well studied. The MUC1, MUC3, MUC12, MUC13, and MUC17 mucins all harbor a single and MUC16 multiple extracellular SEA (sea urchin sperm protein, enterokinase, and agrin) domains, C-terminal of their mucin domain [10].
The SEA domain consists of approximately 100 amino acids and is autocatalytically cleaved during folding in the endoplasmic reticulum (ER) [11–14]. The cleavage site (↓) is N-terminal to the serine residue in the G↓S[V/I]VV motif on the SEA domain of MUC1, 3, 12, and 17 [13]. The conformational strain during folding destabilizes the G-S bond and triggers an N→O acyl shift [12, 15]. Adding four additional glycines N-terminal to the cleavage site lowers the folding strain and destroys the cleavage while the domain is still able to fold correctly, an observation exploited in this study [12]. The SEA domain cleavage products, the N- and C-terminal peptides of the mucin, form a heterodimer that does not dissociate after the cleavage and is held together non-covalently by four β-pleated sheets [12]. The question is what will disrupt and separate the two parts of the SEA domain? To address this, we constructed a recombinant protein harboring three correctly folded but non-cleavable SEA domains organized in tandem. Using an atomic force microscope, we measured the forces required to unfold a single MUC1-derived SEA domain in real time. Our measurements reveal that the MUC1 SEA domain is a relatively stable domain that requires mechanical forces of approximately 168 pN at a loading rate of 25 nNs−1 or thermal energies of above 80°C to dissociate.
Results
The MUC1 SEA Domain is Unstable at Elevated Temperatures
We expressed two recombinant proteins, containing a single MUC1 SEA domain fused to myc- and IgG2a–tags, in CHO-K1 cells (Fig. 1A). The Myc-MUC1SEA-IgG2a/His with a native SEA domain was cleaved, appropriately folded and secreted. Myc-MUC1SEAmutLVPR-IgG2a/His, containing a thrombin cleavage site LVPR↓GS instead of the sequence LFRPG↓S at positions 112–115, was also secreted but not cleaved as it migrated at a molecular mass of 75 kDa and stained both for the myc- and IgG2a-tags (Fig. 1B). Using proteins encoded by these two plasmids, we applied an ELISA-based assay to study conditions that separated the heterodimer associated by the SEA domain. Myc-MUC1SEA-IgG2a/His was exposed to different temperatures prior to binding to Ni-NTA strips and intact protein was detected using monoclonal α-myc antibody. The cleaved SEA protein was fully dissociated at 100°C while a smaller fraction of bound protein dissociated at 80°C (Fig. 1C). Bacterially produced MUC1-SEA had a melting temperature of about 75°C further suggesting that our SEA domain reporter had a native fold [12]. No dissociation of the heterodimer was seen at 60°C or at ambient temperature. Non-cleavable Myc-MUC1SEAmutLVPR-IgG2a/His, boiled at 100°C, did not dissociate, as expected. In similarity to the cleavable SEA domain subjected to low temperature as well as the non-cleavable SEA domain, the cleavable SEA domain was also relatively resistant to harsh chemical conditions such as 4 M guanidinium chloride and 0.3% SDS (results not shown). Levitin et al. have previously titered the SDS concentration for separation of the SEA domain to 0.03% and Ligtenberg et al to 0.1% [11, 14]. The reasons for these differences are not known, but probably have to do with incubation times and temperature differences. In any case, the SEA domain is relatively stable to SDS. As the SEA domain can be exposed to different pH, both intracellular an extracellular, we could show that the native SEA domain was resistant to pH 4.0, pH 5.0 as well as pH 8.8. In order to exclude the involvement of altered luminal ion concentrations in SEA dissociation, we also tested SEA dissociation in presence of 3M NaCl or 15 mM CaCl2. Neither of these treatments induced SEA dissociation. These findings led us to conclude that the SEA heterodimer is neither disrupted by harsh chemical conditions, nor by altered pH and ion concentrations. Instead, we hypothesized that mechanical force could dissociate the two SEA parts in a physiological context.
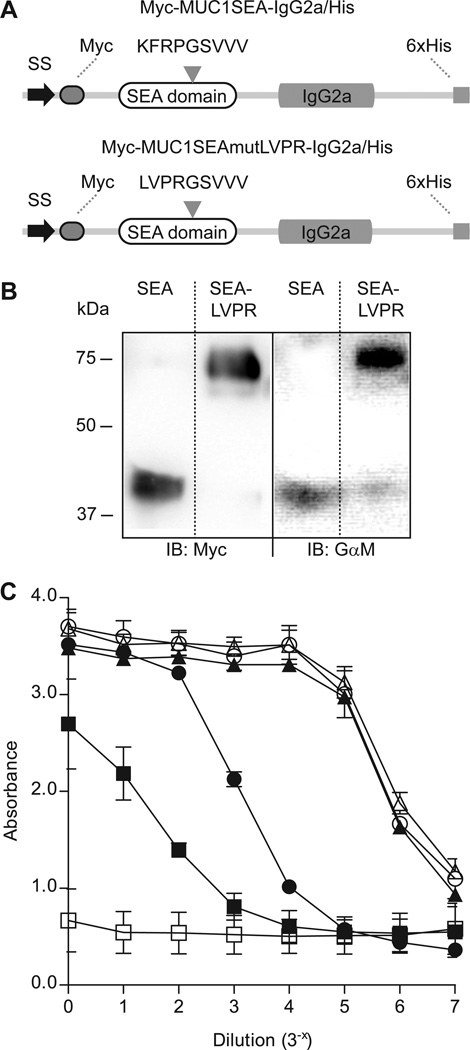
Two recombinant proteins harboring a MUC1-derived SEA domain and their dissociation at high temperatures. A) Myc-MUC1SEA-IgG2a/His contained a SEA domain cleaved at the KFRPG↓SVVV motif, flanked by a N-terminal signal sequence (SS) followed by a Myc-tag (Myc) and a C-terminal IgG2a domain followed by a His-tag consisting of six histidine residues (6×His). Myc-MUC1SEAmutLVPR-IgG2a/His resembled Myc-MUC1SEA-IgG2a/His but it harbored a SEA domain with a mutated LVPR↓GSVVV that could not be cleaved upon biosynthesis. B) Expression of Myc-MUC1SEA-IgG2a/His and Myc-MUC1SEAmutLVPR-IgG2a/His in CHO-K1 cells was assessed by immunoblot post reduction. Left panel represents recombinant proteins detected by Myc mAb (Myc) and right panel represents proteins detected using a Goat anti-mouse secondary antibody (GαM). All lanes were derived from the same PVDF membrane. C) Myc-MUC1SEA-IgG2a/His and Myc-MUC1SEAmutLVPR-IgG2a/His were subjected to different temperatures and stability of SEA domain was assessed using ELISA. Assay buffer (□) served as blank, while Myc-MUC1SEA-IgG2a/His at ambient temperature (Δ) and Myc-MUC1SEAmutLVPR-IgG2a/His at 100°C (○) served as controls. Myc-MUC1SEA-IgG2a/His was heated at 60°C (▲), 80°C (●), and 100°C (■) prior to binding to Ni-NTA HisSorb™ strips via existing His-tag on recombinant proteins. Dissociation of protein at SEA domain was assessed using α-myc mAb and goat anti-mouse secondary antibody coupled to AP.
