Abstract
Background and objectives
Renal biopsies performed in diabetic patients are increasing in number and complexity. This study sought to determine the usefulness of renal biopsy in patients with diabetes and the predictability of diagnosing diabetic nephropathy (DN) versus nondiabetic renal disease (NDRD) from clinical and laboratory data.Design, setting, participants, & measurements
To assess modern trends, a retrospective study was performed of clinical-pathologic findings in all patients with diabetes who had a biopsy in 2011. Among 2642 native kidney biopsies, 620 (23.5%) were from patients with diabetes.Results
The cohort included 371 men (60.7%) aged a median (interquartile range) 62 years (52-69) with 10-year (5-15) duration of diabetes mellitus (DM). Median serum creatinine was 2.5 mg/dl (1.6-4.4), and 52% of patients had stage 4-5 CKD. On biopsy, 37% of patients had DN alone, 36% had NDRD alone, and 27% had DN plus NDRD. In NDRD alone, FSGS (22%), hypertensive nephrosclerosis (18%), acute tubular necrosis (ATN) (17%), IgA nephropathy (11%), membranous GN (8%), and pauci-immune GN (7%) comprised 80% of diagnoses, compared with ATN (43%), hypertensive nephrosclerosis (19%), FSGS (13%), and IgA nephropathy (7%) for DN plus NDRD. In multivariate analyses, longer duration of DM was associated with a greater likelihood of DN and a lower likelihood of NDRD: each added year of DM reduced the odds of NDRD by 5% (odds ratio, 0.95; 95% confidence interval, 0.91 to 0.98; P=0.004). DM duration ≥ 12 years was the best predictor (58% sensitivity, 73% specificity) of DN alone.Conclusions
Approximately one-quarter of all renal biopsies are performed in patients with DM. Judicious use of renal biopsy has uncovered NDRD alone or superimposed on DN in the majority of such biopsies. ATN is emerging as an important category of NDRD, which has not been reported previously.Free full text

The Modern Spectrum of Renal Biopsy Findings in Patients with Diabetes
Summary
Background and objectives
Renal biopsies performed in diabetic patients are increasing in number and complexity. This study sought to determine the usefulness of renal biopsy in patients with diabetes and the predictability of diagnosing diabetic nephropathy (DN) versus nondiabetic renal disease (NDRD) from clinical and laboratory data.
Design, setting, participants, & measurements
To assess modern trends, a retrospective study was performed of clinical-pathologic findings in all patients with diabetes who had a biopsy in 2011. Among 2642 native kidney biopsies, 620 (23.5%) were from patients with diabetes.
Results
The cohort included 371 men (60.7%) aged a median (interquartile range) 62 years (52–69) with 10-year (5–15) duration of diabetes mellitus (DM). Median serum creatinine was 2.5 mg/dl (1.6–4.4), and 52% of patients had stage 4–5 CKD. On biopsy, 37% of patients had DN alone, 36% had NDRD alone, and 27% had DN plus NDRD. In NDRD alone, FSGS (22%), hypertensive nephrosclerosis (18%), acute tubular necrosis (ATN) (17%), IgA nephropathy (11%), membranous GN (8%), and pauci-immune GN (7%) comprised 80% of diagnoses, compared with ATN (43%), hypertensive nephrosclerosis (19%), FSGS (13%), and IgA nephropathy (7%) for DN plus NDRD. In multivariate analyses, longer duration of DM was associated with a greater likelihood of DN and a lower likelihood of NDRD: each added year of DM reduced the odds of NDRD by 5% (odds ratio, 0.95; 95% confidence interval, 0.91 to 0.98; P=0.004). DM duration ≥12 years was the best predictor (58% sensitivity, 73% specificity) of DN alone.
Conclusions
Approximately one-quarter of all renal biopsies are performed in patients with DM. Judicious use of renal biopsy has uncovered NDRD alone or superimposed on DN in the majority of such biopsies. ATN is emerging as an important category of NDRD, which has not been reported previously.
Introduction
As the prevalence of diabetes mellitus (DM) has reached epidemic proportions, the number of renal biopsies performed in diabetic patients is increasing. Patients with diabetes subjected to renal biopsy may manifest diabetic nephropathy (DN) alone, DN with superimposed nondiabetic renal disease (NDRD), or NDRD alone. Differentiating between these diagnostic categories can influence patient management and prognosis. Diagnosing NDRD is especially important when it leads to a specific change in therapy.
