Abstract
Background
Smith-Lemli-Opitz syndrome (SLOS) is an inborn error of cholesterol biosynthesis characterized by diminished cholesterol and increased 7-dehydrocholesterol (7-DHC) levels. 7-Dehydrocholesterol is highly reactive, giving rise to biologically active oxysterols.Methods
7-DHC-derived oxysterols were measured in fibroblasts from SLOS patients and an in vivo SLOS rodent model using high-performance liquid chromatography tandem mass spectrometry. Expression of lipid biosynthesis genes was ascertained by quantitative polymerase chain reaction and Western blot. The effects of an antioxidant mixture of vitamin A, coenzyme Q10, vitamin C, and vitamin E were evaluated for their potential to reduce formation of 7-DHC oxysterols in fibroblast from SLOS patients. Finally, the effect of maternal feeding of vitamin E enriched diet was ascertained in the brain and liver of newborn SLOS mice.Results
In cultured human SLOS fibroblasts, the antioxidant mixture led to decreased levels of the 7-DHC-derived oxysterol, 3β,5α-dihydroxycholest-7-en-6-one. Furthermore, gene expression changes in SLOS human fibroblasts were normalized with antioxidant treatment. The active ingredient appeared to be vitamin E, as even at low concentrations, it significantly decreased 3β,5α-dihydroxycholest-7-en-6-one levels. In addition, analyzing a mouse SLOS model revealed that feeding a vitamin E enriched diet to pregnant female mice led to a decrease in oxysterol formation in brain and liver tissues of the newborn Dhcr7-knockout pups.Conclusions
Considering the adverse effects of 7-DHC-derived oxysterols in neuronal and glial cultures and the positive effects of antioxidants in patient cell cultures and the transgenic mouse model, we believe that preventing formation of 7-DHC oxysterols is critical for countering the detrimental effects of DHCR7 mutations.Free full text

Antioxidant supplementation ameliorates molecular deficits in Smith-Lemli-Opitz Syndrome (SLOS)
Abstract
Background
Smith-Lemli-Opitz syndrome (SLOS) is an inborn error of cholesterol biosynthesis characterized by diminished cholesterol and increased 7-dehydrocholesterol (7-DHC) levels. 7-DHC is highly reactive, giving rise to biologically active oxysterols.
Methods
7-DHC-derived oxysterols were measured in fibroblasts from SLOS patients and an in vivo SLOS rodent model using HPLC-MS-MS. Expression of lipid biosynthesis genes was ascertained by qPCR and Western blot. The effects of an antioxidant mixture, vitamin A, coenzyme Q10, vitamin C and vitamin E were evaluated for their potential to reduce formation of 7-DHC oxysterols in fibroblast from SLOS patients. Finally, the effect of maternal feeding of vitamin E enriched diet was ascertained in the brain and liver of newborn SLOS mice.
Results
In cultured human SLOS fibroblasts the antioxidant mixture led to decreased levels of the 7-DHC-derived oxysterol, DHCEO. Furthermore, gene expression changes in SLOS human fibroblasts were normalized with antioxidant treatment. The active ingredient appeared to be vitamin E, as even at low concentrations, it significantly decreased DHCEO levels. In addition, analyzing a mouse SLOS model revealed that feeding a vitamin E enriched diet to pregnant females led to a decrease in oxysterol formation in brain and liver tissues of the newborn Dhcr7-knockout pups.
Conclusions
Considering the adverse effects of 7-DHC-derived oxysterols in neuronal and glial cultures, and the positive effects of antioxidants in patient cell cultures and the transgenic mouse model, we believe that preventing formation of 7-DHC oxysterols is critical for countering the detrimental effects of Dhcr7 mutations.
