Abstract
Free full text

Brain Volumes in Schizophrenia: A Meta-Analysis in Over 18 000 Subjects
Associated Data
Abstract
Although structural brain alterations in schizophrenia have been demonstrated extensively, their quantitative distribution has not been studied over the last 14 years despite advances in neuroimaging. Moreover, a volumetric meta-analysis has not been conducted in antipsychotic-naive patients. Therefore, meta-analysis on cross-sectional volumetric brain alterations in both medicated and antipsychotic-naive patients was conducted. Three hundred seventeen studies published from September 1, 1998 to January 1, 2012 comprising over 9000 patients were selected for meta-analysis, including 33 studies in antipsychotic-naive patients. In addition to effect sizes, potential modifying factors such as duration of illness, sex composition, current antipsychotic dose, and intelligence quotient matching status of participants were extracted where available. In the sample of medicated schizophrenia patients (n = 8327), intracranial and total brain volume was significantly decreased by 2.0% (effect size d = −0.17) and 2.6% (d = −0.30), respectively. Largest effect sizes were observed for gray matter structures, with effect sizes ranging from −0.22 to −0.58. In the sample of antipsychotic-naive patients (n = 771), volume reductions in caudate nucleus (d = −0.38) and thalamus (d = −0.68) were more pronounced than in medicated patients. White matter volume was decreased to a similar extent in both groups, while gray matter loss was less extensive in antipsychotic-naive patients. Gray matter reduction was associated with longer duration of illness and higher dose of antipsychotic medication at time of scanning. Therefore, brain loss in schizophrenia is related to a combination of (early) neurodevelopmental processes—reflected in intracranial volume reduction—as well as illness progression.
Introduction
Since the seminal neuroimaging study by Johnstone and colleagues,1 brain abnormalities in schizophrenia have been well established. Although multiple meta-analyses—based on voxel-based morphometry (VBM)—have identified gray matter deficits in schizophrenia, especially in the frontal and temporal lobe, cingulate and insular cortex, and the thalamus,2–5 the nature of VBM studies does not allow for the estimation of effect sizes. Remarkably, and in stark contrast to the topicality of the recent meta-analyses on VBM studies, the last general meta-analysis on volumetric Magnetic Resonance Imaging (MRI) studies in schizophrenia that determined effect sizes was published over a decade ago.6 MRI techniques have improved greatly since this last meta-analysis in terms of field strength and corresponding resolution. Moreover, the number of volumetric MRI studies on schizophrenia has expanded greatly over the last 10 years. Despite the relative abundance of evidence for brain abnormalities in schizophrenia, several important questions remain unanswered. First, it is unclear to what extent brain volume alterations are present before antipsychotic treatment is initiated: a meta-analysis on brain volumes in antipsychotic-naive schizophrenia patients has not been conducted. Such analysis is particularly imperative in view of the current debate on the effects of antipsychotics on structural brain changes in schizophrenia.7,8 Second, the extent of intracranial volume reduction in schizophrenia has not been systematically reviewed since its reduction was suggested in an early meta-analysis combining computed tomography (CT) and MRI techniques.9 Intracranial volume is an important variable because a volume reduction would suggest an early developmental cause for (some) of the brain abnormalities in schizophrenia due to the fact that 90% of cranial volume is reached at the age of 5 years.10 Third, the impact of factors such as low intelligence quotient (IQ) and substance abuse on brain volumes in schizophrenia has not been explored in a meta-analysis. These aspects appear highly relevant given the possibility that IQ decrements in schizophrenia11—in view of the correlation between brain volume and IQ12—explain (part of) the brain tissue loss in schizophrenia. Similarly, substance abuse has been shown to affect brain volume in schizophrenia.13
The primary goal of the current meta-analysis was, therefore, to establish to what extent structural brain changes are present in various cerebral regions in patients with schizophrenia by including volumetric MRI studies from September 1998 until January 2012. Moreover, we performed a separate meta-analysis on volumetric MRI studies in antipsychotic-naive schizophrenia patients published prior to January 2012 to examine whether structural brain alterations are present before treatment onset. We also explored the effect of illness duration, sex, and use of antipsychotic medication at time of scanning by performing meta-regression. Finally, we compared effect sizes for left- and right-sided brain structures and investigated the impact of differential matching with regard to IQ scores, current substance abuse, and height.
Methods
Studies that applied MRI to compare brain volumes between patients with schizophrenia and healthy controls were included if they fulfilled all following criteria: (1) the study was written in the English language, (2) the study was published in print between August 1998 and January 2012, (3) patient samples contained at least 10 individuals, (4) diagnosis was established according to the Diagnostic and Statistical Manual of Mental Disorders (version III-R or IV) or International Classification of Diseases (version 9 or 10) criteria, (5) patient and control samples were matched with regard to age and sex, (6) patients with other illnesses than schizophrenia (ie, schizophreniform disorder, schizoaffective disorder or psychosis not otherwise specified) did not account for more than 50% of patient samples, (7) reported volumes represented volumes instead of an area or a volume estimation by means of an inadequate number of slices, and (8) sufficient data were available in order to calculate effect sizes. Brain regions were included when investigated by at least 10 independent study samples.
The same approach was applied to the meta-analysis on antipsychotic-naive patients, with 2 additional inclusion criteria: (1) patients were never exposed to antipsychotic medication before being scanned, (2) articles published before September 1998 were included as well. Because considerably fewer studies on antipsychotic-naive patients were available, brain regions were included in this analysis when studied in at least 5 independent study samples. The key to a meta-analysis is defining an effect size statistic capable of representing the quantitative findings of a set of research studies in a standardized form that permits meaningful comparison and analyses across the studies.14 Therefore, for each region of interest in each study, the effect size statistic Cohen’s d was calculated. In this analysis, the mean volume of a specific brain structure for patients with schizophrenia was subtracted from the mean volume for comparison subjects and divided by the pooled SD of both. According to Cohen, d values of 0.2 represent small effects, values between 0.4 and 0.6 moderate effects, and d values of 0.8 or higher large effects.15 Effect sizes were determined for all eligible brain regions in 2 different samples: a sample of all studies investigating patients taking antipsychotic medication and a sample of all studies investigating antipsychotic-naive patients with schizophrenia. By means of the method described in the online supplementary material, a second measure of effect size was used to calculate a percent difference (ie, average weighted percentage difference in volume) between patient and control groups.