The clinical features that have previously been shown to predict renal involvement by NDRD in the diabetic patient are sudden onset of proteinuria, proteinuria in the absence of retinopathy or neuropathy, short duration of DM, ARF, and hematuria (1,2). Conversely, the factors predicting DN include the presence of retinopathy or neuropathy and longer duration of DM, generally exceeding 10 years (2–4). However, because of the variability of clinical course and the frequency of confounding medical problems in this population, it remains difficult in the individual patient to differentiate between DN and NDRD without the aid of renal biopsy (5).
Therefore, the decision to pursue renal biopsy becomes a pivotal clinical judgment when evaluating diabetic patients with renal disease. The nephrologist must consider whether NDRD is potentially present and the risk/benefit ratio of biopsy (6). To assess modern trends, we undertook the largest reported single-center study of clinical-pathologic findings in all diabetic patients with renal biopsies processed at our center over a one year period. Our aim was to explore the usefulness of renal biopsy in diabetic patients and to assess the predictability of diagnosing NDRD versus DN on the basis of clinical and laboratory findings.
Materials and Methods
The study was approved by the Institutional Review Board of Columbia University Medical Center. All consecutive native renal biopsies accessioned in the Columbia Renal Pathology Laboratory from January through December 2011 were reviewed retrospectively for clinical evidence of DM. Of 2642 native biopsies, 620 diabetic patients (23.5%) were identified, 611 of which had adequate tissue for diagnosis.
All renal biopsies were processed according to standard techniques for light microscopy, immunofluorescence, and electron microscopy and were interpreted by one of four renal pathologists. The major renal biopsy diagnoses provided on the diagnostic line of the biopsy report were tabulated according to three potential categories: DN alone, DN with superimposed NDRD, and NDRD alone.
Patients’ medical records were reviewed for demographics (age, sex, race), duration of diabetes, presenting renal findings, and laboratory and serologic findings. We recorded the referring nephrologist’s indications for renal biopsy as AKI on a baseline of no CKD, AKI on a baseline of CKD, level of proteinuria, any positive serologic test, and active urine sediment. The following laboratory findings at the time of biopsy were recorded: urine protein (measured by 24-hour urine collection or spot urine protein/creatinine ratio), serum creatinine (mg/dl), and estimated GFR (eGFR) (measured by the Modified Diet in Renal Disease study equation). The following clinical definitions were applied: AKI, new development of serum creatinine >1.2 mg/dl or >0.3 mg/dl increase from baseline over a period of <3 months; nephrotic-range proteinuria, 24-hour urine protein >3.5 g/d or spot urine protein/creatinine ratio >3.5 g/g; active urine sediment, hematuria with >5 red blood cells per high-power field; and leukocyturia, >5 white blood cells per high-power field.
The pathologic criteria for DN included diffuse mesangial sclerosis and glomerular basement membrane thickening (≥450 nm) at the light microscopic and ultrastructural levels, with or without mesangial nodularity. Supportive histologic features of DN included thickening of the tubular basement membranes of nonatrophic tubules; diffuse linear staining of glomerular and tubular basement membranes for albumin and IgG; and hyalinosis of glomeruli and vessels producing fibrin caps, capsular drops, and arteriolar hyalinosis. A diagnosis of hypertensive nephrosclerosis in addition to DN was made only in patients with history of longstanding hypertension and findings of mild diffuse diabetic glomerulosclerosis but disproportionate (moderate to severe) arterio- and arteriolosclerosis, extensive global glomerulosclerosis, ischemic-type glomerular tuft retraction, and a tendency to subcapsular scarring. Because some degree of interstitial inflammation is commonly seen in DN, a diagnosis of acute interstitial nephritis (AIN) was made only if interstitial infiltrates involved areas without tubular atrophy/interstitial fibrosis, included eosinophils, and displayed active tubulitis. A diagnosis of acute tubular necrosis (ATN) was made if nonatrophic tubules displayed diffuse acute tubular injury, including epithelial simplification, loss of brush border, and focal cytoplasmic shedding.
Statistical analysis was performed using STATA software (version 11.0). Univariate comparisons between groups were made using the t test (categorical variables) and the Wilcoxon rank-sum test (continuous variables). Multivariate analyses of variables considered as potential predictors of DN versus NDRD were performed using logistic regression.