Introduction
Smith-Lemli-Opitz syndrome (SLOS) is characterized by multiple congenital malformations and defects (with 2/3 toe syndactyly being the most common), photosensitivity, impaired cognitive function, and behaviors of autistic spectrum disorder (1–4). SLOS is caused by mutations in the gene encoding the last enzyme in cholesterol biosynthesis – 7-DHC reductase (DHCR7) (5–7), resulting in accumulation of 7-dehydrocholesterol (7-DHC) in various tissues (8, 9). The physiological concentration of 7-DHC in healthy human plasma is very low (0.005 to 0.05 mg/dl) while in persons with SLOS it is greatly elevated (mean = 25 mg/dl) (9–11). SLOS as a single-gene disorder is recognized as being an appropriate model for understanding the genetic causes of autism (3, 12). In addition it provides a model to study the role of cholesterol in autism spectrum disorder (13, 14).
7-DHC was found to be the most reactive lipid molecule toward free radical peroxidation (15, 16), producing over a dozen oxidation products (i.e., oxysterols) in vitro and in vivo (17–20). These 7-DHC-derived oxysterols exert cytotoxicity, reduce cell proliferation, and induce cell differentiation and gene transcript changes (21, 22). One of the major 7-DHC-derived oxysterols, 3β,5α-dihydroxycholest-7-en-6-one (DHCEO), was established as a biomarker of the peroxidation of 7-DHC in cells and in animal models of SLOS (17, 22). However, DHCEO by itself is also more than a biomarker, as it alters gene expression, promotes differentiation and induces arborization of mouse cortical neurons (23).
Our previous studies suggest that the accumulation of 7-DHC and its related oxysterols may contribute significantly to the pathogenesis of SLOS, which points to a new direction of therapeutic approach – inhibition of the formation of 7-DHC and/or 7-DHC-derived oxysterols. In the current study we report on the effects of antioxidant supplementation on the accumulation of 7-DHC oxysterols in human fibroblasts from SLOS patients and an in vivo rodent model (Dhcr7-KO mice). In summary, we find that antioxidants, and specifically vitamin E supplementation, effectively inhibit the peroxidation of 7-DHC in SLOS human fibroblasts and newborn Dhcr7-KO mice, and reverse the most critical lipid biosynthesis gene expression changes in the fibroblasts.
Materials and Methods
Materials
AquADEKs® and Aqua-E® were purchased from Yasoo® Health Inc, Johnson City, TN. Vitamin C, vitamin A, coenzyme Q and all other chemicals were purchased from Sigma-Aldrich, highest quality grades available. The aqueous solution of vitamins C and A were prepared fresh before each use in dH2O and then added immediately to cell culture medium. All cell culture reagents were from MediaTech and Invitrogen. HPLC grade solvents (hexanes and 2-propanol) were purchased from Thermo Fisher Scientific Inc. Syntheses of [25,26,26,26,27,27,27- d7]-7-DHC, d7-DHCEO, 4α-hydroxy-7-DHC, 4β-hydroxy-7-DHC, and 7-keto-cholesta-5,8-dien-3β-ol were described elsewhere (18, 22, 24). NH2-SPE cartridges (55 μm, 70Å, 500 mg/3mL) were purchased from Phenomenex, Inc.
Cell Cultures
Control (GM05399, GM05565) and SLOS (GM05788, GM03044) human fibroblasts were purchased from the Coriell Institute. GM05788 donor subject is a compound heterozygote: one allele has a G>T transversion at nucleotide 413 of the DHCR7 gene (c.413G>T) resulting in the substitution of valine for glycine at codon 138 [Gly138Val (G138V)] and the second allele has a C>T transition at nucleotide 1213 (c.1213C>T) resulting in the substitution of tyrosine for histidine at codon 405 [His405Tyr (H405Y)]. GM03044 has 45,XY,t(13;14)(13qter>cen>14qter); unbalanced in fibroblasts; clinically affected; increased 7-dehydrocholesterol/cholesterol ratio. All cell lines were maintained in DMEM supplemented with L-glutamine, 10% fetal bovine serum (FBS; Thermo Scientific HyClone, Logan, UT), and penicillin/streptomycin at 37°C and 5% CO2. For antioxidant supplementation, all human fibroblasts were plated in 60 mm cell culture dishes, left to adhere overnight in the cell culture incubator and the following day the medium was completely replaced with fresh DMEM medium without phenol red containing 10% cholesterol-deficient serum (Thermo Scientific HyClone Lipid Reduced FBS) with or without antioxidants. This FBS medium did not have detectable cholesterol level. The cell culture medium was replaced three times a week and the fibroblasts were cultured