The differences in pooled effect sizes between studies that did and did not match for IQ scores and height (for intracranial volume) were calculated and compared by application of a Welch’s t test. Similarly, studies explicitly stating to have excluded comorbid current substance abuse were compared with studies including substance abuse or without mentioning substance abuse as an exclusion criterion. We used unrestricted maximum likelihood weighted random-effects meta-regression on all samples combined to evaluate the effect of mean illness duration, percentage males, dose of antipsychotic medication (where possible subdivided in typical and atypical antipsychotics, see the online supplementary material), slice thickness, year of publication, and the number of investigated brain regions on effect sizes, on the condition that at least 10 different study samples were available. In the sensitivity analysis, the impact of excluding studies that investigated schizophrenia-spectrum disorders, reported relative instead of absolute volumes, applied voxel-based morphometry or shared a control group on effect sizes and heterogeneity was calculated and compared with the combined sample by application of an independent t test. For further details on the applied methods, see the online supplementary material.
Results
In total, over 100 different brain structures were investigated in 352 eligible studies. Thirty-eight of these brain structures fulfilled inclusion criteria, studied in 382 study samples derived from 317 studies.
Meta-Analysis on Volumetric Brain Studies in Medicated Patients With Schizophrenia
For specifications regarding the medicated sample, see table 1. Patients showed a significantly higher proportion of males as compared with controls (P < .0001). No significant difference in age was observed. Details on the 283 contributing studies are given in online supplementary material table 1. As shown in table 2, volumes of 33 of 38 included brain regions were significantly altered in patients compared to controls: consistently decreased in parenchymal brain tissue (except for globus pallidus, which showed a volume increase) and increased in cerebrospinal fluid (CSF) structures. The white matter of the frontal and temporal lobe, total superior temporal gyrus, caudate nucleus, and putamen volumes were not significantly different compared with controls. Volume alterations and specifications for the medicated sample are given in table 2. Forest plots of all investigated brain regions are given in online supplementary material figure 1.
Table 1.
Characteristics of the Medicated and Antipsychotic-Naive Study Samples
| Medicated Sample | Antipsychotic-Naive Sample | |
|---|---|---|
| Number of study samples (unique studies) | 347 (283) | 35 (33) |
| Number of patients/ controls | 8327/8292 | 771/939 |
| Age patients/controls (y) | 32.4 (8.6)/32.1 (8.7) | 28.0 (4.7)/27.4 (3.9) |
| Duration of illness (y) | 10.0 (6.8) | 3.1 (2.5) |
| Sex patients/controls (% male) | 72.0/67.0* | 64.8/60.6 |
| Current dose of antipsychotic medication1 | 413.7 (240.5) | 0 (0) |
| Number of investigated brain regions | 5.4 (6.1) | 4.5 (3.1) |
| Magnetic field strength 1.5T (% of study samples) | 87.8% | 88.9% |
| Slice thickness in mm (range) | 0.82–5.5 | 1.0–5.0 |
| Segmentation on contiguous slices (% of study samples)2 | 96.4 | 95.5 |
Table 2.
Comparison of Brain Volumes Between Medicated Patients With Schizophrenia and Controls