Results
Key clinical and laboratory data of the cohort, stratified by whether biopsy showed DN alone, DN plus NDRD, or NDRD alone, are summarized in Table 1. The median age of the cohort at biopsy was 62 (interquartile range [IQR], 52–69) years, although patients with DN alone were slightly younger than patients with NDRD. Approximately 61% (n=371) of patients were men. Race was unknown in nearly half of patients (n=269, 44.0%). Among the 342 patients with known race/ethnicity information, the majority were Caucasian (n=195, 57.0%), followed by African American (n=101, 29.5%), Hispanic (n=27, 7.9%), and Asian (n=15, 4.4%). Neither sex nor race appeared to differ between patients with DN alone, DN plus NDRD, or NDRD alone. Only 16 patients had type 1 DM, nine of whom had DN alone. Median duration of DM (5 years) was significantly shorter in patients with NDRD alone versus patients with DN alone (13 years) and patients with DN plus NDRD (10 years). Data on retinopathy were missing for 524 patients (85.8%); 70 of the remaining 87 patients had retinopathy.
Table 1.
Key demographic and clinical data at time of kidney biopsy
| Characteristics | DN Alone | DN Plus NDRD | NDRD Alone |
|---|---|---|---|
| Participants (n) | 227 | 164 | 220 |
| Age (yr) | 59 (49–65) | 63 (55–72)a | 63 (54–70)b |
| Male sex | 129 (56.8) | 100 (61.0) | 142 (64.6) |
| Race | |||
 Unknown Unknown | 108 (47.6) | 57 (34.8)a | 104 (47.3)c |
 White White | 62 (27.3) | 63 (38.4)a | 70 (31.8) |
 African American African American | 39 (17.2) | 33 (20.1) | 29 (13.2) |
 Hispanic Hispanic | 12 (5.3) | 7 (4.3) | 8 (3.6) |
 Asian Asian | 4 (1.8) | 4 (2.4) | 7 (3.2) |
 Other Other | 2 (0.9) | 0 (0.0) | 2 (0.9) |
| DM type 1 | 9 (4.0) | 5 (3.1) | 2 (0.9)b |
| Duration of DM (yr) | 13 (8–17) | 10 (7–18) | 5 (3–10)b,c |
| Serum creatinine (mg/dl) | 2.3 (1.6–3.8) | 3.1 (1.7–5.2)a | 2.3 (1.5–4.4)c |
| eGFR (ml/min per 1.73 m2) | 31.3 (17.5–55.2) | 21.4 (12.5–46.6)a | 32.5 (14.3–60.0)c |
| Proteinuria (g/d) | 5.0 (2.8–8.8) | 5.0 (2.0–8.0) | 2.9 (1.4–7.1)b,c |
Of 620 patients with diabetes who underwent biopsy, 611 had adequate tissue for diagnosis. Categorical variables are expressed as n (%); continuous variables are expressed as median (interquartile range). DN, diabetic nephropathy; NDRD, nondiabetic renal disease; DM, diabetes mellitus; eGFR, estimated GFR.
The entire cohort was marked by significant renal dysfunction, with median creatinine of 2.5 mg/dl (IQR, 1.6–4.4) and eGFR 29.1 ml/min per 1.73 m2 (IQR, 14.5–54.5) at time of biopsy; just over half of patients had eGFR <30 ml/min per 1.73 m2. Patients with DN alone had significantly lower creatinine and higher eGFR than patients with DN plus superimposed NDRD. Median proteinuria for the entire cohort was in the nephrotic range (4.3; IQR, 1.9–8.0 g/d); patients with DN alone (median 5.0 g/d) or with concomitant NDRD (median 5.0 g/d) had significantly higher proteinuria than patients with NDRD alone (median 2.9 g/d). Nephrotic-range proteinuria was significantly more prevalent in patients with DN alone (54.2%) and DN plus NDRD (47.0%) compared to patients with NDRD alone (31.4%).
DN alone was detected in 227 of 611 patients; in the remaining 384 patients with NDRD, ATN, FSGS, and hypertensive nephrosclerosis were the leading diagnoses, followed by IgA nephropathy (IgAN) (Figure 1) and membranous GN (MGN) (Table 2). ATN was more likely to be found with, rather than without, concomitant DN. Glomerular diseases such as FSGS, IgAN, MGN, and pauci-immune GN, were more likely to be present in the absence of, rather than superimposed on, DN. This was particularly true when proteinuria was in the subnephrotic range: All 4 patients with MGN and subnephrotic proteinuria, 12 of 16 patients with IgAN and subnephrotic proteinuria, and 25 of 27 patients with FSGS and subnephrotic proteinuria had no evidence of DN on biopsy.
Table 2.