H12SEA(Gly)4IgG2a Protein is not Cleaved
Atomic force microscopy is a useful tool for high resolution measurements of protein unfolding forces. In order to determine the mechanical force necessary to break a cleavable SEA domain, we designed a recombinant protein, H12SEA(Gly)4IgG2a, containing three SEA domains in tandem where the G↓SVVV motifs were extended with four glycines (Gly-loop) to yield GGGGGSVVV. Furthermore, we flanked the three SEA domains with an N-terminal 12 amino acid long histidine-tag and a C-terminal IgG2a-tag (Fig. 2A). Insertion of a Gly-loop eliminates autocatalytic cleavage due to relieved strain, while it allows correct folding of the SEA domain which adopts a folded conformation comparable to the native SEA domain [12, 16]. The Gly-looped SEA domains arranged in tandem render a distinct fingerprint represented by a force-distance curve composed of at least three peaks upon unfolding of each of three Gly-looped SEA domains, while the stretching and subsequent dissociation of a single native, cleavable SEA domain does not generate force-distance curves that can be differentiated from background adhesion and unfolding of multiple domains. This strategy allowed us to distinguish between unfolding of a single, immobilized recombinant protein and background adhesion or stretching of bundles of immobilized recombinant proteins. The recombinant protein was expressed in CHO-K1 cell where silver staining and immunoblot of the from spent culture medium purified recombinant protein proved that it was correctly processed, secreted and that none of the three Gly-looped SEA domains dissociated when protein sample was boiled at 95°C in presence of SDS and DTT (Fig. 2B).
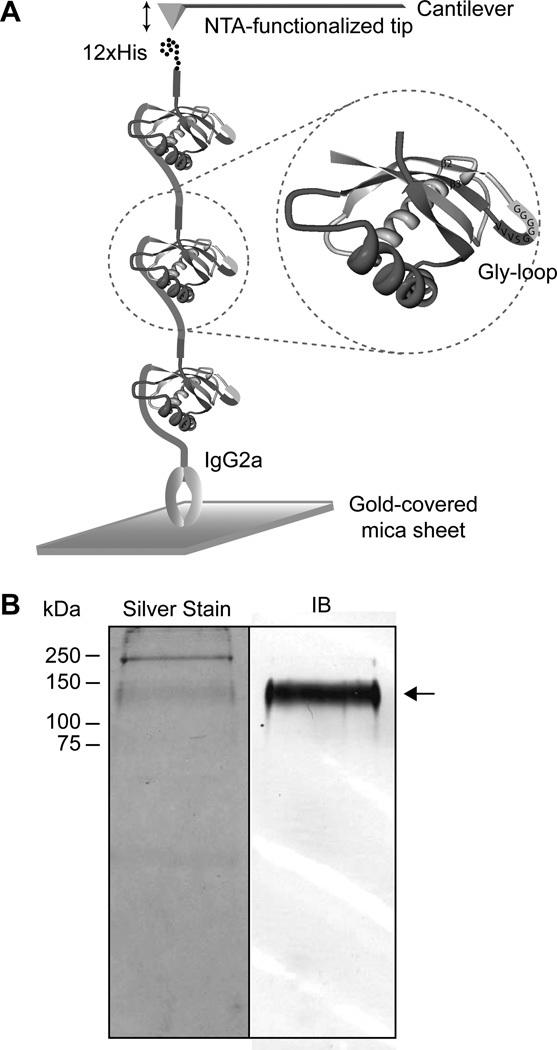
A non-cleaved recombinant protein H12SEA(Gly)4IgG2a was expressed in CHO-K1 cells. A) A recombinant protein H12SEA(Gly)4IgG2a, harboring three MUC1-derived SEA domains, was designed. The GSVVV motif in each individual SEA domain was extended with four additional glycines to GGGGGSVVV in order to generate a Gly-loop. The recombinant protein was flanked by an N-terminal His-tag consisting of 12 histidine residues (12×His) and a C-terminal constant region of IgG2a (IgG2a). B) H12SEA(Gly)4IgG2a was expressed and secreted by CHO-K1 cells. Spent medium from CHO-K1 cells stably expressing H12SEA(Gly)4IgG2a was separated using SDS-PAGE. The protein was detected using Silver stain (left panel) and by immunoblot via goat anti-mouse secondary antibody coupled to AP (right panel). Arrow points to separated H12SEA(Gly)4IgG2a recombinant protein. Both panels were derived from the same PVDF membrane.