| Bilateral Brain Structure | Number of Samples (Studies) | Number of Patients | Number of Controls | Mean Weighted Effect size Cohen’s d (95% CI) | P Value for d | AWD in % | Cochran’s Q (P Value) | I² (Index in %) | FSN | ERT (P Value) | Trim and Fill Adjusted Cohen’s d (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Intracranial volume | 108 (91) | 3461 | 3542 | −0.17 (−0.23 to −0.12) | < 1 × 10−9 | −2.0 | 133.4 (.043) | 19.8 | 1246 | .15 | −0.12 (−0.18 to −0.05) |
| Total brain volume | 119 (95) | 3547 | 3894 | −0.30 (−0.36 to −0.25) | < 1 × 10−9 | −2.6 | 155.4 (.012) | 24.1 | 4731 | .011 | −0.23 (−0.29 to −0.17) |
| Total gray matter | 63 (50) | 2010 | 2316 | −0.49 (−0.57 to −0.41) | < 1 × 10−9 | −4.3 | 96.0 (.0037) | 35.4 | 3637 | .027 | −0.39 (−0.48 to −0.29) |
| Cortical gray matter | 15 (12) | 495 | 492 | −0.43 (−0.56 to −0.30) | < 1 × 10−9 | −4.8 | 13.0 (.52) | 0.0 | 136 | .43 | −0.43 (–0.56 to −0.30) |
| Total white matter | 61 (49) | 2007 | 2163 | −0.17 (−0.25 to −0.10) | 2.1 × 10−6 | −1.8 | 76.1 (.078) | 21.2 | 399 | .38 | −0.11 (−0.19 to −0.04) |
| Total CSF | 34 (27) | 1142 | 1184 | 0.36 (0.21 to 0.50) | 1.2 × 10−6 | 8.7 | 89.9 (3.6 × 10−7) | 63.3 | 526 | .62 | 0.36 (0.21 to 0.50) |
| Lateral ventricles | 46 (34) | 1595 | 1558 | 0.45 (0.37 to 0.54) | < 1 × 10−9 | 26.7 | 60.4 (.06) | 25.5 | 1662 | .40 | 0.43 (0.34 to 0.53) |
| Third ventricle | 25 (20) | 820 | 773 | 0.60 (0.50 to 0.70) | < 1 × 10−9 | 34.5 | 18.5 (.78) | 0.0 | 821 | .06 | 0.51 (0.40 to 0.61) |
| Frontal lobe | |||||||||||
| Frontal lobe, GM | 17 (13) | 613 | 675 | −0.49 (−0.64 to −0.34) | < 1 × 10−9 | −4.8 | 26.3 (.050) | 39.1 | 295 | .037 | −0.49 (−0.64 to −0.34) |
| Frontal lobe, WM | 15 (11) | 506 | 546 | −0.13 (−0.30 to 0.04) | .14 | N/A | 25.5 (0.030) | 45.2 | 0 | .15 | −0.13 (−0.30 to 0.04) |
| Prefrontal GM | 16 (14) | 659 | 604 | −0.44 (−0.58 to −0.31) | < 1 × 10−9 | −6.1 | 17.9 (0.27) | 16.0 | 193 | .82 | −0.44 (−0.58 to −0.31) |
| Prefrontal WM | 12 (11) | 511 | 454 | −0.29 (−0.42 to −0.16) | 1.0 × 10−5 | −4.8 | 6.4 (0.85) | 0.0 | 56 | .039 | −0.24 (−0.36 to −0.12) |
| Anterior cingulate gyrus | 29 (23) | 946 | 973 | −0.34 (−0.46 to −0.22) | 5 × 10−8 | −5.8 | 46.3 (0.016) | 39.6 | 333 | .80 | −0.32 (−0.44 to −0.19) |
| Posterior cingulate gyrus | 10 (7) | 311 | 324 | −0.32 (−0.56 to −0.09) | 6.2 × 10−3 | −6.1 | 18.5 (0.030) | 51.4 | 31 | .31 | −0.32 (−0.56 to −0.09) |
| Superior frontal gyrus | 12 (9) | 422 | 452 | −0.29 (−0.43 to −0.16) | 2.8 × 10−5 | −4.5 | 10.4 (0.49) | 0.0 | 42 | .69 | −0.25 (−0.40 to −0.11) |
| Middle frontal gyrus | 11 (9) | 352 | 325 | −0.32 (−0.48 to −0.17) | 3.2 × 10−5 | −4.7 | 6.4 (0.78) | 0.0 | 26 | .19 | −0.32 (−0.48 to −0.17) |
| Inferior frontal gyrus | 10 (8) | 342 | 315 | −0.41 (−0.56 to −0.25) | 2.9 × 10−7 | −6.1 | 6.8 (0.66) | 0.0 | 49 | .75 | −0.41 (−0.56 to −0.25) |
| Orbitofrontal cortex | 19 (15) | 550 | 591 | −0.21 (−0.37 to −0.05) | .010 | −3.0 | 29.7 (0.040) | 39.4 | 36 | .51 | −0.21 (−0.37 to −0.05) |
| Temporal lobe | |||||||||||
| Temporal lobe | 25 (20) | 642 | 586 | −0.22 (−0.34 to −0.09) | 6.9 × 10−4 | −2.5 | 27.6 (0.28) | 13.2 | 65 | .48 | −0.20 (−0.33 to −0.08) |
| Temporal lobe, GM | 19 (17) | 717 | 716 | −0.43 (−0.60 to −0.26) | 6.6 × 10−7 | −4.1 | 42.6 (9.1 × 10−4) | 57.8 | 271 | .96 | −0.43 (−0.60 to −0.26) |
| Temporal lobe, WM | 14 (12) | 489 | 519 | −0.04 (−0.25 to 0.17) | .69 | N/A | 32.7 (0.0019) | 60.2 | 0 | .045 | −0.04 (−0.25 to 0.17) |
| Hippocampus | 87 (68) | 2487 | 2654 | −0.52 (−0.60 to −0.44) | < 1 × 10−9 | −6.3 | 169.5 (< 1 × 10−9) | 49.3 | 6486 | .008 | −0.36 (−0.46 to −0.27) |
| Amygdala | 40 (34) | 1019 | 1186 | −0.31 (−0.43 to −0.19) | 5.5 × 10−7 | −4.8 | 72.1 (9.9 × 10−4) | 45.9 | 449 | .33 | −0.25 (−0.38 to −0.12) |
| Parahippocampal gyrus | 20 (15) | 561 | 568 | −0.24 (−0.39 to −0.09) | 2.1 × 10−3 | −4.5 | 28.8 (0.07) | 33.9 | 57 | .31 | −0.13 (−0.30 to 0.04) |