for 5–7 days in the presence or absence of antioxidants. AquADEKs® is an antioxidant-rich multi-vitamin and mineral supplementation. The nominal concentration of all isoforms of tocopherols in AquADEKs® was calculated to be approximately 100 mM, and it was used as a stock solution. This stock was further diluted in cell culture medium to the final concentrations shown in the Figures. AquADEKs® contains in mM: β-carotene 6.4, vitamin C 255, total tocopherol 112 (77 mM of d-α-tocopherol and 35 mM of other mixed tocopherols), coenzyme Q10 2.3, vitamin D3 0.03, vitamin K 0.9, niacin 49, vitamin B6 2.4. Other ingredients include thiamin, riboflavin, biotin, pantothenic acid, selenium, sodium, and zinc. The concentration of Aqua-E® was determined to be 67 mM of total vitamin E (ca. 32 mM of d-α-tocopherol and 35 mM of other mixed tocopherols). This stock was further diluted in cell culture medium to the final concentrations shown in the Figures. Different batches of cultured cells were used for oxysterol measurement, RNA extraction and protein analysis. For each type of experiment, three to five independent batches of cultures were prepared and the data presented in the graphs shows the average of different experiments. For oxysterol measurements we used wide range of concentrations of antioxidant mixture and water soluble vitamin E: 1 nM to 10 μM. For gene expression and protein analysis we used 50 nM and 500 nM of antioxidant mixture and vitamin E. All cultured SLOS and control human fibroblasts used were passage 8–20. The statistical significance was measured using two tailed t-test in MS-Excel 2007.
Dhcr7-KO Mice – Dhcr7-HET (Dhcr7tm1Gst/J) mice were purchased from Jackson Laboratories (catalogue # 007453). The mice were kept and bred in Division of Animal Care facilities at the Vanderbilt University. Fifteen female mice, 2–3 months of age, were paired with males (2 females with one male) and at that time, mice were randomly allocated to one of three dietary treatments. A) Control (CNT) - Laboratory Rodent Diet 5001 (LabDiet); B) Control diet D10001 (vitamin E containing =VEC) (Research Diets, Inc) containing standard vitamin mix (V1001), including vitamin E (50 IU/kg diet; as vitamin E acetate) and no vitamin C; C) Vitamin C and E rich diet (VER) D04101103 (Research Diets, Inc) containing 1g vitamin C and 350 IU vitamin E per kg diet in addition to the standard vitamin mix for a total of 400 IU/kg diet vitamin E. Although mice are capable of synthesizing their own vitamin C in the liver, vitamin C is included in the diet to decrease the possibility that high levels of vitamin E could begin to play a pro-oxidant role since vitamin C can recycle vitamin E from its radical form (25, 26). Excess vitamin C is excreted in urine. Samples were obtained from multiple litters for each treatment. VEC diet: 4 litters with total 34 pups (6 WT, 19 Het, 9 KO); VER diet: 5 litters with total 41 pups (8 WT, 22 Het, 11 KO), CNT diet: 5 litters with total 30 pups (8 WT, 17 Het, 5 KO). Pups were collected shortly after birth at P0 (and in some cases at E20) because Dhcr7-KO pups do not survive long past birth, and brain and liver were harvested and frozen instantly in dry ice-precooled 2-methyl butane at −80°C until needed for analyses. Tails were saved for genotyping. The female mice were allowed one week of rest after delivery and mated again to obtain more litters. This process took about 6 months during which time all mice were kept on the diet that was initially assigned. The oxysterols were measured in whole brains and livers from WT and KO mice. The vitamin E content was measured in heterozygous littermates of Dhcr7-WT and Dhcr7-KO mice used for oxysterol analysis. This was done because there is not sufficient amount of tissue to reliably measure both oxysterols and vitamin E content from the same sample (the average wet weight of the brain from newborn KO mouse is ~60 mg). The genomic DNA from mouse tails was extracted using RED Extract-N-Amp Tissue PCR kit (Sigma-Aldrich). Genotyping was performed using the following PCR primers: forward - ggatcttctgagggcagcctt, reverse - tctgaacccttggctgatca, neo: ctagaccgcggctagagaat. The statistical significance was measured using two tailed t-test in MS-Excel 2007. All procedures were performed in accordance with the Guide for the Humane Use and Care of Laboratory Animals. The use of mice in this study was approved by the IACUC of Vanderbilt University.