AFM Force Curves on H12SEA(Gly)4IgG2a Show Three Unfolding Events
For force spectroscopy studies, the recombinant protein H12SEA(Gly)4IgG2a was immobilized on a gold-coated mica sheet via covalent interaction between thiols found only in the IgG2a Fc part of the protein and the gold surface. An AFM scan rendering a topographic image of the surface after protein attachment confirmed that the recombinant protein was immobilized on the mica sheet. Next, pulling force measurements were performed in PBS, PBS/Ni2+ and PBS/Ni2+/EDTA, where the presence of Ni2+ resulted in a His-NTA-interaction and EDTA, chelating Ni2+, broke the His-NTA-interaction. The recombinant protein H12SEA(Gly)4IgG2a with its three SEA domains resembles a semi-flexible polymer, and we thus attempted to describe its unfolding behavior with the worm-like chain (WLC) model in order to determine contour lengths (L) and unfolding forces of individual SEA domains [17, 18]. Although the WLC model is not ideal for describing unfolding of the immobilized SEA domain, as the original WLC theory of Kratky and Porod [19] refers to free and unconstrained solution conditions, this model is frequently used for the analysis of AFM experiments [20, 21]. The unfolding events in the force-distance curves of the H12SEA(Gly)4IgG protein followed the WLC equation (See Material and Methods, equation 1) as exemplified by the WLC fit shown in a typical force-distance curve generated in presence of PBS/Ni2+ (Fig. 3). In the presence of PBS/Ni2+, unfolding of the three SEA domains could be observed as a specific fingerprint, represented by three distinct peaks (designated peaks 1–3). Unspecific interaction of the cantilever tip and the gold-coated mica sheet gave rise to peak 0. Stretching of an individual SEA domain generated a gradually increasing pulling force until it reached a maximum, corresponding to the force required for unfolding of that specific SEA domain. After unfolding of the domain, the pulling force approached the baseline as the protein relaxed, just before the next SEA domain was stretched out by the AFM cantilever and the force increased again. Unfolding forces displayed a tendency to increase during unfolding of the three individual SEA domains, where the first unfolding event displayed the lowest force and the third the highest. Peak 3 was a convolution of two events, namely unfolding of the third SEA domain and rupture of the His-NTA-interaction as the pulling force hit baseline after peak 3. In conclusion, force curves obtained from single-molecule AFM experiments showed that stretching of H12SEA(Gly)4IgG2a gave rise to a saw-tooth pattern with three distinct peaks describing the unfolding of each one of the three SEA domains and the rupture of the His-NTA-interaction.
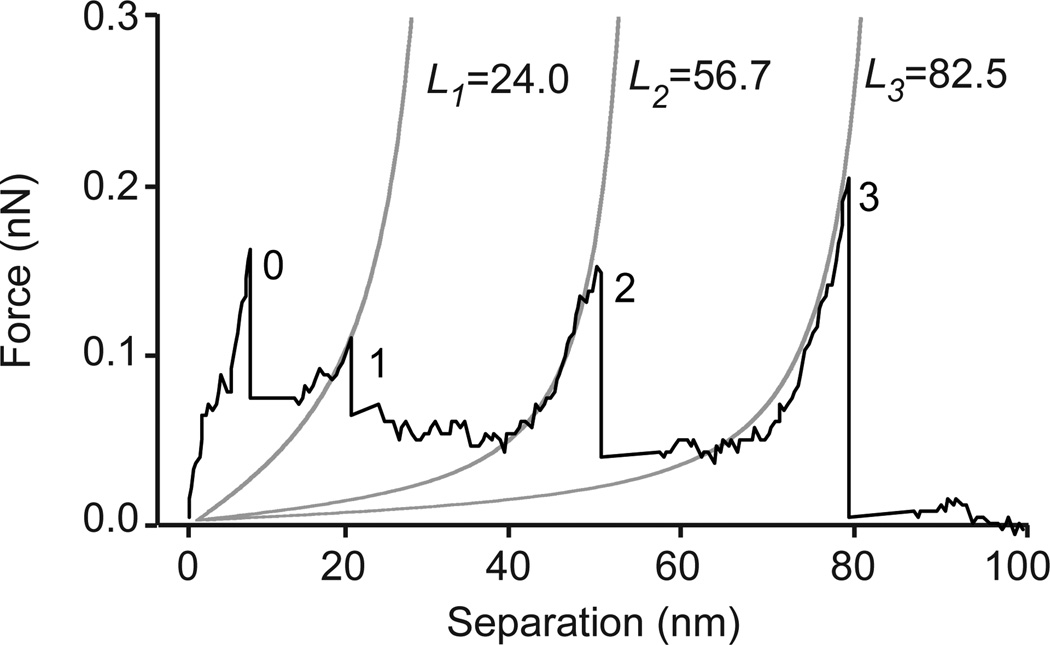
Fitting of a force-distance curve to the WLC model. A single force-distance curve recorded upon unfolding of H12SEA(Gly)4IgG2a revealed four characteristic peaks (numbered 0–3). Peak 0 represents unspecific interaction of the AFM-tip with the surface while peaks 1–3 represented subsequent unfolding of three SEA domains. Peak 3 also included the NTA-His rupture. The force-distance curve was fitted to the WLC model, represented by gray solid lines, to determine contour lengths (L) of each unfolded SEA domain.
Quantitative Data Analysis Using the Worm-Like Chain Model
For further data analysis, we identified a number of criteria that when applied to our data set exclusively extracted force-distance curves generated from unfolding of one single recombinant protein. The first criterion was based on the physical limitations of the H12SEA(Gly)4IgG2a protein. Structural studies mapped the SEA domain to the amino acid residues 1041–1144 of MUC1 (SWISS PROT entry P15941) [12]. This unfolded stretch of 104 amino acids corresponds to a theoretical contour length of 41.6 nm (the persistence length for a single amino acid is 0.4 nm) while the distance between N- and C-terminal amino acids of the folded SEA domain is approximately 3.5 nm. The second criterion was based on the number of force peaks obtained upon stretching. Peak 0 was assumed to be generated by unspecific interactions of the cantilever tip with the gold-coated mica and was disregarded in our data analysis and so were all force-distance curves displaying more than four peaks.
Contour Length of the MUC1 SEA Domain
Contour lengths of each unfolded SEA domain as obtained by WLC fitting were averaged and plotted against peak numbers 1–3. Unfolding of a single protein generated average contour lengths for unfolded domains that increased proportionally to the corresponding peak number (Fig. 4A). Linear regression analysis performed on the average contour length of each unfolded domain plotted against peak number showed linearity (r2=0.99), thus indicating consistency in the unfolding of the three identical SEA domains. To determine the contour length of an individual unfolded SEA domain in presence of PBS/Ni2+, contour lengths of peak 1 (L1) were subtracted from contour lengths of peak 2 (L2) and the differences averaged. The same operation was performed for peaks 2 and 3. The averaged differences in contour lengths obtained, L2- L1 and L3- L2 for each stretched protein, gave an average contour length of an unfolded SEA domain of 32 ± 7 nm (Fig. 4B).