| Fusiform gyrus, GM | 10 (8) | 337 | 353 | −0.52 (−0.76 to −0.29) | 1.2 × 10−5 | −7.6 | 19.0 (0.0025) | 52.6 | 96 | .23 | −0.42 (−0.66 to −0.19) |
| Superior temporal gyrus, total | 14 (12) | 394 | 406 | −0.27 (−0.55 to 0.01) | .058 | N/A | 45.7 (1.6 × 10−5) | 71.5 | 40 | .23 | −0.27 (−0.55 to 0.01) |
| Superior temporal gyrus, GM | 16 (14) | 580 | 601 | −0.58 (−0.75 to −0.41) | < 1 × 10−9 | −8.2 | 29.5 (0.014) | 49.2 | 342 | .32 | −0.53 (−0.72 to −0.35) |
| Planum temporale | 14 (14) | 444 | 405 | −0.42 (−0.68 to −0.16) | 1.5 × 10−3 | −9.5 | 41.2 (9.0 × 10−5) | 68.4 | 104 | .63 | −0.42 (−0.68 to −0.16) |
| Heschl’s gyrus | 12 (11) | 301 | 336 | −0.29 (−0.50 to −0.09) | 4.3 × 10−3 | −7.7 | 16.3 (0.13) | 32.5 | 23 | .10 | −0.29 (−0.50 to −0.09) |
| Insula | 14 (11) | 415 | 441 | −0.44 (−0.67 to −0.20) | 2.7 × 10−4 | −5.2 | 35.2 (8.0 × 10−4) | 63.0 | 116 | .75 | −0.38 (−0.62 to −0.14) |
| Parietal lobe, GM | 9 (8) | 354 | 404 | −0.31 (−0.54 to −0.08) | 7.6 × 10−3 | −2.5 | 17.9 (0.022) | 55.3 | 29 | .66 | −0.20 (−0.46 to 0.06) |
| Occipital lobe, GM | 9 (8) | 339 | 361 | −0.22 (−0.37 to −0.07) | 4.1 × 10−3 | −2.7 | 7.6 (0.47) | 0.0 | 13 | .33 | −0.22 (−0.37 to −0.07) |
| Subcortical structures | |||||||||||
| Thalamus | 35 (28) | 1168 | 1351 | −0.31 (−0.40 to −0.22) | < 1 × 10−9 | −3.4 | 43.3 (0.13) | 21.5 | 480 | .45 | −0.26 (−0.36 to −0.16) |
| Caudate nucleus | 35 (28) | 1101 | 1154 | −0.03 (−0.14 to 0.07) | .50 | N/A | 47.3 (0.06) | 28.1 | 0 | .24 | −0.03 (−0.14 to 0.07) |
| Putamen | 28 (21) | 950 | 1006 | 0.10 (−0.07 to 0.26) | .24 | N/A | 81.8 (2.0 × 10−7) | 67.0 | 1 | .56 | 0.02 (−0.15 to 0.19) |
| Nucleus accumbens | 13 (11) | 426 | 478 | −0.29 (−0.53 to −0.05) | .017 | −5.5 | 35.1 (4.5 × 10−4) | 65.8 | 40 | .28 | −0.29 (−0.53 to −0.05) |
| Globus pallidus | 15 (11) | 510 | 634 | 0.26 (0.02 to 0.50) | .034 | 5.2 | 53.9 (1.3 × 10−6) | 74.0 | 44 | .13 | 0.26 (0.02 to 0.50) |
| Cerebellum | 20 (17) | 726 | 676 | −0.15 (−0.30 to −0.01) | .035 | −1.5 | 29.4 (0.06) | 35.3 | 15 | .77 | −0.15 (−0.30 to −0.01) |
Note: AWD, average weighted percentage difference; FSN, fail-safe number for publication bias; ERT, Egger’s regression test for publication bias; CSF, cerebrospinal fluid; GM, gray matter; WM, white matter
Meta-Analysis on Volumetric Brain Studies in Antipsychotic-Naive Patients With Schizophrenia
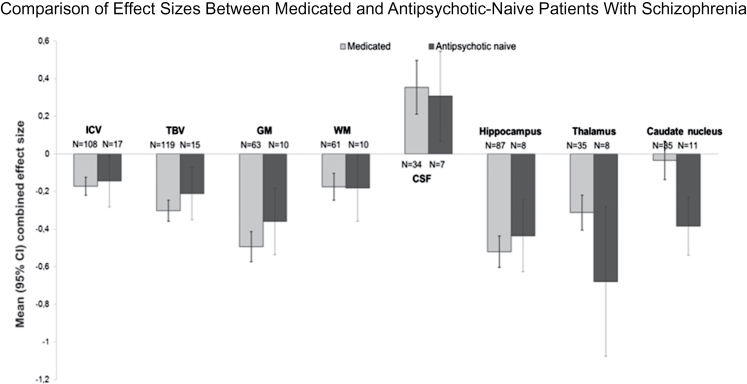
Eight brain structures were studied in at least 5 independent study samples and, thus, included in the antipsychotic-naive analysis. Specifications on the antipsychotic-naive sample are given in table 1. Details on the 33 contributing studies are given in online supplementary material table 2. As shown in table 3, intracranial (d = −0.14), total brain (d = −0.21), and total gray matter volume (d = −0.36) were significantly decreased in antipsychotic-naive patients, yet somewhat less pronounced than the reduction observed in medicated patients. Total white matter volume reduction showed a similar effect size to that observed in the medicated sample (d = −0.18). Volumes of the thalamus (d = −0.68; P = 8.3 × 10−4) and caudate nucleus (d = −0.38; P = 9.5 × 10−7) showed a more pronounced reduction in antipsychotic-naive patients than in the medicated sample (see figure 1), even when excluding 1 outlier for thalamus volume (see online supplementary material figure 2). For other volume alterations in the antipsychotic-naive sample, see table 3. Figure 1 depicts the observed effect sizes in the medicated and antipsychotic-naive samples. Forest plots of all investigated brain regions are given in online supplementary material figure 2.
Table 3.