Summary of NDRD, with and without DN, found on biopsies of patients with diabetes
| Types of NDRD (n) | NDRD Alone (n=220) | DN Plus NDRD (n=164) | P Valuea |
|---|---|---|---|
| Acute tubular necrosis (109) | 38 (17.3) | 71 (43.3) | <0.001 |
| FSGS (69) | 48 (21.8) | 21 (12.8) | 0.02 |
 Primary FSGS (6) Primary FSGS (6) | 6 (2.7) | 0 (0.0) | 0.03 |
 Secondary FSGS (63)b Secondary FSGS (63)b | |||
  HTN related HTN related | 19 (8.6) | 10 (6.1) | 0.35 |
  HTN plus obesity related HTN plus obesity related | 16 (7.3) | 10 (6.1) | 0.65 |
  Obesity related Obesity related | 4 (1.8) | 1 (0.6) | 0.30 |
  Otherc Otherc | 3 (1.4) | 0 (0.0) | 0.13 |
| Hypertensive nephrosclerosis (70)b | 39 (17.7) | 31 (18.9) | 0.77 |
| IgA nephropathy (35) | 23 (10.5) | 12 (7.3) | 0.29 |
| Membranous GN (23) | 18 (8.2) | 5 (3.0) | 0.04 |
| Pauci-immune crescentic GN (19) | 15 (6.8) | 4 (2.4) | 0.05 |
| Acute interstitial nephritis (18) | 11 (5.0) | 7 (4.3) | 0.73 |
| Amyloidosis (10) | 10 (4.5) | 0 (0) | 0.01 |
| Myeloma cast nephropathy (10) | 8 (3.6) | 2 (1.2) | 0.14 |
| Acute postinfectious GN (6) | 3 (1.4) | 3 (1.8) | 0.72 |
| Atheroembolic disease (5) | 2 (0.9) | 3 (1.8) | 0.43 |
| Others (10) | 5 (2.3) | 5 (3.0) | 0.64 |
Values are expressed as n (%). NDRD, nondiabetic renal disease; DN, diabetic nephropathy; HTN, hypertension.
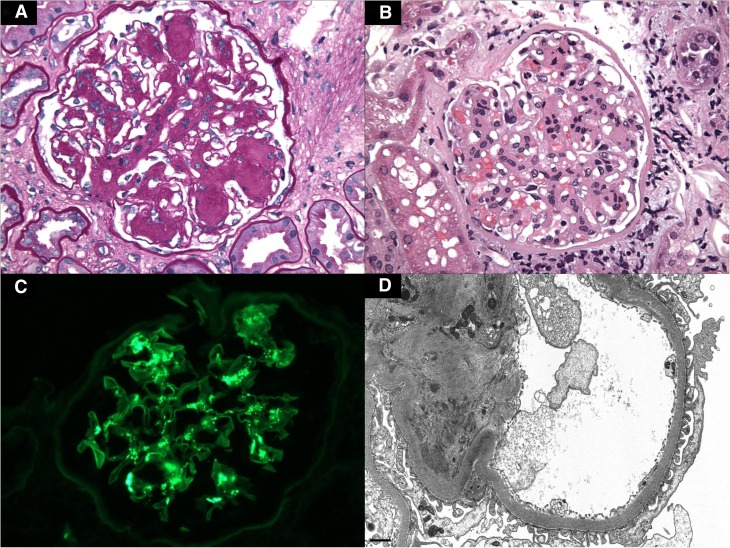
Diabetic nephropathy with superimposed IgA nephropathy. (A) A glomerulus with moderate nodular mesangial sclerosis and glomerular basement membrane thickening exhibits mild segmental mesangial hypercellularity (staining with periodic acid–Schiff). (B) A representative glomerulus shows mesangial expansion by increased mesangial cell number and matrix (staining with hematoxylin and eosin). (C) Immunofluorescence staining for IgA shows global mesangial immune deposits within the expanded mesangium. (D) By electron microscopy, there is a paramesangial electron dense deposit typical of IgA nephropathy associated with mesangial sclerosis and mild uniform glomerular basement membrane thickening owing to underlying diabetic nephropathy. Original magnification, ×400 in A–C; ×10,000 in D.
