Abstract
Free full text

Sarcopenia and Sarcopenic Obesity
Abstract
The aging process is associated with progressive loss of muscle mass and strength, as well as decline in physical functioning. Although consensus diagnosis has not been reached, sarcopenia is increasingly defined by both loss of muscle mass and loss of muscle function or strength. The cause of sarcopenia is suggested as multifactorial, including hormonal changes, inflammatory pathway activation, fatty infiltration, poor nutrition, and decreased physical activity. Sarcopenia is often associated with visceral obesity. Sarcopenic obesity in the elderly impacts metabolic complications and represents a major public health challenge in a rapidly aging society. Further research about sarcopenia and sarcopenic obesity may be needed to confront the influence of aging society in Korea.
INTRODUCTION
Aging is associated with a progressive loss of muscle mass, quality and strength, which results in a condition known as sarcopenia. In 1989, Rosenberg and Roubenoff [1] proposed the term sarcopenia, originating from the Greek words sarx (flesh) and penia (loss). Sarcopenia has been defined as "the age-associated loss of skeletal muscle mass, which results in decreased strength and aerobic capacity and thus functional capacity" [2]. Lean muscle mass contributes up to 50% of total body weight in young adults but declines with age to 25% at 80 years old [3]. After 50 years of age, approximately 1% to 2% of muscle mass is expected to be lost per year, and muscle strength decreases at an even greater rate [4]. Sarcopenia is characterized by atrophy of type II muscle fiber and reduction in muscle fiber satellite cells with aging [5]. Interestingly, young men have twice as much muscle mass as fat mass, whereas this ratio is almost reversed in older men [6]. Aging is also related to increased visceral fat mass, which is an important factor in the development of metabolic syndrome, type 2 diabetes and cardiovascular disease. Sarcopenia and visceral obesity may have a synergistic impact on both chronic metabolic disorders and physical disability [7].
DEFINITION OF SARCOPENIA
Several different definitions of sarcopenia and sarcopenic obesity have been proposed in previous studies. Baumgartner et al. [8] defined sarcopenia as a two or greater standard deviation (SD) reduction in appendicular skeletal muscle (ASM) divided by height squared (ASM/height2) below the normal mean for a young reference group measured using dual X-ray absorptiometry [8]. Janssen et al. [9] proposed a definition of sarcopenia as skeletal muscle mass index (skeletal muscle mass [kg]/weight [kg]×100) one or two SD below the mean for a younger reference group. Newman et al. [10] introduced an alternative definition of sarcopenia, using appendicular lean mass adjusted for height and body fat mass (residuals). Recently, the European Working Group on Sarcopenia in Older People developed a practical clinical definition and recommends using the presence of both low muscle mass and low muscle function (strength or performance) for the diagnosis of sarcopenia [11]. Table 1 summarizes the measurement of muscle mass, strength, and function. The International Working Group on Sarcopenia proposed that a diagnosis of sarcopenia is consistent with a gait speed of less than 1 m/sec and an objectively measured low muscle mass (e.g., ASM mass relative to height2 that is ≤7.23 kg/m2 in men and ≤5.67 kg/m2 in women) [2].
