Abstract
Free full text

Transforming growth factor-β: transforming plaque to stability
Lievens and colleagues report an important finding that helps to elucidate the role of transforming growth factor β (TGFβ)β in atherosclerotic plaque formation.1 TGFβ is a widely expressed cytokine produced by smooth muscle cells, endothelial cells, monocytes, macrophages, and T cells.2,3 Clinical studies suggest that TGFβ is antiatherosclerotic, citing a negative correlation between plasma TGFβ concentration and the extent of atherosclerosis.4–7 In mouse models of atherosclerosis, inhibition of TGFβ signalling causes the formation of unstable plaques. This plaque phenotype is probably caused by two consequences of inhibiting TGFβ: first, blockade of TGFβ's role in promoting plaque fibrosis would make the plaques less stable, and, secondly, the absence of TGFβ signalling results in increased numbers of pro-inflammatory T cells that populate atherosclerotic lesions.8–12 Because dendritic cells (DCs) express the TGFβ receptor (TGFβR) and have a central role in regulating immune responses, studying the potential role of the TGFβR in DCs and how it might influence T-cell activation and modulate atherosclerosis is an area of great interest.
To study the effect of TGFβ signal inhibition on DCs and its eventual effect on atherosclerosis development, Lievens et al. backcrossed Apoe–/– mice with mice whose TGFβ-β signalling in DCs is disrupted by a dominant negative TGFβRII transgene (that encodes the extracellular and transmembrane, but not intracellular, regions of the TGFβ type II receptor) inserted into the CD11c promoter.1 CD11c is expressed on most mouse DCs and some macrophages, and the CD11c promoter, although not DC specific, drives expression in many DCs. Previous publications using similar constructs have reported the transgene's presence in CD11c-expressing DCs and natural killer (NK) cells, but not in T cells, NKT cells, or B cells.13 These CD11c-dnTGFβRII (CD11cDNR) mice were kept on chow diet and euthanized at 20 weeks of age.
In both Apoe–/–CD11cDNR and Apoe–/– mice, most CD11c+ cells in atherosclerotic lesions were either DCs or macrophage-derived foam cells (CD11c+CD11b−). In spleen and lymph nodes, there were slightly more pro-inflammatory CD8−CD4− DCs in Apoe–/–CD11cDNR mice, which produced more tumour necrosis factor α (TNFα α) and interleukin-12 (IL-12) after lipopolysaccharide (LPS) stimulation than in control Apoe–/– mice. More importantly, bone marrow-derived CD11c+ cells from Apoe–/–CD11cDNR mice strongly induced T-cell proliferation, along with interferon γ (IFNγ γ) and IL-4 production, when co-cultured with CD4+ T cells from wild-type mice previously stimulated with anti-CD3/CD28. Similarly, when splenic DCs from Apoe–/–CD11cDNR mice were stimulated with anti-CD3/CD28, there was a significant increase in the production IFNγ, IL-4, IL-17, and IL-10 in supernatants compared with their Apoe–/– counterparts. When examining lymphoid organs, Apoe–/–CD11cDNR mice had fewer naïve T cells (CD44lowCD62Lhigh), and more effector memory T cells (CD44highCD62Llow) within both the CD4+ and CD8+ T-cell compartments. These findings suggest that TGFβ signal inhibition in DCs leads to interactions with T cells that are biased towards the maturation of an inflammatory repertoire of T cells.
Consistent with this finding is the observation that Apoe–/–CD11cDNR mice exhibited a doubling of atherosclerotic plaque area in the aortic root, as well as nearly three times as many CD3+ cells in plaque when compared with Apoe–/– controls. Both CD4+ and CD8+ T cells were found in greater number, without a difference in the number of regulatory T cells. When examining the macrophage compartment, disruption of TGFβ signalling in CD11c+ cells did not affect macrophage phenotype or the number of MOMA-2+ cells within the plaque lesions, or alter the M1/M2 balance, but immunohistochemistry revealed a more advanced stage of plaque in Apoe–/–CD11cDNR mice, with decreased collagen content, and decreased α-smooth muscle actin staining. Matrix metalloproteinase-9 (MMP-9) expression was also significantly increased in lesions of Apoe–/–CD11cDNR mice, reflecting increased matrix turnover.