Lipid extraction, separation, and HPLC-MS-MS analyses of oxysterols in cells and tissues
This method has been described previously (18, 22) and we provide detailed description in the Supplement.
RNA Preparation, Quantitative PCR and Western Blotting
These methods have been described previously (21, 27) and we provide detailed description in the Supplement. Statistical analyses of the qPCR data were performed using pairwise Student t-test in MS-Excel 2010, while false discovery for multiple testing was performed by calculating the individual q-value (28) for transcript using the Benjamini-Hochberg approach (29).
Determination of alpha-tocopherol and ascorbic acid levels
Vitamin E was measured as alpha-tocopherol in cortex and liver from heterozygous mice, from at least two litters, from dams fed with the control diet, the vitamin E containing diet, and the vitamin E rich diet as described above in this section. Cortical samples within a litter were sometimes combined in order to yield sufficient sample for analysis leading to overall fewer samples for analysis. Tissue samples were homogenized in an extraction buffer of water, reagent alcohol and 3% SDS with 10 mg/ml butylated hydroxytoluene as an antioxidant. Samples were mixed thoroughly with hexane, and the top organic layer was then collected and dried down under nitrogen. This sample, containing the alpha-tocopherol, was then re-eluted in a 1:1 mixture of methanol and reagent alcohol and measured by HPLC with electrochemical detection as described previously (30). Values were calculated as nmol per gram of tissue (wet weight). The same method was adapted for use with ground up food pellets using 100 mg of powder for extraction.
Vitamin C (ascorbic acid) levels were determined by HPLC following homogenization of samples in extraction buffer of 5% metaphosphoric acid and 100 mM sodium phosphate buffer and appropriate dilution of the supernatant was diluted with dH2O as described previously (31). Differences were analyzed by Univariate ANOVA using Graph Pad Prism 5 for Mac.
RESULTS
Antioxidants inhibit accumulation of DHCEO, a 7-DHC-derived oxysterol in SLOS human fibroblasts
Our previous studies showed that 7-DHC-derived oxysterols accumulate in cell cultures derived from SLOS patients (22) and that they are toxic to neuronal and glial cells (23). Due to these negative effects of oxysterols, ways to reduce such effects with antioxidants have been sought. We found that cultured human fibroblasts derived from SLOS patients, when exposed to an antioxidant mixture, AquADEKs® (containing vitamin A as 87% β-carotene, vitamin D as cholecalciferol, vitamins C as sodium ascorbate and, vitamin E as a mixture [d-alpha-tocopherol, γ-tocopherol, and other tocopherols], and coezyme Q10; see Materials and Methods) led to significant decrease in the levels of DHCEO in a dose-dependent manner (Figure 1A). This reduction was already significant at concentrations as low as 1 nM (85% of control, p<0.05), and showed a remarkable and highly significant effect at 30nM (30% of control, p<0.005). Further increase of concentration to 50nM and 100nM provided no additional effect over the 30 nM exposure.