Table 1
Measurements of Muscle Mass, Strength, and Function
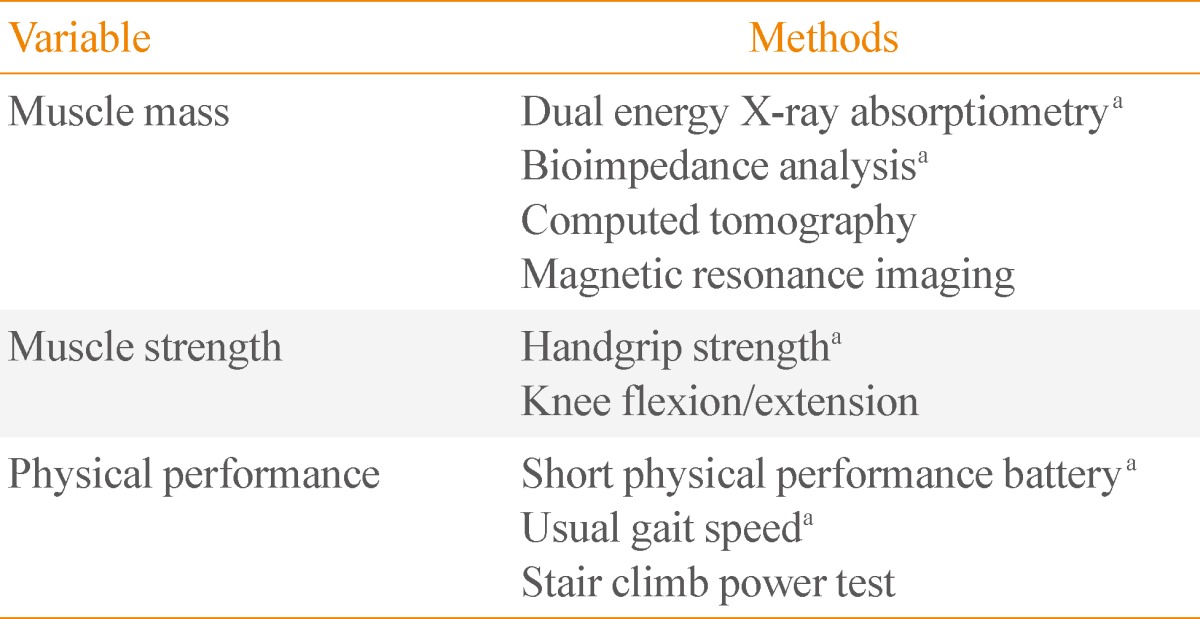
aRecommended for clinical practice.
MECHANISMS OF SARCOPENIA
Changes in hormone levels
Aging is associated with changes in hormone levels, such as those of growth hormone (GH), insulin-like growth factor (IGF)-I, insulin, androgens, estrogens, and corticosteroids, all of which affect the anabolic and catabolic conditions for muscle protein metabolism [12]. Elderly people show a decrease in GH and IGF-I levels that is paralleled by changes in body composition [13]. Reduced testosterone and estrogen levels might cause decreased muscle mass as well as diminishing bone strength. Increased insulin resistance with aging is associated with augmentation of intramyocellular fat mass and loss of muscle function [12].
Inflammation
Cytokines play a pivotal role not only in muscle homeostasis, but also in the pathogenesis of clinical conditions characterized by alterations in protein metabolism [14]. An increase in proinflammatory cytokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6, results in muscle breakdown, which is caused by decreasing muscle protein synthesis and increasing myofibrillar protein degradation [15]. TNF-α induces nuclear factor-κB, which leads to increasing proteolysis by activating the ubiquitin-dependent proteolysis system. IL-6 is considered to be a catabolic cytokine due to its participation in the regulation of muscle protein turnover [14].
Other risk factors
Several previous studies have demonstrated that serum 25-hydroxyvitamin D (25(OH)D) levels are inversely correlated with various parameters of obesity [16]. 25(OH)D levels decrease longitudinally with aging [17]. Recently, we found that insulin resistance and 25(OH)D levels were independently associated with sarcopenic obesity in men, while insulin resistance and high sensitivity C-reactive protein were significant factors predicting sarcopenic obesity in women [18]. On the other hand, oxidative metabolism generates reactive oxygen species, which damage cell components, particularly mitochondria. Alterations to mtDNA increase with age in skeletal muscle and are affected by sarcopenia [19]. Another important factor in the regulation of skeletal muscle mass is myostatin, which suppresses differentiation and proliferation of myocytes [20]. Myostatin suppression may have therapeutic potential for improvement of sarcopenia [21].
INFLUENCE OF SARCOPENIA AND SARCOPENIC OBESITY
Previous studies have reported that sarcopenia is associated with risk of adverse outcomes, such as physical disability, poor quality of life, and death. In an early study by Baumgartner et al. [8], sarcopenia was significantly associated with a 3-fold to 4-fold increased risk of physical disability in both men and women [8]. They also reported a relationship between sarcopenia and falls in the previous year. After adjustment for other confounding factors, the odds ratio for falls was statistically significant in men at 2.58 (95% confidence interval [CI], 0.60 to 2.67) but not in women at 1.28 (95% CI, 0.60 to 2.67) [8]. In the Korean Sarcopenic Obesity Study (KSOS) of 810 subjects (414 patients with type 2 diabetes and 396 control subjects), we found that type 2 diabetes was independently associated with increased risk of sarcopenia [22]. Furthermore, we observed that sarcopenic obesity was associated with the risk of metabolic syndrome [23]. Park et al. [24] showed that type 2 diabetes is associated with excessive loss of skeletal muscle, and that older women with type 2 diabetes in the Health ABC cohort are at especially high risk for loss of skeletal muscle mass [24]. Recently, Lim et al. [25] found that sarcopenic obesity, defined by ASM/weight, was more closely associated with metabolic syndrome than either sarcopenia or obesity alone. In a study including 1,396 men and women aged 70 years and older, low arm muscle area was associated with an elevated mortality rate during an 8-year follow-up period (hazard ratio, 1.95; 95% CI, 1.25 to 2.00) [26]. Heitmann et al. [27] reported that lower levels of fat-free mass were associated with an increased risk of mortality among 787 men aged 60 years and older who were followed for 22 years. Recent studies suggest that subjects with less common body-size phenotypes, such as metabolically abnormal but normal weight or metabolically healthy obese, seem to be more prone or more resistant, respectively, to the development of obesity-associated metabolic disorders. We observed that low muscle mass might be a factor associated with different metabolic consequences according to body-size phenotype in the KSOS cohort [28].