Blockade of TGFβ signalling in CD11c+ cells affected atherosclerotic plaque in several ways. First, plaque burden was greater in mice whose TGFβ signalling was inhibited. This confirms findings by others who reported increased atherosclerosis in Apoe–/– mice administered either a monoclonal antibody directed against TGFβ[8],9 or a recombinant soluble TGFβRII:Fc construct unable to carry out TGFβ signalling downstream9,10 (see Figure 1). Secondly, plaques in mice with defective TGFβ signalling (either systemically9,10 or in T cells only8,11,12) resulted in increased cellularity, T-cell infiltration, and a more unstable plaque phenotype (less collagen, less α-smooth muscle actin, and in some studies increased foam cells and MMP). Lievens et al. report increased pro-inflammatory cytokine production in supernatants of DCs from Apoe–/–CD11cDNR after LPS stimulation or when co-cultured with wild-type CD4+ T cells, a finding that supports data from two other studies that found higher levels of IFNγ and IL-4 production from T cells of mice whose TGFβRII signalling was inhibited.8,11 Figure 1 summarizes the major aspects of each experimental model and the observed plaque phenotype.
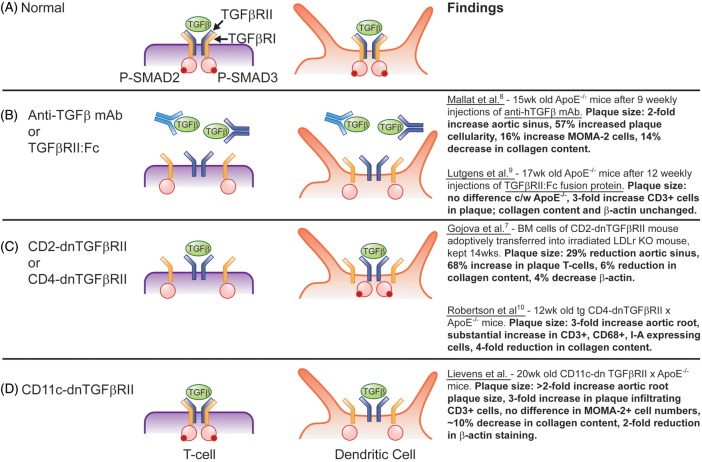
(A) Soluble transforming growth factor β (TGFβ) binds to TGFβ receptor II (TGFβRII), which recruits TGFβ receptor I (TGFβRI) to form a tetramer. Once the TGFβR complex is formed, TGFβRII phosphorylates SMAD 2/3 (red circles) and causes transcription of effector genes. (B) Addition of anti-TGFβ monoclonal antibody (light blue)8 or a truncated soluble TGFβRII:Fc protein (dark blue)10 binds soluble TGFβ, leading to loss of TGFβ signalling in all cells that express TGFβR. (C) A dominant negative TGFβRII transgene driven by a CD28 or CD411 promoter abrogates TGFβRII signalling in all T cells, but not in dendritic cells (DCs). (D) In the study of Lievens et al.,1 CD11c promoter was used to drive dominant negative TGFβRII in DCs and CD11c-expressing macrophages.
Conversely, a study published in 2009 used tetracycline-regulated cardiac-specific expression of constitutively active TGFβ1, and found reduction in the size and number of atherosclerotic plaques in the aorta, increased collagen content, decreased aortic plaque T cell/macrophage numbers, and 40–50% less mRNA for IFNγ, TNFα, macrophage inflammatory protein-1α (MIP-1α, α), MIP-1β, MMP-9, and MMP-13.14
Taken together, these studies constitute a solid body of evidence supporting a protective role for TGFβ in atherosclerotic plaque development. Prior to this publication, the data suggested that TGFβ plays a role in plaque formation and stabilization through generalized pro-fibrotic effects of TGFβ, and/or through its direct inhibitory action on T cells [possibly via induction of regulatory T cells, which suppress lineage commitment of naïve T cells towards the T helper 1 (TH1) phenotype]. The work of Lievens and colleagues suggests that an additional function of TGFβ lies in its ability to affect DCs within atherosclerotic plaque, where DCs that are able to bind and signal through the TGFβ pathway can interact with T cells, thereby inhibiting their commitment toward a pro-inflammatory lineage, thus decreasing pro-inflammatory cytokine production and probably also preventing their overpopulation of the plaque itself.