The frequent finding of ATN in this cohort prompted a query in our database during the same 1-year period for the incidence of ATN as a separate diagnostic entry in other major categories of renal disease typically associated with nephrotic-range proteinuria or nephrotic syndrome. The incidence rates were as follows: ATN in minimal change disease, 16 of 88 (18.2%); glomerular tip lesion, 2 of 17 (11.8%); FSGS not otherwise specified, 10 of 368 (2.7%); MGN, 15 of 315 (4.7%); and amyloidosis, 2 of 60 (3.3%). The biopsy incidence of ATN superimposed on DN (43.3%) was significantly greater than in any of these categories. In this cohort, ATN was found more commonly in patients who had a biopsy for AKI compared with another indication: Of the 109 cases of ATN in this cohort, 104 patients had a biopsy for indications of AKI (77 with AKI and normal baseline renal function, and 27 with AKI on top of baseline CKD).
Serologic testing for systemic diseases (e.g., hepatitis B surface antigen or hepatitis C virus antibodies, antinuclear antibody, ANCA, serum protein electrophoresis, urine protein electrophoresis) was positive in at least one parameter in approximately one-third of all diabetic patients who underwent biopsy, without a clear distinction among those with DN alone, DN plus NDRD, and NDRD alone (Table 3). When examining specific tests, however, we found an association between low complement levels (C3 and/or C4) and NDRD (alone or with coexistent DN) and an association between M-spike in either serum or urine and NDRD (alone) compared with DN alone. These serologies were not entirely predictive of disease findings on biopsy and instead were commonly found in the setting of ATN, secondary FSGS, and hypertensive nephrosclerosis. Only 24% of patients with positive M-spike had related pathologies including myeloma cast nephropathy or amyloidosis, and only 25% of patients with low complements were diagnosed with hypocomplementemic glomerulonephritides, including lupus nephritis, membranoproliferative GN, cryoglobulinemic GN, or acute postinfectious GN.
Table 3.
Reported indications and notable laboratory values in patients with diabetes who underwent kidney biopsy
| Variables | DN Alone | DN plus NDRD | NDRD Alone |
|---|---|---|---|
| Patients (n) | 227 | 164 | 220 |
| Proteinuria (mg/d) | |||
 Data unavailable Data unavailable | 42 (18.5) | 39 (23.8) | 54 (24.6) |
 <500 <500 | 13 (5.7) | 9 (5.5) | 21 (9.6) |
 500–3500 500–3500 | 49 (21.6) | 39 (23.8) | 76 (34.6)a,b |
 >3500 >3500 | 123 (54.2) | 77 (47.0) | 69 (31.4)a,b |
| Active urine sediment | 63 (27.8) | 62 (37.8)c | 74 (33.6) |
| AKI (baseline no CKD) | 101 (44.5) | 85 (51.8) | 110 (50.0) |
| AKI (baseline CKD) | 37 (16.3) | 43 (26.2)c | 37 (16.8)b |
| All AKI | 138 (60.8) | 128 (78.1)c | 147 (66.8)b |
| Any positive serologic test | 69 (30.4) | 60 (36.6) | 77 (35.0) |
| (+) ANA, dsDNA, or cardiolipin antibody | 28 (12.3) | 26 (15.9) | 20 (9.1)b |
| (+) ANCA | 7 (3.1) | 10 (6.1) | 15 (6.8) |
| Low C3 and/or C4 | 2 (0.9) | 16 (9.8)c | 12 (5.5)a |
| (+) HBsAg or HCV antibody | 22 (9.7) | 16 (9.8) | 13 (5.9) |
| M-spike (serum or urine) | 16 (7.1) | 13 (7.9) | 30 (13.6)a |
| (+) ASLO | 2 (0.9) | 3 (1.8) | 3 (1.4) |
| (+) HIV | 1 (0.4) | 5 (3.1)c | 4 (1.8) |
Variables are expressed as n (%). DN, diabetic nephropathy; NDRD, nondiabetic renal disease; ANA, antinuclear antibody; dsDNA, double-strand DNA; HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; ASLO, anti-streptolysin O.
To assess the strongest predictors of NDRD versus DN alone, we then used multivariate logistic regression models, adjusting for degree of proteinuria, baseline eGFR, age, sex, race, duration of DM, AKI, complement levels, and serum and urine protein electrophoresis results (Table 4). Longer duration of DM was associated with a greater likelihood of DN and a lower likelihood of NDRD: each added year of DM reduced the odds of NDRD by 5% (odds ratio [OR], 0.95; 95% confidence interval [95% CI], 0.91 to 0.98; P=0.004). In secondary analyses, duration of DM was only significantly associated with NDRD alone (OR, 0.90; 95% CI, 0.85–0.95; P<0.001) and not with NDRD plus coexistent DN (OR, 1.02; 95% CI, 0.98 to 1.05; P=0.39). Nephrotic-range proteinuria (OR, 0.32; 95% CI, 0.11 to 0.95; P=0.04) was also inversely associated with finding NDRD alone on biopsy.