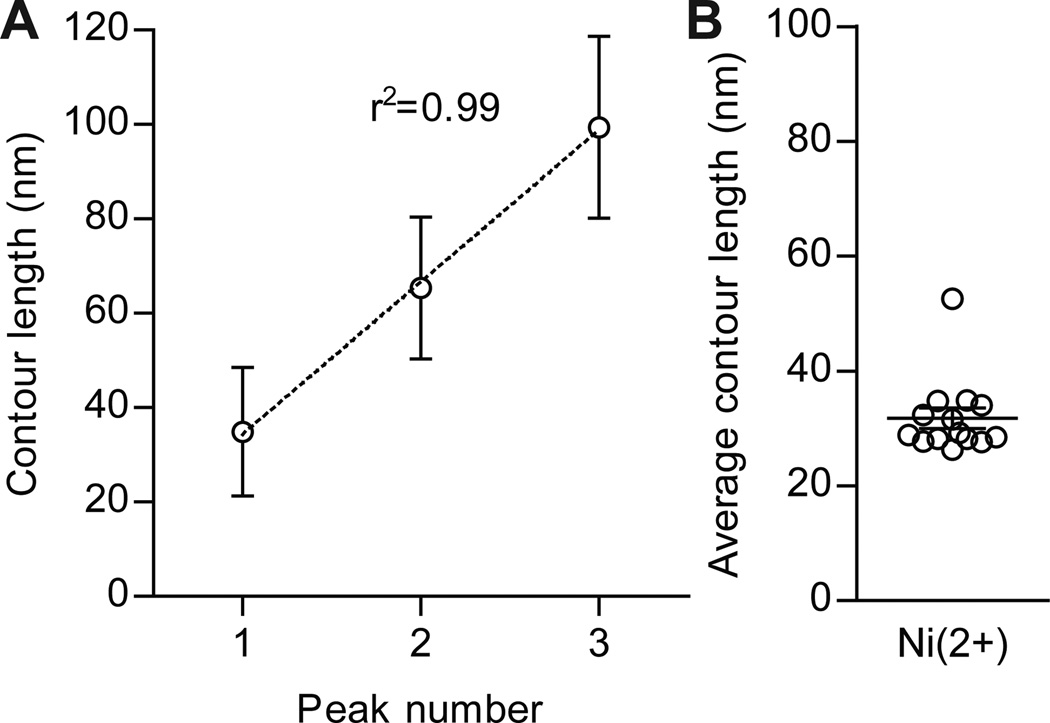
Distribution of contour lengths obtained by fitting force-distance curves to the WLC model. A) The average contour lengths from each SEA domain were plotted against peak number. Regression analysis revealed a linear correlation between the average contour length sorted by peak and peak number (n=14 for each group, r2= 0.99). B) Average contour length of a single unfolded SEA domain was calculated by subtracting contour lengths of peak 1 from peak 2 and contour lengths of peak 2 from peak 3. Data points were displayed in a scatter plot revealing an average contour length of 32 ± 7 nm (n=14, mean ± SD).
Unfolding Force of the MUC1 SEA Domain
Force-distance curves from unfolding of a single recombinant protein revealed a saw-tooth pattern featuring 3–4 peaks, where the force typically increased successively with peak order. After the last peak, the force relaxed to the baseline, indicating that this last peak was a convolution of unfolding of the third SEA domain and rupture of the NTA-His-complex, i.e. detachment. Therefore, forces associated with the last unfolded domain were excluded in our calculations. Analysis of unfolding forces from the two remaining SEA domains resulted in an average unfolding force of 168 ± 73 pN (loading rate 25 nNs−1) per SEA domain in presence of PBS/Ni2+ (Fig. 5A). Obtained forces from the three experimental setups were visualized in a force frequency histogram (Fig. 5B). Control experiments, PBS and PBS/ Ni2+/EDTA, generated force curves recording either weak interactions or one single adhesion peak due to unspecific interaction of cantilever with gold-coated mica sheet. Forces generated in experimental setups PBS and PBS/Ni2+/EDTA were 26 ± 21 pN and 35 ± 34 pN, respectively. The here obtained average force required to unfold a single SEA domain in presence of PBS/ Ni2+ can be compared to previously published forces required to break the NTA-6×His-complex (≈200 pN for a loading rate of 25nNs−1) and 1400 pN, the force necessary to break the thiol-mediated IgG-Au bond [22, 23].