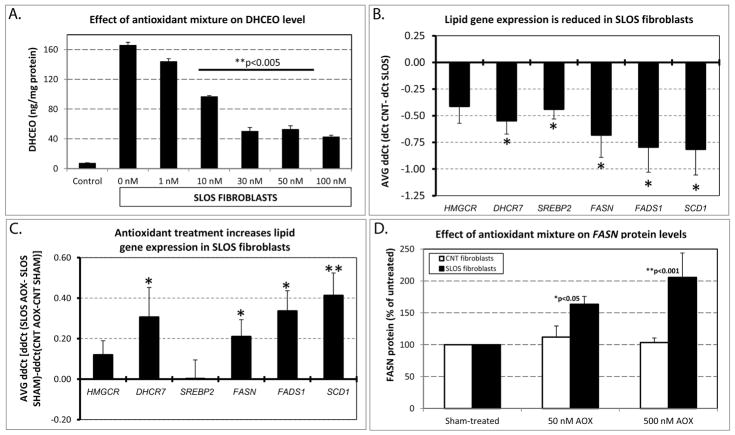
A) Effect of antioxidant mixture on DHCEO levels. X axis – Control refers to human fibroblasts taken from healthy individuals, rest of the categories denote increasing antioxidant mixture concentrations in fibroblasts taken from SLOS patients. Y axis – DHCEO levels measured in ng/mg of protein. Bars denote average of n=3/group, error bars indicate standard deviation. Note that antioxidant mixture effectively reduced DHECO levels even at concentration as low as 10nM. B) Lipid gene expression is decreased in SLOS fibroblasts. X axis – six lipid gene transcripts, Y-axis – AVGΔΔCt between SLOS and control fibroblasts cultured under normal conditions. Asterisk denotes significance at p<0.05, n=4 replicates, error bars represents SEM. Note that five out of six genes reported a significant decrease of transcript levels in the fibroblasts obtained from SLOS patients. C) Antioxidant treatment increases lipid gene expression in SLOS fibroblasts. X axis – same lipid genes as in panel B, Y axis - qPCR measured difference in response of control vs. SLOS fibroblasts to 500 nM of antioxidant treatment. n=4/group, *p<0.05; **p<0.005, error bars represent SEM. Note that four of six genes preferentially responded to antioxidant treatment in SLOS fibroblasts. D) FASN protein levels increase in response to 50 and 500 nM of antioxidant treatment. X axis – antioxidant mixture concentration, Y axis - % of change in FASN levels measured by Western blot in comparison to sham-treated cultures. n=4 group, error bars indicate SEM. Note that antioxidant exposure had no effect on control fibroblasts, but FASN protein levels showed a dose-dependent increase.
Antioxidants reverse gene expression changes in fibroblasts from SLOS patients
Previous studies in Neuro2a cells revealed that Dhcr7 is a potent regulator of lipid biosynthesis transcripts (27). We hypothesized that some of the transcriptome changes observed in Dhcr7-deficient Neuro2a cells would be present in SLOS human fibroblasts. To test this hypothesis, using qPCR we analyzed mRNA expression of Hmgcr (the enzyme promoting the rate limiting step in the cholesterol biosynthesis pathway), Dhcr7 (the last enzyme in the pathway), Srebp2 (the transcription factor that regulates transcription of all cholesterol biosynthesis transcripts), Fasn (responsible for the conversion of acetyl-CoA and malonyl-CoA into long-chain saturated fatty acids), Fads1 (catalyst of biosynthesis of highly unsaturated fatty acids from linoleic acid and alpha-linolenic acid), and Scd1 (involved in synthesis of oleate and palmitoleate). Our qPCR results revealed that Dhcr7, Srebp2, Fasn, Fads1 and Scd1 were all significantly downregulated (Dhcr7: ΔΔCt= − 0.55, p=0.016, q=0.045; Srebp2: ΔΔCt= − 0.44, p=0.013, q=0.045; Fasn: ΔΔCt= − 0.68, p=0.037, q=0.045; Fads1: ΔΔCt= − 0.79, p=0.034, q=0.045; Scd1: ΔΔCt= − 0.81, p=0.034, q=0.045) in SLOS patient-derived fibroblasts compared to fibroblasts derived from unaffected individuals (Figure 1B). Furthermore, Hmgcr also showed a downregulation trend in the patient fibroblasts (ΔΔCt= −0.41, p=0.065, q=0.065).