Table 4.
Association of key clinical predictors and biopsy findings of nondiabetic renal disease
| Variables | OR (95% CI) | P Value |
|---|---|---|
| Proteinuria (mg/d) | ||
 <500 <500 | 1.00 (reference) | |
 500–3500 500–3500 | 1.28 (0.39 to 4.20) | 0.68 |
 >3500 >3500 | 0.55 (0.19 to 1.66) | 0.29 |
| eGFR (ml/min per 1.73 m2) | ||
 >60 >60 | 1.00 (reference) | |
 30–60 30–60 | 0.89 (0.35 to 2.25) | 0.81 |
 15–30 15–30 | 1.42 (0.53 to 3.82) | 0.49 |
 ≤15 ≤15 | 1.54 (0.48 to 4.96) | 0.47 |
| Age | 1.03 (1.00 to 1.06) | 0.06 |
| Male sex | 1.05 (0.54 to 2.02) | 0.89 |
| Race | ||
 Unknown Unknown | 1.00 (reference) | |
 White White | 0.93 (0.46 to 1.91) | 0.85 |
 Black Black | 1.38 (0.49 to 3.84) | 0.54 |
 Hispanic Hispanic | 1.07 (0.27 to 4.23) | 0.93 |
 Asian Asian | 1.66 (0.26 to 10.67) | 0.59 |
| Duration of diabetes | 0.95 (0.91 to 0.98) | 0.004 |
| AKI | 1.44 (0.67 to 3.07) | 0.35 |
| Low complements | 4.70 (0.49 to 45.42) | 0.18 |
| M-spike (serum or urine) | 1.50 (0.51 to 4.37) | 0.46 |
Analysis performed using multivariate logistic regression. OR, odds ratio; 95% CI, 95% confidence interval; eGFR, estimated GFR.
Of the 384 patients with NDRD (220 alone, 164 with concomitant DN), 186 revealed lesions that, in general, would alter treatment decisions. This 186-patient subgroup excludes participants with FSGS, ATN, and hypertensive nephrosclerosis and includes patients with diagnoses of AIN, pauci-immune GN, acute postinfectious GN, MGN, IgAN, amyloidosis, cast nephropathy, fibrillary GN, and lupus nephritis. The majority of these cases (69%) were found in patients with NDRD alone. In a multivariate logistic regression model, duration of DM remained an important predictor of outcome (OR, 0.94; 95% CI, 0.90 to 0.99; P=0.01) alongside a significant association between low complements (OR, 13.43; 95% CI, 2.28 to 79.15; P=0.004) and M-spikes (OR, 4.10; 95% CI, 1.46 to 11.50; P=0.01) with these forms of NDRD.
Given the emergence of duration of DM as the consistent predictor in our logistic regression models, we applied receiver operating characteristic curves to further define the importance of duration of DM. For all cases of DN, duration of DM ≥8 years was the best predictor (76.8% sensitivity, 63.2% specificity, 78.5% positive predictive value, and 61.0% negative predictive value; area under the curve, 0.75; 95% CI, 0.69 to 0.81), and for DN alone, duration of DM ≥12 years was the best predictor (57.5% sensitivity, 73.3% specificity, 56.0% positive predictive value, and 74.5% negative predictive value; area under the curve, 0.66; 95% CI, 0.60 to 0.73).
Discussion
In this cohort of 620 patients with DM, the largest study to date of renal biopsy findings in diabetic patients, NDRD was identified in >60% of biopsies: 220 patients with NDRD alone and 164 patients with NDRD and coexistent DN. We found that duration of diabetes was the strongest predictor of whether NDRD or DN was identified on biopsy. The median duration of DM in patients with NDRD alone was 5 years, which was significantly shorter than in patients with DN alone (13 years) and DN plus NDRD (10 years), and DM duration ≥12 years emerged as the best predictor of DN alone. In addition, although the median proteinuria for the entire cohort was in the nephrotic range, heavier proteinuria was associated with a lower likelihood of finding NDRD alone. Taken together, these results suggest that the diabetic patient who is most likely to have NDRD alone has a short duration of DM and subnephrotic proteinuria.