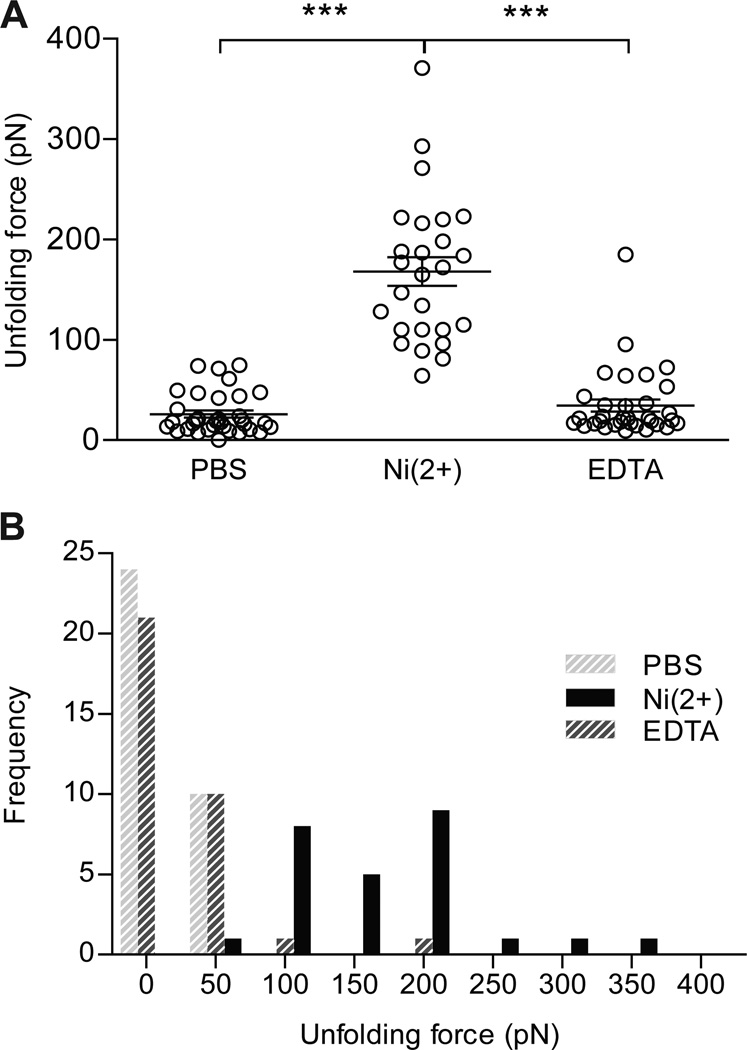
Distribution of unfolding forces obtained by fitting force-distance curves to the WLC model. A) Unfolding forces were recorded during three experimental settings; PBS, PBS/Ni2+, and PBS/Ni2+/EDTA. In PBS (PBS), forces recorded an average of 26 ± 21 pN, while the same experiment PBS/Ni2+ (Ni(2+)) generated forces with an average of 168 ± 73 pN. Upon addition of EDTA to PBS/Ni2+ (EDTA), an average force of 35 ± 34 pN was measured. The loading rate was 25 nN s−1 in all experiments. (n=32 for PBS and EDTA, n=26 for Ni(2+), mean ± SD, *** significant at p<0.001) B) Forces generated under the three experimental settings, PBS, PBS/Ni2+ and PBS/Ni2+/EDTA, were displayed in a frequency histogram. Bright grey bars represent unfolding forces generated in PBS (PBS), black bars represent forces recorded in PBS/Ni2+ (Ni(2+)) and dark grey bars represent forces in PBS/Ni2+/EDTA (EDTA).
Discussion
Using AFM, we studied a recombinant protein harboring three MUC1-derived SEA domains. We immobilized the recombinant protein on a gold surface and applied a pulling force mediated through a NTA-His-complex between a functionalized AFM tip and a 12×histidine-tag on the recombinant protein. Upon pulling, we were able to record a maximum of four events, corresponding to the unspecific interaction of the functionalized tip with the protein-covered gold surface and unfolding of each of the three SEA domains. Data obtained from force-distance curves were fitted to a WLC model and analyzed by constructing scatter plots and frequency histograms. Average unfolding force and average contour length of one SEA domain were determined to be 168 ± 73 pN and 32 ± 7 nm, respectively.
The peak force typically increased successively for each unfolded domain, as displayed in Fig. 3. This can be explained by the fact that more unstable SEA domains, having a lower unfolding barrier, unfold first followed by domains with higher unfolding barriers [24]. The average unfolding forces for the first and second unfolded domains were approximately 162 and 175 pN (loading rate=25nNs−1), while the average force associated with the third domain was approximately 194 pN, indicating that the final peak was a combination of SEA domain unfolding and NTA-His rupture. The final peak was therefore excluded from data analysis when calculating the average unfolding force for a single SEA domain.
In some cases, the force-distance curves showed additional peaks between the three main unfolding maxima, probably generated by partial unfolding of SEA domains (Fig. 3). These additional peaks could be caused by stable intermediate unfolding states, stabilized by residual non-covalent interactions. Similar unfolding intermediates have been identified during unfolding of the modular muscle protein titin [25, 26].
A characteristic feature of membrane mucins is that they are cleaved during biosynthesis but still held together by strong non-covalent forces as they are presented on the cell surface. This can be obtained by two types of domains characteristic for membrane mucins; the NIDO-AMOP-VWD of the MUC4 mucin or the SEA domain in the MUC1, MUC3, MUC12, MUC16, and MUC17 mucins [12]. The SEA domain is cleaved in an autocatalytic mechanism using the folding-strain energy during biosynthesis in the endoplasmic reticulum. Cleavage and correct folding has been shown to be important for passage of SEA domain-containing glycoproteins out of the endoplasmic reticulum [27]. A non-cleavable variant of the SEA domain cleavage site, where the serine was replaced by alanine (GAVVV, S1098A), resulted in a poorly folded protein when produced in bacteria [12]. If instead four additional glycines were added to generate a Gly-loop, the SEA domain was not cleaved as the strain was completely relieved. Still the Gly-looped SEA domain folded into its native conformation as proven by NMR spectroscopy and Circular Dichroism spectroscopy [16]. These data suggest that the non-covalent interactions that hold together the native, cleavable SEA domain are also present in the Gly-looped domain. Our recombinant protein with three Gly-looped SEA domains, but lacking a transmembrane domain, was secreted from CHO-K1 cells, suggesting that the protein is correctly processed and folded. Thus, our data on unfolding of the Gly-looped SEA domain can be extrapolated to the native SEA domain.
SEA domain membrane mucins are expressed on luminal epithelial surfaces in the lung and the gastro-intestinal tract and are suggested to be important components of the glycocalyx barrier protecting the apical surfaces. MUC1 and MUC16 are expressed in lung epithelium, MUC1 on the gastric epithelium whereas MUC3, MUC12, and MUC17 are expressed in the intestine. Membrane mucins have been suggested to monitor the extracellular milieu although no proof of such functions has been offered [12, 28]. In S. cerevisiae, mucin-type proteins have been attributed a role as osmosensors. The signaling mucins Msb2 and Hkr1 act upstream in the high-osmolarity glycerol (HOG) and filamentous growth (FG) signaling pathways and modulate intracellular signals that generate responses to alterations in osmolarity and nutrition [29, 30]. The mucin domain of the MUC5B gel-forming mucin exhibits change in layer thickness due to variations in ion concentration of the liquid. Changes in dissipation were ascribed to shielding of adjacent electrostatic charges on oligosaccharides of the mucin domain [31]. Thus, mucin domains present on membrane mucins have the capacity to act as sensors of at least luminal osmolarity.