A recent paper by the late Ralph Steinman et al. described the presence of FLT3/FLT3-L-dependent dendritic cells which appear to inhibit atherosclerosis, at least in part, by supporting the differentiation and accumulation of T-regulatory (Treg) cells.15 Steinman did not comment specifically on these cells' ability to respond to TGFβ. It seems plausible that they would be able to. The new Lievens publication would lend credence to the idea that these FLT3/FLT3-L-dependent DCs may be the link between the effects of TGFβ signalling and downstream inhibitory effects on effector T cells. Clearly, TGFβ's anti-atherosclerotic role has many facets.
At least some of the DCs expressing TGFβR are expected to be tolerogenic, but others promote pro-inflammatory T-cell responses including TH1 and TH17.16 With current experimental techniques, it is difficult or impossible to distinguish between tolerogenic and pro-inflammatory DCs. Although the study of Lievens et al. does not relieve this uncertainty, it clearly shows that expressing a dominant negative TGFβR under the CD11c promoter has pro-inflammatory and pro-atherosclerotic consequences.
References
Articles from European Heart Journal are provided here courtesy of Oxford University Press
Full text links
Read article at publisher's site: https://doi.org/10.1093/eurheartj/ehs228
Read article for free, from open access legal sources, via Unpaywall:
https://academic.oup.com/eurheartj/article-pdf/34/48/3684/1309592/ehs228.pdf
Citations & impact
Impact metrics
Citations of article over time
Article citations
Cancer-associated foam cells hamper protective T cell immunity and favor tumor progression in human colon carcinogenesis.
J Immunother Cancer, 12(10):e009720, 12 Oct 2024
Cited by: 0 articles | PMID: 39395839 | PMCID: PMC11474856
Macrophages use apoptotic cell-derived methionine and DNMT3A during efferocytosis to promote tissue resolution.
Nat Metab, 4(4):444-457, 31 Mar 2022
Cited by: 54 articles | PMID: 35361955 | PMCID: PMC9050866
Galectin-3 Identifies a Subset of Macrophages With a Potential Beneficial Role in Atherosclerosis.
Arterioscler Thromb Vasc Biol, 40(6):1491-1509, 16 Apr 2020
Cited by: 42 articles | PMID: 32295421 | PMCID: PMC7253188
Atheroprotective Vaccination with MHC-II Restricted Peptides from ApoB-100.
Front Immunol, 4:493, 27 Dec 2013
Cited by: 58 articles | PMID: 24416033 | PMCID: PMC3873602
T cells in atherosclerosis.
Int Immunol, 25(11):615-622, 01 Nov 2013
Cited by: 97 articles | PMID: 24154816 | PMCID: PMC3806170
Review Free full text in Europe PMC
Go to all (6) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Abrogated transforming growth factor beta receptor II (TGFβRII) signalling in dendritic cells promotes immune reactivity of T cells resulting in enhanced atherosclerosis.
Eur Heart J, 34(48):3717-3727, 21 May 2012
Cited by: 45 articles | PMID: 22613345 | PMCID: PMC3869966
Heteromeric and homomeric transforming growth factor-beta receptors show distinct signaling and endocytic responses in epithelial cells.
J Biol Chem, 273(48):31770-31777, 01 Nov 1998
Cited by: 33 articles | PMID: 9822641
Activation of a serine/threonine kinase signaling pathway by transforming growth factor type beta.
Proc Natl Acad Sci U S A, 92(26):12110-12114, 01 Dec 1995
Cited by: 15 articles | PMID: 8618854 | PMCID: PMC40306
No major contribution of the TGFBR1- and TGFBR2-mediated pathway to Kabuki syndrome.
Am J Med Genet A, 140(8):903-905, 01 Apr 2006
Cited by: 4 articles | PMID: 16528739
Review