Once it was established that this panel of genes involved in lipid metabolism and cholesterol biosynthesis is downregulated in SLOS fibroblasts, we inquired if antioxidant supplementation could be successfully used to normalize gene expression changes. Indeed, treatment of SLOS fibroblasts with an antioxidant mixture was more effectively increasing four of the six investigated lipid transcript levels in fibroblasts derived from SLOS patients than in those derived from control subjects (Dhcr7: 1.3-fold, ΔΔCt=0.31, p=0.043, q=0.066; Fasn: 1.2-fold, ΔΔCt=0.21, p=0.018, q=0.056; Fads1: 1.3-fold, ΔΔCt=0.34, p=0.029, q=0.058; and Scd1: 1.4-fold, ΔΔCt=0.41, p<0.001, q=0.006) (Figure 1C). To determine if transcript changes translate into protein expression change, we tested expression of the fatty acid synthase protein (FASN) in a separate set of antioxidant treated cultures (Supplement: Figure S1 and Figure 1D). Under control untreated conditions, FASN protein expression is downregulated in SLOS fibroblasts compared to control fibroblasts by 52%, p<0.01 (Supplement: Figure S1). While the antioxidants did not affect significantly expression of FASN in the control cultures, they greatly upregulated the expression level of FASN in SLOS fibroblasts (>2-fold, p<0.001).
Vitamin E is sufficient for normalizing DHCEO levels in fibroblasts from SLOS patients
To establish if the observed effects were mediated by the antioxidant mixture or by one of the specific ingredients, we evaluated the efficacy of individual components of the antioxidant mixture to decrease DHCEO formation. For these experiments we used vitamin A, coenzyme Q10, vitamin C and a water-soluble formulation of vitamin E (Aqua-E®) (Figure 2). Our results revealed that only vitamin E significantly decreased the levels of DHCEO (Figure 2A), while coenzyme Q10 (Figure 2B), and vitamins A (Figure 2C) and C (Figure 2D), did not show any detectable effect on DHCEO levels even at high doses. The effect of vitamin E was also dose-dependent, and at concentration of 25 nM resulted in an 80% reduction of DHCEO levels (p<0.0005). Furthermore, concentrations of 1μM resulted in a 95% reduction of DHCEO (p<0.0005), resulting in DHCEO levels that were comparable to those observed in fibroblasts of unaffected, control fibroblasts.
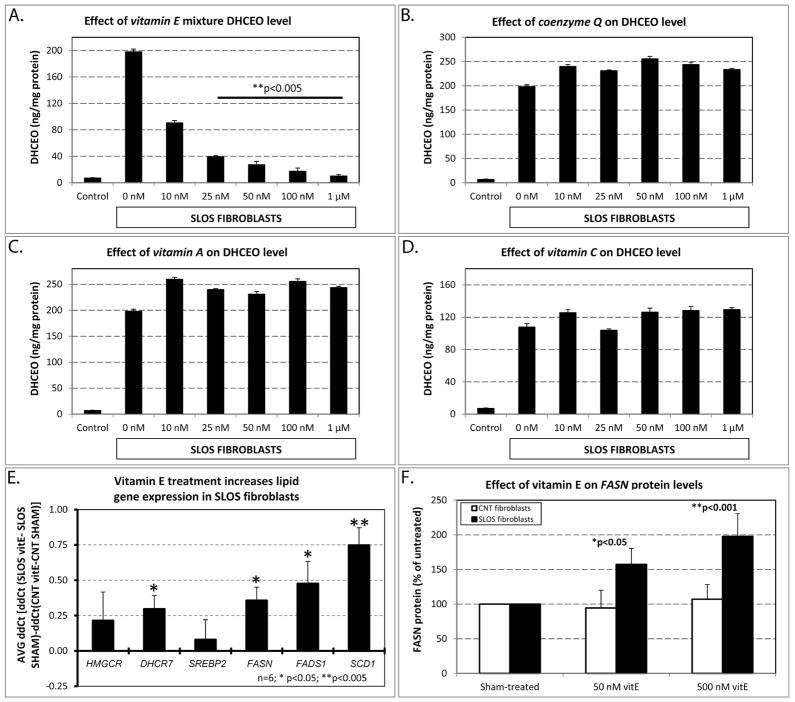
A–D) Effect of the four individual components of the antioxidant mixture on DHCEO levels. Figure layout and experimental design similar to that in Figure 1A. Note that treatment with vitamin E showed a comparable, dose-dependent decrease in DHCEO level to that seen with the antioxidant mixture in Figure 1A, while the other antioxidants showed no effect even at high doses. E) Vitamin E treatment increases lipid gene expression in SLOS fibroblasts. Figure layout and experimental design similar to that in Figure 1C. Note that the same four lipid gene transcripts showed significant and comparable change in both the antioxidant and vitamin E treated cultures of SLOS patients. F) FASN protein levels increase in response to antioxidant treatment. Figure layout and experimental design similar to that in Figure 1D. Note that antioxidant exposure had no effect on control fibroblasts, but FASN protein levels showed a dose-dependent increase similar to that seen in the antioxidant-treated cultures. *, p<0.05; **, p<0.005.