Up until now, no specific function of the SEA domain is known. Here we hypothesize that the SEA domain can act as a sensor for mechanical manipulation consisting of tangential or perpendicular forces applied on membrane mucins, i.e. shear stress or forces generated by for example attachment of bacteria. Previous structure analysis of the SEA domains revealed a network of intermolecular interactions, such as hydrogen bonds, that stabilize the cleaved, correctly folded SEA domain [12]. The extracellular part of the membrane mucins mainly consists of a highly glycosylated mucin domain which could for example act as docking site for bacteria via glycan-binding adhesins. When the bonds holding the SEA domains together are broken, the membrane mucin heterodimer dissociates in its two parts. The outer part is lost to the lumen and the membrane-anchored smaller fragment remains in the membrane, potentially transducing signals via its CT. In fact signal transduction mediated by MUC1 has been extensively studied in cancer cells that have lost polarization [28]. The cytoplasmic tail of MUC1 has been shown to translocate to the cell nucleus via association with β-catenin, p120 catenin, p53, and estrogen receptor α [32]. Binding of P. aeruginosa to MUC1 can spark signaling via the MAP kinase pathway [4]. Many ion channels have PDZ-binding motifs in their CTs and are regulated by different PDZ proteins. We have previously shown that the murine orthologue of MUC17, designated Muc3(17), harbors a apical retention signal in its CT region, which enables the mucin to bind to PDZK1, a PDZ protein that entraps the ion channel CFTR [33]. Furthermore, protein levels of human MUC3 were regulated by CFTR via a competitive mechanism involving a PDZ protein called GOPC [34]. Together these facts make it likely that SEA membrane mucins, like MUC3, MUC12, and MUC17, can be involved in luminal sensor mechanisms that transduce signals to the cell, generating cellular responses.
Studies on covalent bonds have revealed that forces required for breaking them lie in the picoNewton range [23]. The frequently exploited biotin-avidin-interaction is broken at 160 pN [35]. AFM measurements on the giant muscle protein titin, involved in passive muscle elasticity, assembly, and stress-sensing, showed that titin unfolded at approximately 700 pN [36]. The SEA domain mucin MUC1 is readily expressed on the apical cell membrane between the cilia on the epithelial cells of the trachea [37]. Interestingly, the coordinated action of cilia generates a force equivalent to approximately 60 pN [38]. This suggests that the orchestrated beating of cilia generates liquid flow without dissociation or shedding of the mucin at the SEA domain as this would require forces above 168 pN for every SEA domain. The force required to extract a membrane protein from the plasma membrane is dependent on hydrophobic bonds between membrane spanning segments of the protein and the lipid membrane, as well as interactions between the cytoplasmic tail of the membrane protein and the cytoskeleton. This force has been determined to 400–600 pN [39]. This means that only forces above 168 pN, i.e. the unfolding force of a single SEA domain, and below 400–600 pN applied on cell surfaces, result in the dissociation of the SEA domain before the membrane mucin is extracted from the cell surface and the cell membrane ruptured. The out-stretched and extended conformation of SEA-type membrane mucins renders them the first proteins that are encountered by agents exerting mechanical forces while approach cell membranes. Therefore, it is probable that SEA domains absorb mechanical forces that otherwise would disrupt the cell surface. In the intestine, where a constant flow towards distal segments takes place, the mass of a single E. Coli bacteria exerts forces in the 10−14 pN range [40]. Given our results, it is unlikely that bacterial interactions with membrane mucins result in SEA domain dissociation. In regards of pathogens, it is more reasonable that intestinal parasites that interact with cell surfaces, generate forces sufficient to damage cell membranes and that SEA-type membrane mucins help protect these membranes from parasitic insult. Cell surface protection by SEA-type membrane mucins may also be useful in disease. The dehydrated and viscous mucus layer in the intestine and lungs of cystic fibrosis patients tends to collapse, exerting shear stress on the underlying epithelial cells. SEA domains of membrane mucins may protect cell surfaces by absorbing these forces, followed by dissociation of the SEA domain and detachment of collapsed mucus.
The mucin domain of membrane mucins protrudes up to a micrometer into the extracellular milieu and can absorb mechanical energy by their gel properties. This large mucin domain is anchored to the membrane via the SEA domain that must be held together by sufficiently strong non-covalent forces. The SEA domain must at the same time break by strong mechanical forces to function as a breaking point as a way to protect the cell. Thus, the physical strength holding the SEA domain together is well balanced.
Material and Methods
Construction of Plasmid Vectors
A sequence containing the cleavage site of MUC1, located in the SEA-domain of the protein, was inserted into the the pSM plasmid described previously [41]. A myc-tag and an IgG2aFc-sequence were incorporated followed by a histidine (6×His)-tag and an enterokinase site. The new plasmid, named pSM-MUC1SEA-IgG2a/His, was used as starting material for construction of pSH12SEA(Gly)4IgG2a. Firstly, a stop codon was introduced after the IgG2a-tag in order to delete the existing His-tag. Secondly, a glycine loop with four additional glycine residues was inserted into the G↓SVVV motif of the SEA domain. Thirdly, a His-tag harboring 12 histidine residues, was inserted N-terminal of the SEA domain. Finally, two additional SEA domains, harboring the GGGGGSVVV motif, were inserted after the existing SEA domain.
A pSM-MUC1SEAmutLVPR-IgG2a/His expression vector was constructed by mutation of seven residues of the MUC1 fragment in the pSM-MUC1-IgG2a/His plasmid using site directed mutagenesis (QuickChange™ site-directed mutagenesis kit, Stratagene). The following oligonucleotides were used; 5´ CTG GGC CTC TCC AAT CTG GTC CCT CGA GGA TCT GTG GTG GTA C 3´ and 5´ GTA CCA CCA CAG ATC CTC GAG GGA CCA GAA TAT TGG AGA GGC CCA G 3´ (mismatches are underscored).
Tissue Culture
CHO-K1 (CCL-64, ATCC) were cultured in Iscoves’ modified Dulbecco’s medium (Invitrogen) containing 10% (v/v) fetal calf serum and supplemented with sodium pyruvate (110 mg/L), L-arginine (116 mg/L), L-glutamine (290 mg/L), L-asparagine (36 mg/L), folic acid (10 mg/L), and β-mercaptoethanol (3.49 µL/L) at 37°C in 5% CO2.