CONCLUSIONS
Both sarcopenia and obesity are becoming major threats to aging society. The concept of sarcopenic obesity may help to elucidate the interrelationship between physical disability, metabolic disorders and mortality in the elderly population. More research about sarcopenia and sarcopenic obesity is needed to improve morbidity and mortality as consequences of rapidly aging society.
ACKNOWLEDGMENTS
This research was supported by the Basic Science Research Program, through the National Research Foundation of Korea, funded by the Ministry of Education, Science and Technology (2012006363) and by the Brain Korea 21 Project of the Ministry of Education and Human Resources Development, Republic of Korea (A102065-1011-1070100).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
Articles from Endocrinology and Metabolism are provided here courtesy of Korean Endocrinology Society
Full text links
Read article at publisher's site: https://doi.org/10.3803/enm.2013.28.2.86
Read article for free, from open access legal sources, via Unpaywall:
https://www.e-enm.org/upload/pdf/enm-28-86.pdf
Citations & impact
Impact metrics
Article citations
Sarcopenia-related traits and 10 digestive system disorders: insight from genetic correlation and Mendelian randomization.
Front Public Health, 12:1412842, 10 Jul 2024
Cited by: 0 articles | PMID: 39050602 | PMCID: PMC11267997
Sex-Specific Effects of Dietary Factors on Sarcopenic Obesity in Korean Elderly: A Nationwide Cross-Sectional Study.
Nutrients, 16(8):1175, 15 Apr 2024
Cited by: 0 articles | PMID: 38674866 | PMCID: PMC11054115
Association between sarcopenia and hearing impairment in middle-aged and elderly people in China: a prospective cohort study.
Sci Rep, 14(1):6061, 13 Mar 2024
Cited by: 0 articles | PMID: 38480872
Influence of Sugar-Sweetened Beverages Intake on Sarcopenic Obesity, Visceral Obesity, and Sarcopenia in Lebanese Patients with MASLD: A Case-Control Study.
Healthcare (Basel), 12(5):591, 05 Mar 2024
Cited by: 0 articles | PMID: 38470703 | PMCID: PMC10931226
Correlation of Sarcopenic Obesity on Various Cardiometabolic Risk Factors and Fracture Risk in Mid-Aged Korean Women.
J Menopausal Med, 29(2):58-65, 01 Aug 2023
Cited by: 1 article | PMID: 37691313 | PMCID: PMC10505515
Go to all (54) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Sarcopenia and sarcopenic obesity.
Korean J Intern Med, 31(6):1054-1060, 01 Nov 2016
Cited by: 137 articles | PMID: 27809450 | PMCID: PMC5094937
Review Free full text in Europe PMC
Health Consequences of Sarcopenic Obesity: A Narrative Review.
Front Endocrinol (Lausanne), 11:332, 21 May 2020
Cited by: 105 articles | PMID: 32508753 | PMCID: PMC7253580
Review Free full text in Europe PMC
Association between Sarcopenia, Sarcopenic Obesity, and Chronic Disease in Korean Elderly.
J Bone Metab, 25(3):187-193, 31 Aug 2018
Cited by: 34 articles | PMID: 30237999 | PMCID: PMC6135652
Muscle contractile and metabolic dysfunction is a common feature of sarcopenia of aging and chronic diseases: from sarcopenic obesity to cachexia.
Clin Nutr, 33(5):737-748, 29 Mar 2014
Cited by: 197 articles | PMID: 24785098