Comparison of Brain Volumes Between Antipsychotic-Naive Patients With Schizophrenia and Controls
| Bilateral Brain Structure | Numbers of Samples (Studies) | Number of Patients | Number of Controls | Mean Weighted Effect Size Cohen’s d (95% CI) | P Valu for d | AWD in % | Cochran’s Q (P Value) | I² (Index in %) | FSN | ERT (P Value) |
|---|---|---|---|---|---|---|---|---|---|---|
| Intracranial volume | 17 (17) | 414 | 575 | −0.14 (−0.28 to −0.01) | .041 | −1.7 | 17.8 (.34) | 9.9 | 3 | .82 |
| Total brain volume | 15 (15) | 364 | 490 | −0.21 (−0.35 to −0.07) | 3.0 × 10−3 | −2.0 | 8.9 (.84) | 0.0 | 20 | .75 |
| Total gray matter | 10 (10) | 238 | 292 | −0.36 (−0.53 to −0.18) | 6.6 × 10−5 | −3.8 | 7.7 (.56) | 0.0 | 28 | .58 |
| Total white matter | 10 (10) | 238 | 292 | −0.18 (−0.36 to −0.01) | .042 | −2.4 | 6.8 (.66) | 0.0 | 0 | .58 |
| Total CSF | 7 (7) | 182 | 286 | 0.31 (0.07 to 0.55) | .011 | 7.6 | 8.6 (.20) | 30.1 | 10 | .81 |
| Hippocampus | 8 (8) | 194 | 251 | −0.43 (−0.63 to −0.24) | 7.6 × 10−6 | −4.3 | 2.4 (.93) | 0.0 | 34 | .56 |
| Thalamus | 8 (7) | 152 | 260 | −0.68 (−1.08 to −0.28) | 8.3 × 10−4 | −9.6 | 21.3 (3.3 × 10−3) | 67.2 | 60 | .12 |
| Caudate nucleus | 11 (10) | 299 | 422 | −0.38 (−0.54 to −0.23) | 9.5 × 10−7 | −5.9 | 8.3 (.60) | 0.0 | 62 | .22 |
Note: AWD, average weighted percentage difference; FSN, fail-safe number for publication bias; ERT, Egger’s regression test for publication bias; CSF, cerebrospinal fluid.
Exploration of Heterogeneity
In the analysis on the medicated sample, application of Cochran’s Q test indicated significant heterogeneity in 21 studied regions (see table 2). As outlined in online supplementary material table 3, factors such as illness duration, current use of antipsychotic medication, and sex did show significant associations with certain brain regions, partially explaining heterogeneity. Meta-regression on slice thickness revealed that smaller slice thickness was associated with larger effect sizes in a few subregions, particularly the superior frontal gyrus. With a few exceptions, year of publication did not significantly influence effect sizes.
In the analysis on antipsychotic-naive patients, significant heterogeneity was only observed for the thalamus (P = 3.3 × 10−3; I ² = 67%). In the sensitivity analysis, exclusion of studies that partly included schizophrenia-spectrum disorders, exclusion of the small minority of studies that reported only relative instead of absolute volumes, exclusion of VBM studies, or exclusion of the few samples that shared a control group did not generally change the significance of findings presented above. In addition, sensitivity analysis generally did not reduce heterogeneity, with minor exceptions (see online supplementary material table 4).
Meta-Regression, Differential Matching and Asymmetry
For complete results of meta-regression, see online supplementary material table 3.
Duration of Illness.
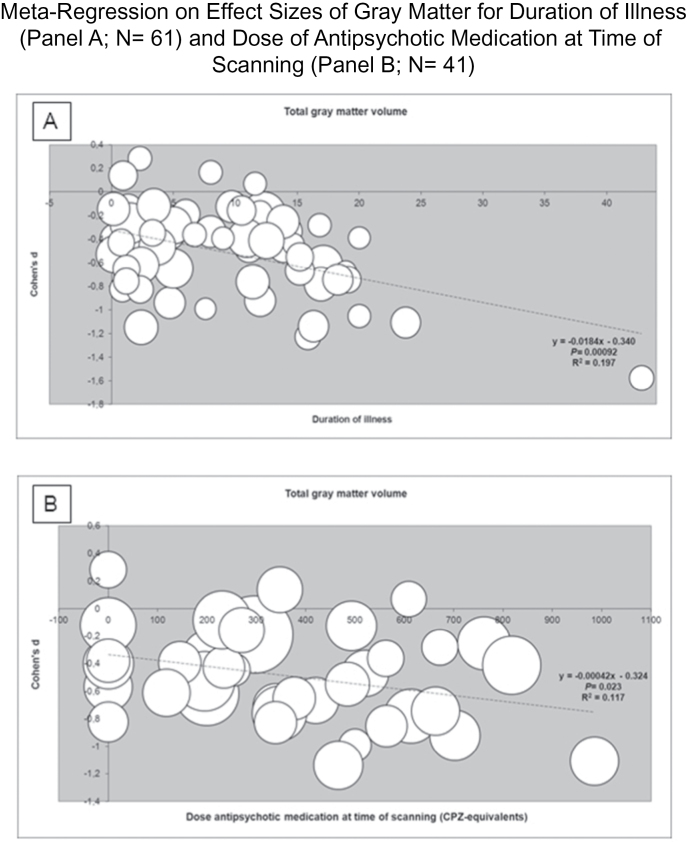
Total gray matter showed larger volume reductions with advancing duration of illness (see figure 2). The only gray matter subregion sharing this association with increasing illness duration was the prefrontal region, whereas meta-regression on several more posterior gray matter regions (superior temporal gyrus, Heschl’s gyrus, and parietal lobe) indicated attenuated volume reductions with advancing illness duration.
Current Dose of Antipsychotic Medication.
Total gray matter loss was more pronounced in patients using a higher dose of antipsychotic medication at time of scanning (see figure 2). This association was significant only for atypical antipsychotics. Dose at time of scanning was significantly correlated with duration of illness (r = .67; P < .001). Total brain volume showed larger reductions with higher dose of antipsychotic medication at time of scanning as well, with significant associations for both typical and atypical antipsychotic medication. For total white matter, no association with dose of antipsychotic medication at time of scanning was observed. Higher dose of antipsychotic medication was associated with volume increase of the caudate nucleus, an effect observed only for atypical antipsychotics.
Sex.
Sex did not affect effect sizes, with 1 major exception: Reductions in intracranial volume were associated with male sex.
Intelligence Quotient.
We investigated whether volume differences could be observed between studies that did or did not match for IQ scores between patients and controls. Approximately 25% of studies provided information regarding IQ. As displayed in online supplementary material table 5, only trends were observed toward larger reductions in intracranial and total gray matter volume in studies with significantly lower IQ scores for patients compared with controls, and a nonsignificant trend in the opposite direction for the hippocampus.