Transfections and Protein Expression
pSM-MUC1SEA-IgG2a/His, pSM-MUC1SEAmutLVPR-IgG2a/His, and pSH12SEA(Gly)4IgG2aStop were transfected into CHO-K1 cells using Lipofectamine 2000 (Invitrogen) according to manufacturer’s instructions. Positive clones were selected by adding G418 (250 µg/mL) post-transfection. High-expressing positive clones were recloned and selected for further investigation.
SDS-PAGE, Immunoblots and Silver Staining
Protein samples were reduced in SDS-PAGE sample buffer [25 mM Tris/HCl, 10% (v/v) glycerol, 2.5% (w/v) SDS] with 100 mM 1,4-ditriotheitol for 5 min. at 95°C, separated on 10% polyacrylamide gels with 3% stacking gels for 1 hour at 4 V cm−1. Procedure for silver-staining was described previously [42]. Immunoblots were performed using semi-dry transfer of the separated proteins to PVDF membrane (0.2 µm Immobilon, pSQ, Millipore) at 2.5 mA/cm2 in a Transfer-Blot SD-Dry Transfer Cell (Bio Rad Laboratories). Transfer buffer contained 48 mM Tris, 39 mM glycine, 1.3 mM SDS, and 15% methanol. A blocking solution [PBS containing 0.2% (w/v) non-fat milk powder, 0.1% Tween 20, 0.05% (w/v) NaN3] was used to prevent unspecific binding to the PVDF membrane. Antibodies (α-myc mAb [hybridoma supernatant] 1:50, goat anti-mouse IgG coupled to horseradish peroxidase 1:500) were dissolved in PBS containing 0.2% BSA. Antibody detection was performed using a SNAP i.d. Protein Detection System (Millipore).
Protein Purification
Spent culture medium from CHO-K1 cells stably expressing pSH12SEA(Gly)4IgG2aStop was desalted and the buffer changed to PBS. 500 mM NaCl was added and the sample was loaded on a HiTrap Chelating HP column (GE Healthcare) charged with Co2+. The column was washed with 20 mM Na-Phosphate buffer, pH 7.4, and 500 mM NaCl followed by 20 mM Na-Phosphate buffer, pH 7.4, 500 mM NaCl, 20 mM Imidazole. Finally, the H12SEA(Gly)4IgG was eluted with 20 mM Na-Phosphate buffer, pH 7.4, 500 mM NaCl, 200 mM Imidazole. The fractions positive for H12SEA(Gly)4IgG2a were pooled, dialyzed against PBS, and stored at −80°C.
Enzyme-linked Immunosorbent Assay (ELISA)
ELISA was performed on Ni-NTA HisSorb™ strips (Qiagen). Strips were blocked in 2% BSA, 100mM NaCl, 0.1% Tween-20, 0.05% NaN3, 10mM Tris-HCl pH 7.5 prior to assay. PBS containing 0.05% Tween-20 was used to wash strips between each incubation step. Both media and antibodies were diluted in PBS containing 0.1% BSA and 0.1% NaN3. Bound protein was detected using α-myc antibody, goat anti-mouse secondary antibody coupled to alkaline phosphatase (AP), and AP substrate containing p-nitrophenylphosphate in diethylamine buffer (Bio Rad Laboratories). The strips were incubated in 21°C in darkness and read on a Victor 2 Microplate Reader (Perkin Elmer) at 450 nm after 15, 30, and 45 min. Strips were subjected to different temperatures (100°C for 10 min., 80°C for 30 min., 60°C for 30 min., 22°C for 30 min.) prior to incubation with antibodies. Also, strips were subjected to PBS containing 0.1% BSA, with either citric acid or borate buffer, at pH 4.0, 5.0, or 8.8 for 30 min.. Moreover, strips were treated with PBS containing 0.1% BSA, with either 0.3% SDS, 4M guanidium hydrochloride, 3 M NaCl, or 15 mM CaCl2 for 30 min.. PBS containing 0.1% BSA and incubation for 10 or 30 min. at 37°C was used as control.
Atomic Force Microscopy / Force Spectroscopy
Freshly cleaved mica sheets (V1 grade, 1" × 3", Ted Pella Inc.) were coated with a 5 nm thick chromium adhesion layer and a 25 nm thick gold layer in a resistively heated PVD system (Edwards HPTS, Model Auto 306) with a base pressure of 2·10−6 mbar. Prior to use, gold substrates were cleaned in a homemade UV/ozone chamber for two times 25 min., with rinsing and blow-drying with nitrogen in between, and then stored in Milli-Q water (resistivity > 18.2 MΩcm−1; Millipore S.A.) overnight. The larger sheets were then cut into 1" × 1" pieces, rinsed extensively with Milli-Q water, blow-dried with nitrogen and mounted into the AFM fluid cell. The substrate was immersed in PBS (0.14 M NaCl, 2.7 mM KCl, 10 mM phosphate buffer pH 7.4) immediately after mounting. Double-side gold-coated CSC38/Cr-Au cantilevers chips (kspec = 0.03 N/m; Mikromasch) were treated in a homemade UV/ozone cleaner for 20 min. and subsequently stored in hot ethanol at 65°C for 40 min.. After storing them in ethanol at room temperature for 20 min., the cantilever chips were rinsed several times in ethanol and then stored in thiolated-NTA (1mM HS-(CH2)11-EG3-NTA in ethanol; ProChimia Surfaces) overnight in order to form an NTA-terminated self-assembled monolayer (SAM) on the gold surface. The functionalization procedure was concluded by rinsing the chips several times in ethanol, followed by careful blow-drying with nitrogen. The AFM tip holder and fluid cell were cleaned by immersing them in isopropyl alcohol (p.a. grade; Sigma-Aldrich) for 15 min., rinsing with Milli-Q water, immersing in PBS for 45 min., rinsing with water and drying with nitrogen gas. All force spectroscopy experiments were carried out at 23.0±0.5°C using PicoScan software (version 5.3.3; Molecular Imaging) together with a PicoSPM atomic force microscope (Molecular Imaging) equipped with a small-area scanner and a fluid cell. Cantilever spring constants were determined using the thermal noise method [43]. The approach/retraction speed for all experiments reported here was v ≈ 820 nm s−1, corresponding to a force-loading rate of vk ≈ 25 nNs−1. H12SEA(Gly)4IgG2a protein was diluted to 3 µg/mL, reduced in 0.1 M (tris(2-carboxyethyl)phosphine) (TCEP), and bound to gold-covered mica sheets by exposing the gold surface to the protein solution for 10 minutes. After protein adsorption, the sample was rinsed carefully with PBS buffer. Control experiments were performed in PBS, while actual force measurements were performed in the presence of Ni2+ after incubation of bound protein with 0.1 M NiCl2. Regeneration was performed in 0.05 M EDTA. Force curves were analyzed using Punias software (version 1.0; freeware provided by Dr. Philippe Carl) and the scanning probe image processer (SPIP version 3.0.0.9, Image Metrology, Inc.). Baselines were corrected using SPIP.