The water-soluble vitamin E treatment also had a beneficial effect on gene expression. Similarly to the antioxidant mixture, 500 nM of vitamin E exposure had a greater effect on increasing mRNA expression levels in SLOS fibroblasts that in matched controls (Figure 2E). Dhcr7, Fasn, Fads1 and Scd1 all showed significant upregulation over the transcription response of control fibroblasts (Dhcr7: 1.3-fold, ΔΔCt=0.30, p=0.035, q=0.053; Fasn: 1.4-fold, ΔΔCt=0.36, p=0.025, q=0.052; Fads1: 1.5-fold, ΔΔCt=0.48, p=0.024, q= 0.052; and Scd1: 1.8-fold, ΔΔCt=0.75, p=0.005, q=0.033). Furthermore, 50 nM of vitamin E exposure increased FASN protein expression by 57% (p<0.05) and 500 nM of vitamin E exposure resulted in a 98% increase in FASN protein levels (p<0.001) (Figure 2F). At the same time, these concentrations of vitamin E had no effect on the FASN levels of fibroblasts derived from control subjects. These combined experiments clearly suggested that vitamin E was a critical and active component mediating the beneficial effects of the antioxidant mixture, and that their effect was preferential on the fibroblasts derived from the SLOS patients.
Antioxidants decrease 7-DHC oxidation in mouse model of SLOS
To evaluate the effectiveness of antioxidant supplementation in vivo on reduction of 7-DHC oxidation, pregnant Dhcr7Gst1-HET female mice were fed with either a 50 IU/kg vitamin E containing diet (VEC diet), 400 IU/kg vitamin E-rich (VER diet) or control (CNT) diet that contained no vitamin E. The vitamin E rich diet was selected because this specific diet has been tested in another mouse model where it has been shown to reduce oxidative stress (32). The brain and liver of newborn pups were collected and processed for HPLC analyses of vitamin C and vitamin E. We found that vitamin E levels were increased in both the liver and the brain of newborn pups whose mothers were fed VEC and VER diets, although this difference was only significant in the VER diet group (Figure 3). In contrast, measurement of vitamin C (ascorbic acid) levels in tissues showed no difference among the three diet groups (F=0.65, p=0.53; data not shown).
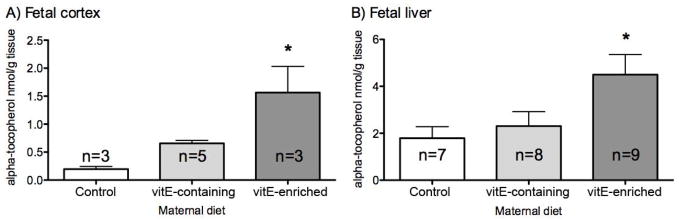
Maternal diet increases fetal vitamin E concentration in brain (A) and liver (B) of newborn mice. X axis - maternal diet, Y axis – vitamin E (alpha-tocopherol) levels in nmol/gr of tissue. Error bars denote SD, asterisk indicates p<0.05. Note that vitamin E enriched diet lead to significantly increased levels of vitamin E in both brain and liver.