Current Substance Abuse.
Effect sizes from studies explicitly excluding subjects because of current substance abuse were not significantly different for any of the brain structures compared with studies that did not mention current substance abuse as exclusion criterion. Remarkably, the trend for volume reductions pointed toward larger effect sizes in studies excluding for current substance abuse (see online supplementary material table 6).
Height.
Study samples that matched for height (n = 14) showed an effect size of −0.13 (P = .018) for intracranial volume, which is comparable to the general effect size of −0.17.
Asymmetry.
Significant differences in effect sizes between left- and right-sided brain structures were observed only for the planum temporale, where left-sided reduction significantly exceeded right-sided volume reduction (d = −0.75 vs d = −0.29; P = .0034) (see online supplementary material table 7 and figure 3).
Publication Bias
As also shown in table 2, the fail-safe number in the analysis on the medicated sample surpassed the number of actual studies by a factor 0.75 (cerebellum) up to a factor 75 (hippocampus). Egger’s regression test indicated publication bias for total brain, total gray matter, third ventricle, frontal gray matter, prefrontal and temporal white matter, hippocampus, and Heschl’s gyrus volumes. After performing meta-regression, the number of explored brain structures per study was generally not associated with the observed effect sizes with the exception of caudate nucleus and putamen, as shown in online supplementary material table 3. Although fail-safe numbers were generally smaller in the analysis on antipsychotic-naive patients, Egger’s regression test did not indicate publication bias here. Significance of effect sizes did not change after adjustment by the Trim and Fill method, with the exception of the parahippocampal gyrus and gray matter of the parietal lobe.
In addition, we applied a previously developed test16 that recently suggested an excess of significant findings in meta-analyses on neuroimaging in psychiatry.17 As shown in online supplementary material table 8, the number of observed significant findings for both samples combined did not significantly differ from the number of expected significant findings in any brain region.
Discussion
This meta-analysis collected volumetric data from 317 MRI studies in schizophrenia until January 2012, including over 18 000 patients and controls. Because our meta-analysis included 33 studies comprising 771 antipsychotic-naive patients and 939 controls, we were also able to examine the extent of brain volume changes prior to the initiation of antipsychotic treatment. In addition, we addressed the question to what extent effect sizes were affected by illness duration, antipsychotic use at time of scanning and sex. The impact of IQ matching status between patients and controls and presence or absence of substance abuse was explored as well.
Intracranial volume showed a small but highly significant reduction in patients with schizophrenia compared with controls (d = −0.17; −2.0%), whereas the reduction of total brain volume was considerably larger (d = −0.30; −2.6%). This difference was explained by a marked reduction in total gray matter (d = −0.49; −4.3%) because white matter loss was equivalent to the decrease in intracranial volume. Focal gray and white matter volume reductions largely paralleled these global effects: Generally, reductions in gray matter structures showed effect sizes of approximately 0.4. Total CSF, lateral ventricle, and especially third ventricle (d = 0.60) volumes were increased.
In the antipsychotic-naive sample, brain changes reflected those observed in the medicated samples, albeit to a lesser extent. For total brain and gray matter volume, effect sizes were up to 30% lower in antipsychotic-naive compared with the medicated patients. The reduction in intracranial volume was comparable in both groups as was global white matter volume reduction.
These data suggest that part of the reduction in brain volume observed in schizophrenia must be present prior to the age when the cranium reaches its final size, which is in early adolescence.18 Interestingly, white matter reduction was similar in the medicated and medication-naive samples, whereas gray matter reduction was larger in the former group. This suggests in turn that the reduction in white matter—present before treatment initiation—progresses little if at all, during the subsequent course of the illness, whereas gray matter reduction—although also reduced at treatment onset—does worsen. Indeed, we found that effect sizes for gray matter volume loss increased with illness duration. Although based on cross-sectional data only, this observation is consistent with a recent meta-analysis on longitudinal volumetric studies in schizophrenia where progressive loss of gray matter—but not total white matter—was found in the schizophrenia patients.19 Similar to that study, we found that this effect was confined to prefronal gray matter. That meta-analysis did however find progressive volume decreases for frontal, temporal, and parietal white matter. In agreement with the current analysis, Olabi and colleagues19 did not find evidence for volume change over time for hippocampus and amygdala but did report progressive volume increase for lateral ventricles in contrast to what we report here in our cross-sectional meta-regression. Given their significant correlation, determining whether the larger gray matter volume reduction with increasing duration of illness is due to the effect of illness or of medication is almost impossible to disentangle, certainly in a meta-analysis of cross-sectional studies. We did find that higher dose of antipsychotic treatment at time of scanning was associated with larger volume reductions in total gray, but not in white matter. We found no support for the notion that typical antipsychotics are exclusively responsible for this effect, as suggested previously.20 Thalamic and caudate nucleus reductions were more pronounced in the studies including antipsychotic-naive patients than in the medicated sample. This finding corroborates the evidence suggesting that antipsychotics increase basal ganglia volumes, an effect associated with typical antipsychotics.21 Curiously, we found such an association only for atypical antipsychotics. Taken together, these data suggest that subcortical volume reductions are present early in the illness before antipsychotic medication is initiated and is attenuated by antipsychotic treatment. Indeed, thalamus volume reductions have been reported in subjects at high risk of developing schizophrenia22 and in relatives of patients with schizophrenia,23 suggesting that thalamic volume reduction is associated with (the risk to develop) schizophrenia. The fact that antipsychotic medication attenuates this volume loss may well account for the comparable thalamus reduction in (medicated) first episode and chronic patients,24 and the absence of caudate nucleus volume decrease both in the medicated sample in the current analysis and in the sample of Wright and colleagues.6 Interestingly, intracranial volume reduction showed a significant association with male sex. As indicated, intracranial volume reaches 90% of its final volume at the age of 5 years10 and does not change after reaching its final volume at 14 years of age.18 It is therefore tempting to speculate that the more pronounced intracranial decrement in male patients may be the result of a more severe (or earlier) disruption in neurodevelopment, which in turn could contribute to the well-documented poorer outcome in male compared with female patients.25
Because IQ shows a correlation of 0.4 with total brain volume26 and lowered premorbid intelligence is well established in schizophrenia,11 determining to what extent brain tissue alterations can be attributable to IQ decrements seems highly relevant. This analysis, by studying the differential impact of IQ matching and meta-regression on IQ scores, did not allow for clear conclusions because merely trends were observed for attenuated total gray matter and enlarged hippocampal volumetric differences between patients and controls in IQ matched studies. Studies excluding for current substance abuse did not show any trend toward effect size reduction, arguing against the possibility that brain volume alterations in schizophrenia can be largely attributable to current substance abuse.