The Worm-Like Chain Model
The Igor Pro software (version 4.19; WaveMetrics) were used to fit the experimental data with the Worm-Like Chain (WLC) model. According to the WLC model semi-flexible polymers are characterized by an intrinsic stiffness termed persistence length [17, 18]. The model predicts that the applied stretching force (F) is related to the fractional extension (x/L) of the polymer chain by,
where A is the persistence length, measuring the chains bending rigidity or stiffness, kB is the Boltzmann constant, T is the absolute temperature, x is the extension, and L is the contour length which is defined as a polymer’s length at maximum physically possible extension.
Acknowledgment
This work was supported by the Swedish Research Council (no. 7461, 21027, and 342-2004-4434), the Swedish Cancer Foundation, the Knut and Alice Wallenberg Foundation, IngaBritt and Arne Lundberg Foundation, Sahlgren's University Hospital (LUA-ALF), Wilhelm and Martina Lundgren’s Foundation, Torsten och Ragnar Söderbergs Stiftelser, the Swedish CF Foundation, Lederhausen’s Center for CF Research at Univ. Gothenburg, the Sahlgrenska Academy Foundations, the Swedish Foundation for Strategic Research - The Mucosal Immunobiology and Vaccine Center (MIVAC), the Mucus-Bacteria-Colitis Center (MBC) of the Innate Immunity Program (2010–2014), and The Foundation for Strategic Environmental Research (Mistra Dnr 2004-118).
Glossary
| AFM | atomic force microscopy |
| AMOP | adhesion associated domain in MUC4 and other proteins |
| AP | alkaline phosphatase |
| CHO | Chinese hamster ovary |
| CFTR | cystic fibrosis transmembrane conductance regulator |
| CT | cytoplasmic tail |
| ER | endoplasmic reticulum |
| FG | filamentous growth |
| HOG | high-osmolarity glycerol |
| MAP | mitogen-activated protein |
| MUC | mucin |
| NIDO | nidogen |
| NTA | nitrilotracetic acid |
| PDZK1 | PDZ domain containing 1 |
| PTS | proline-, serine- and threonine-rich |
| PVD | physical vapor deposition |
| SEA | sea urchin sperm protein, enterokinase, and agrin |
| TCEP | tris(2-carboxyethyl)phosphine |
| vWD | von Willebrand factor type D |
| WLC | worm-like chain |
Footnotes
Author contributions: Å.P., M.Z., T.P., and G.C.H. designed research; T.P., M.Z., Å.P., F.S., and D.G.A.J performed research; T.P., M.Z., and G.C.H. analyzed data; and T.P., M.Z., and G.C.H. wrote the paper.
References
Full text links
Read article at publisher's site: https://doi.org/10.1111/febs.12144
Read article for free, from open access legal sources, via Unpaywall:
https://onlinelibrary.wiley.com/doi/pdfdirect/10.1111/febs.12144
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1111/febs.12144
Article citations
The dimerized pentraxin-like domain of the adhesion G protein-coupled receptor 112 (ADGRG4) suggests function in sensing mechanical forces.
J Biol Chem, 299(12):105356, 18 Oct 2023
Cited by: 0 articles | PMID: 37863265 | PMCID: PMC10687090
Bacterial SEAL domains undergo autoproteolysis and function in regulated intramembrane proteolysis.
Proc Natl Acad Sci U S A, 120(40):e2310862120, 27 Sep 2023
Cited by: 4 articles | PMID: 37756332 | PMCID: PMC10556640
Discovery of a MUC3B gene reconstructs the membrane mucin gene cluster on human chromosome 7.
PLoS One, 17(10):e0275671, 18 Oct 2022
Cited by: 2 articles | PMID: 36256656 | PMCID: PMC9578598
Emergence of MUC1 in Mammals for Adaptation of Barrier Epithelia.
Cancers (Basel), 14(19):4805, 30 Sep 2022
Cited by: 15 articles | PMID: 36230728 | PMCID: PMC9564314
Review Free full text in Europe PMC
Chronic activation of MUC1-C in wound repair promotes progression to cancer stem cells.
J Cancer Metastasis Treat, 8:12, 31 Mar 2022
Cited by: 15 articles | PMID: 35539431 | PMCID: PMC9083497
Go to all (23) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Genes & Proteins
- (1 citation) UniProt - P15941
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
SEA domain autoproteolysis accelerated by conformational strain: mechanistic aspects.
J Mol Biol, 377(4):1130-1143, 30 Jan 2008
Cited by: 18 articles | PMID: 18314133
Autoproteolysis coupled to protein folding in the SEA domain of the membrane-bound MUC1 mucin.
Nat Struct Mol Biol, 13(1):71-76, 20 Dec 2005
Cited by: 152 articles | PMID: 16369486
The role of the SEA (sea urchin sperm protein, enterokinase and agrin) module in cleavage of membrane-tethered mucins.
FEBS J, 272(11):2901-2911, 01 Jun 2005
Cited by: 39 articles | PMID: 15943821
Mechanical design of proteins studied by single-molecule force spectroscopy and protein engineering.
Prog Biophys Mol Biol, 74(1-2):63-91, 01 Jan 2000
Cited by: 214 articles | PMID: 11106807
Review