Our results are an important addition to previously reported data from smaller cohorts on biopsy findings in diabetic patients and the significance of disease duration and proteinuria. A number of smaller studies, with cohort sizes ranging from 52 to 370, have reported no differences in the duration of DM between patients with DN and NDRD (7–9). Conversely, Tone et al. (3) found that short duration of diabetes (<5 years) carried high sensitivity (75%) and specificity (70%) for predicting NDRD, and Chang et al. (2) reported a mean DM duration of 5.9 years in patients with NDRD versus 10.6 years in patients with DN alone (P<0.001 for comparison). Similarly, previous reports have provided conflicting findings on the relative weight of proteinuria in predicting DN versus NDRD. Whereas some investigators have reported nephrotic-range proteinuria in patients with DN alone, DN plus NDRD, and NDRD alone (1,2), the majority of reports have suggested that a higher degree of proteinuria is found in DN than in NDRD (3,4,8,10,11).
In clinical settings, a number of serologic tests are often performed on diabetic patients with proteinuria, particularly those with either an acute onset of proteinuria or an abrupt increase in proteinuria. The value of such serologic testing in predicting NDRD has not been reported by others (1–4,8,10,11). In our cohort, although the finding of any positive serologic test performed at the time of biopsy was not predictive of NDRD, the specific findings of low complement levels (low C3 and/or low C4) and M-spike in either serum or urine, on univariate analyses, were associated with NDRD on biopsy. These serologies, however, were only predictive of biopsy findings in about 25% of cases, and in multivariate models, low complements and M-spikes were associated with NDRD only when cases of ATN, FSGS, and hypertensive nephrosclerosis were excluded. We hypothesize that some of these low serum complements may have been spuriously low due to the methods of serum collection and delayed delivery of the sample for laboratory analysis. We interpret the M-spikes in patients without relevant renal pathologies as representing monoclonal gammopathy of uncertain significance. Our patients are in an age group (median age 62 years) when monoclonal gammopathy of uncertain significance becomes increasingly prevalent in the general population.
Perhaps the most important aspect of this study is not only that NDRD was identified in the majority of diabetic patients who underwent kidney biopsy, but also the spectrum of NDRD seen on biopsies, either alone or superimposed on DN. In the cohort of patients with NDRD alone, FSGS (22%), hypertensive nephrosclerosis (18%), ATN (17%), IgAN (11%), MGN (8%), and pauci-immune GN (7%) comprised 80% of diagnoses, compared with ATN (43%), hypertensive nephrosclerosis (19%), FSGS (13%), and IgAN (7%) for NDRD with coexistent DN (Table 2). The overwhelming majority of these diagnoses would yield some change in treatment, ranging from the use of immunosuppression to titration of renin-angiotensin-aldosterone system blockade. The most common NDRDs previously reported in patients with DM are IgAN (3,11–13), FSGS (1,10), and MGN (10,14). In our series, FSGS was the most common finding in NDRD alone, identified in 48 patients (22%) and, in all but 6 patients, read as secondary or adaptive FSGS related to such comorbidities as obesity and systemic hypertension. Among all cases of NDRD in this cohort (i.e., with and without concomitant DN), however, ATN was the most common biopsy finding, seen in 28.4% of all NDRD patients. This is a unique finding of our study, because ATN has not been reported previously as a common NDRD, either alone or superimposed on DN. The higher incidence of ATN as a superimposed disease on DN may reflect reduced renal reserve and the greater potential for renal hypoperfusion and hemodynamic instability under renin-angiotensin-aldosterone system blockade, typically used as standard therapy to allay progression of DN. The high prevalence of ATN carries even more weight in light of evidence from a number of epidemiologic cohorts that AKI episodes are associated with a cumulative risk for progression to ESRD, particularly in patients with diabetes (15–17).
The strength of this retrospective study lies in its size and generalizability. Because our renal pathology laboratory is a referral laboratory for nephrologists over a broad geographic region, these findings are likely representative of current national trends and illustrate the spectrum of renal pathology in the modern era of the type 2 diabetes epidemic. Notably, approximately one of every four native renal biopsies is performed in a diabetic patient. The high frequency of NDRD in such biopsies suggests that nephrologists are appropriately selecting for biopsy those diabetic patients with clinical features considered atypical of DN alone or suspicious for NDRD. In addition, the high rates of FSGS and hypertensive nephrosclerosis highlight the importance of comorbidities such as obesity and hypertension.