We observed significant asymmetry only for the planum temporale, where left-sided reduction was more pronounced. The planum temporale is anatomically and functionally lateralized, with left-sided structures in particular subserving language processes, being part of Wernicke’s region.27 Reduced asymmetry of the planum temporale was recently associated with more severe positive symptoms in schizophrenia patients.28
The current analysis extends the findings of the previous general volumetric meta-analysis in schizophrenia, and for the medicated sample, we included studies from the last third of 1998 when that analysis ended.6 The effect size found for intracranial volume was comparable to the observed effect size found in the previous meta-analysis on intracranial volume (−0.18; P = .001), that shared no contributing studies with the current analysis and combined CT and MRI imaging techniques.9 More recently, Woods and colleagues reported a significant reduction of intracranial volume of 20 cc in patients compared with controls (based on 20 studies that partly overlap with the current analysis).29 With a mean intracranial volume of 1460 cc in controls in the current analysis, this represents a decrease of 1.4% (current analysis: 2.0%). For total brain, white matter, and lateral and third ventricles, effect sizes were quite comparable to those reported by Wright and colleagues.6 However, we found more pronounced reductions in total gray matter and hippocampus volumes. Presumably, these differences are at least partly due to greater statistical power in the current analysis as we were able to include up to 15 times as many studies in some regions. Regions most consistently showing clusters of gray matter decreases in VBM based meta-analyses such as insula, anterior cingulate cortex, inferior and medial frontal gyrus, amygdala, and thalamus2–5 all showed significant volume decreases in the current analyses although none surpassed the general effect size for overall gray matter reduction.
A meta-analysis is as valuable as its constituting studies, which mainly determine the major limitations of the current analysis. Selective publishing of significant results can never be ruled out, as was recently emphasized.17 Egger’s regression test for publication bias did suggest the existence of such bias for several regions, although not for any brain structure in the antipsychotic-naive subsample. However, fail-safe numbers for these regions exceeded the actual number of studies by up to a factor 75 so that it is unlikely that publication bias alone generated volumetric alterations. We cannot exclude the possibility that authors selectively reported significant findings. However, the number of investigated brain regions generally did not affect effect sizes, and application of a recently recommended test17 did not indicate major excess significance. On the other hand, the majority of explored brain regions showed significant heterogeneity, complicating the interpretation of the results, although it is conceivable that Cochran’s test is too strict for large meta-analyses to be suitable.30 Indeed, application of the I² test showed considerably less heterogeneity. Meta-regression on illness duration, sex, and current dose of antipsychotic treatment partly explained heterogeneity, whereas performing sensitivity analysis generally did not reduce heterogeneity. IQ matching did reduce heterogeneity, although this effect may also be explained by the smaller sample sizes in the IQ matching analysis. Except for thalamus volume, no significant heterogeneity was found in the antipsychotic-naive analysis. We assume that at least part of this difference in heterogeneity is due to the large number of studies included in the general analysis.
It is conceivable that different brain imaging techniques had an impact on observed effect sizes. However, applied MRI techniques (whether manual, semiautomatic, or automatic) are not uniform and, therefore, unsuitable for group comparison. In addition, applied segmentation techniques likely relate to year of publication and image resolution that would have a confounding impact on the relation between segmentation technique and observed effect sizes. For these reasons, we were unable to explore the relation between imaging technique and effect sizes.
The differentiation of studies based on IQ matching and the exclusion of current substance abuse was somewhat imprecise. For example, IQ estimation was generally based on vocabulary and block design subtasks on the Wechsler Adult Intelligence Scale or on the National Adult Reading Test, representing an estimate of current or premorbid IQ, respectively. Numbers of studies that matched on IQ were relatively small, increasing the possibility of type II errors. In addition, even in the IQ matched sample, all contributing studies showed lower IQ scores in patients, thus limiting comparability.
Illness duration was substantially lower in antipsychotic-naive patients compared with medicated patients, and whether these patients suffered from less severe illness could not be established. Although this limits the comparability of both groups, it did not affect the primary aim of this comparison, which was establishing to what extent brain volume changes could be observed in schizophrenia that were not attributable to antipsychotic medication use. To examine the effect of antipsychotic medication on brain volumes, we were confined to dose at time of scanning as variable because cumulative antipsychotic dose was inconsistently reported in contributing studies. This limits the interpretability of the effect of antipsychotic use on brain volumes in this meta-analysis.
Furthermore, because we did not correct for multiple testing in the meta-regression analysis due to the conservative nature of the applied statistical method, significant findings should be interpreted with caution given the amount of variables tested. It should be stressed that regression on illness duration is a crude way of investigating disease progression and longitudinal designs are needed to draw definitive conclusions. Finally, despite the fact that all contributing studies were matched for sex, the medicated sample showed significantly more males in the patient group compared with controls. In our analysis, in both patient and control groups, intracranial volume was 11% smaller in females, representing an effect size of approximately −1.6 (data available upon request). It seems reasonable to assume that, as the sex difference between patients and controls equaled 4.7%, reported brain reductions were underestimated and volume increases were overstated by an effect size of approximately 0.075.
In summary, this meta-analysis in over 18 000 subjects explored the extent of brain volume alterations in schizophrenia. It showed a small but highly significant reduction in intracranial volume (most pronounced in male patients) with a similar decrease in white matter volume, suggesting a neurodevelopmental origin to some of the brain volume reductions observed in schizophrenia. Total brain volume loss was nearly twice as large as intracranial volume reduction, this difference being completely attributable to a decrease in gray matter. The latter showed an association with longer duration of illness and higher dose of antipsychotic medication at the time of scanning. Effect sizes for total brain and gray matter volumes in the samples of antipsychotic-naive patients were approximately 75% of those found in the medicated samples, indicating that the largest part of brain volume reduction in schizophrenia is present before treatment is initiated. Caudate nucleus and thalamus volumes showed remarkably large reductions in antipsychotic-naive patients.
Thus, on the basis of this meta-analysis including over 8000 medicated and nearly 800 medication-naive patients, the conclusion appears warranted that brain loss in schizophrenia is related to a combination of (early) neurodevelopmental processes—reflected in intracranial volume reduction—and illness progression.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Acknowledgments
The authors thank the following authors who responded to our request for additional information: S. Arndt, S. Bachmann, G. Bartzokis, D. Berge, P. Brambilla, A. Brennan, A.M. Brickman, R.W. Buchanan, M.S. Buchsbaum, M. Chakos, S.L. Collinson, B. Crespo-Facorro, T.J. Crow, C. Davatzikos, A. David, S. Ehrlich, L.A. Flashman, W.W. Fleischhacker, G. Frisoni, B. Ganeshan, N. Gogtay, R.C. Gur, A. Hasan, J. Hietala, R. Highley, W.G. Honer, H.J. Horn, D. Hubl, A. James, E.M. Joyce, G.W.K. Juckel, C. Junque, E. Kemether, M. Keshavan, J.-J. Kim, A.C. Krabbendam, M. Kubicki, J.S. Kwon and W.H. Jung, D. Lang, S.M. Lawrie, J.A. Lieberman, D. Mamah, M. Martín-Loeches, R.W. McCarley, E.M. Meisenzahl, S. Mitelman, P. Moberg, V. Molina, R. Nesvag, G. Okugawa, P. Premkumar, A. Prestia, R. Rajarethinam, G. Rametti, M.L. Reite, L.M. Rimol, R. Roiz Santiánez, P. Sachdev, D.F. Salisbury, P.C. Sallet, N. Schork, M.J. Smith, G. Spalletta, M. Suzuki, P. Szeszko, T. Takahashi, Y. Takayanagi, P. Tanskanen, G. Venkatasubramanian, L. Wang, H. Witthaus, H. Yamasue, M. Zarei, and R.B. Zipursky. None of the authors report any financial interest in the study.
References
Articles from Schizophrenia Bulletin are provided here courtesy of Oxford University Press
Full text links
Read article at publisher's site: https://doi.org/10.1093/schbul/sbs118
Read article for free, from open access legal sources, via Unpaywall:
https://academic.oup.com/schizophreniabulletin/article-pdf/39/5/1129/16975520/sbs118.pdf
Citations & impact
Impact metrics
Article citations
Caudate nucleus volume in medicated and unmedicated patients with early- and adult-onset schizophrenia.
Sci Rep, 14(1):22755, 01 Oct 2024
Cited by: 0 articles | PMID: 39353988 | PMCID: PMC11445249
Peripheral Lipid Signatures, Metabolic Dysfunction, and Pathophysiology in Schizophrenia Spectrum Disorders.
Metabolites, 14(9):475, 28 Aug 2024
Cited by: 0 articles | PMID: 39330482 | PMCID: PMC11434505
Review Free full text in Europe PMC
Peering into the mind: unraveling schizophrenia's secrets using models.
Mol Psychiatry, 08 Sep 2024
Cited by: 0 articles | PMID: 39245692
Review
Cognitive subgroups of affective and non-affective psychosis show differences in medication and cortico-subcortical brain networks.
Sci Rep, 14(1):20314, 02 Sep 2024
Cited by: 0 articles | PMID: 39223185 | PMCID: PMC11369100
Dysregulation of myelination-related genes in schizophrenia.
J Neurochem, 168(9):2227-2242, 31 Jul 2024
Cited by: 0 articles | PMID: 39086020
Review
Go to all (466) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Progressive brain changes in schizophrenia related to antipsychotic treatment? A meta-analysis of longitudinal MRI studies.
Neurosci Biobehav Rev, 37(8):1680-1691, 14 Jun 2013
Cited by: 268 articles | PMID: 23769814 | PMCID: PMC3964856
The Effect of Antipsychotic Treatment on Cortical Gray Matter Changes in Schizophrenia: Does the Class Matter? A Meta-analysis and Meta-regression of Longitudinal Magnetic Resonance Imaging Studies.
Biol Psychiatry, 78(6):403-412, 16 Feb 2015
Cited by: 120 articles | PMID: 25802081
Brain volume changes in first-episode schizophrenia: a 1-year follow-up study.
Arch Gen Psychiatry, 59(11):1002-1010, 01 Nov 2002
Cited by: 209 articles | PMID: 12418933
Progressive loss of cortical gray matter in schizophrenia: a meta-analysis and meta-regression of longitudinal MRI studies.
Transl Psychiatry, 2:e190, 20 Nov 2012
Cited by: 227 articles | PMID: 23168990 | PMCID: PMC3565772
Review Free full text in Europe PMC