Our study has a number of limitations. First, selection bias is inherent in any biopsy-based clinicopathologic study. Our results can only be interpreted as applicable to those patients whom a treating nephrologist would consider biopsy candidates. This bias should be toward the null, however, because most nephrologists would not biopsy patients for whom the pre-test probability of finding NDRD is low. Diabetic retinopathy has classically been shown to predict DN, but the clinical history provided at the time of biopsy only reported retinopathy results on 14% of this cohort. If the common clinical practice to not biopsy diabetic patients with retinopathy holds for our cohort, then including retinopathy data in our analyses may have introduced significant selection bias. Given the importance of proteinuria in this cohort and its association with biopsy findings, ideal analyses would not only include proteinuria at time of biopsy but trends in proteinuria before biopsy. Such data were not available and precluded us from analyzing whether changes in proteinuria were equally important as degree of proteinuria. Similarly, hemoglobin A1c values were missing for most patients and thus could not be included in our analyses.
In summary, our data indicate that approximately one-quarter of all renal biopsies in the modern era are performed in patients with diabetes. As the diabetes epidemic prevails, there is an increasing need for nephrologists to remain vigilant and weigh the risks and benefits of renal biopsy in the diabetic patient. In this cohort, >60% of biopsies in diabetic patients revealed NDRD, alone or alongside concomitant DN. Whereas AKI, low complements, and positive M-spike suggest an increased likelihood of finding NDRD on biopsy, long duration of DM emerged as the strongest predictor of finding DN alone. Therefore, the threshold to pursue kidney biopsy should increase in parallel with DM duration.
Footnotes
S.G.S. and A.S.B. contributed equally to this work.
Published online ahead of print. Publication date available at www.cjasn.org.
References
Articles from Clinical Journal of the American Society of Nephrology : CJASN are provided here courtesy of American Society of Nephrology
Full text links
Read article at publisher's site: https://doi.org/10.2215/cjn.02510213
Read article for free, from open access legal sources, via Unpaywall:
https://cjasn.asnjournals.org/content/clinjasn/8/10/1718.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.2215/cjn.02510213
Article citations
Artificial Intelligence-Assisted Perfusion Density as Biomarker for Screening Diabetic Nephropathy.
Transl Vis Sci Technol, 13(10):19, 01 Oct 2024
Cited by: 0 articles | PMID: 39388177 | PMCID: PMC11472892
Kidney Biopsy Findings Among Patients With Diabetes in the Cleveland Clinic Kidney Biopsy Epidemiology Project.
Kidney Med, 6(10):100889, 13 Aug 2024
Cited by: 0 articles | PMID: 39310117 | PMCID: PMC11414546
Potential Role of Mineralocorticoid Receptor Antagonists in Nondiabetic Chronic Kidney Disease and Glomerular Disease.
Clin J Am Soc Nephrol, 19(11):1499-1512, 22 Jul 2024
Cited by: 0 articles | PMID: 39037799
Review
Rationale and design of the Innsbruck Diabetic Kidney Disease Cohort (IDKDC)-a prospective study investigating etiology and progression of early-stage chronic kidney disease in type 2 diabetes.
Clin Kidney J, 17(5):sfae109, 11 Apr 2024
Cited by: 0 articles | PMID: 38726211 | PMCID: PMC11079669
Non-diabetic nephropathy in diabetic patients: incidence, HbA1c variability and other predictive factors, and implications.
Int Urol Nephrol, 56(9):3091-3100, 25 Apr 2024
Cited by: 1 article | PMID: 38662267
Go to all (143) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
[THE SPECTRUM OF RENAL DISEASE IN KIDNEY BIOPSIES OF DIABETIC PATIENTS: A SINGLE CENTER EXPERIENCE].
Harefuah, 155(3):158-62, 196, 01 Mar 2016
Cited by: 0 articles | PMID: 27305749
Clinicopathological study of nondiabetic renal disease in type 2 diabetic patients: A single center experience from India.
Saudi J Kidney Dis Transpl, 28(6):1330-1337, 01 Nov 2017
Cited by: 4 articles | PMID: 29265044
Clinicopathological characteristics of non-diabetic renal disease in patients with type 2 diabetes mellitus in a northeastern Chinese medical center: a retrospective analysis of 273 cases.
Int Urol Nephrol, 48(10):1691-1698, 06 Jun 2016
Cited by: 21 articles | PMID: 27272256 | PMCID: PMC5031732
Non-diabetic renal disease (NDRD) in patients with type 2 diabetes mellitus (type 2 DM).
J Assoc Physicians India, 61(3):194-199, 01 Mar 2013
Cited by: 17 articles | PMID: 24475681
Review