The brain and liver of the three additional groups of newborn pups (VER, VEC, CNT maternal diets) were analyzed for oxysterols DHCEO, 4α-OH-7-DHC, 4β-OH-7-DHC, 7-keto-cholesta-5,8-dien-3β-ol (7-kDHC), and 3β,5α,9α-trihydroxycholest-7-en-6-one (THCEO) (Figure 4A). All of these oxysterols are derived from 7-DHC oxidation as described in detail in previous publications (18, 20, 22, 24). As hypothesized, due to the distinct oxysterol profiles of brain and liver (17, 18, 22), the vitamin E diet had a tissue specific effect. DHCEO, the most abundant 7-DHC derived oxysterol in the brain was significantly decreased in the brains of the Dhcr7-KO mice on VEC and VER diet (28% decrease, p<0.05) (Figure 4B), but this decrease was not statistically significant in the liver where DHCEO is formed at very low levels (Figure 4C). The levels of the oxysterol THCEO were significantly reduced in both brain and liver tissues of the Dhcr7-KO mice (brain 60% decrease in mice on VER diet, p<0.05; liver 75% decrease in mice on both VEC and VER diets, p<0.005). In contrast, 7-kDHC was significantly decreased only in the liver of pups from mothers fed vitamin E rich diet (more than 50% decrease in both diets, p<0.05). In addition, we observed a decreasing trend for the levels of 4α-OH-7-DHC and 4β-OH-7-DHC in the liver as a result of vitamin E enriched diet, but the changes did not show statistical significance. However, previous study suggests that 4α-OH-7-DHC and 4β-OH-7-DHC may be formed from enzymatic oxidation of 7-DHC (18).
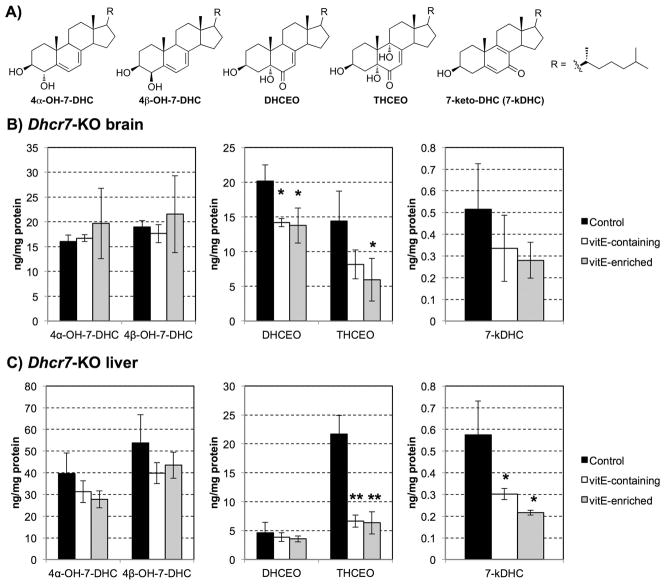
A) Chemical structures of five 7-DHC-derived oxysterols. B–C) Oxysterol levels in the Dhcr7-KO newborn mice brain (B) and liver (C) as a function of maternal diet. X axis – five 7-DHC-derived oxysterols; Y axis – oxysterol levels expressed in ng/mg of protein. n=3/group; error bars denote SD, *p<0.05, **p<0.01. Note that vitamin E-containing and vitamin E-enriched maternal diet significantly decreased oxysterol formation in newborn Dhcr7-KO mice in a tissue-specific manner.
DISCUSSION
In summary, our experiments revealed that: 1) the antioxidant mixture decreased accumulation of DHCEO oxysterol in human fibroblasts from SLOS patients; 2) the antioxidant decreased oxysterol-induced gene expression changes in a dose-dependent manner, thus normalizing the lipid transcription profile in SLOS-derived fibroblasts; 3) the active ingredient in the antioxidant mixture appears to be vitamin E, as vitamin E application was as effective in lowering oxysterol levels, restoring lipid gene mRNA and protein expression changes as was the antioxidant mixture; 4) vitamin E-containing and vitamin E-enriched diets resulted in increased vitamin E content in the brain and liver of Dhcr7Gst1-transgenic newborn mice carrying a SLOS-derived mutation; and 5) vitamin E enriched diets reduced oxysterol levels in both the brain and liver of Dhcr7Gst1-KO newborn mice, in a tissue-specific manner.
Vitamin E is a chain-breaking antioxidant against free radical peroxidation, with α-tocopherol being the most common and active form (33–35). In addition to its well-documented antioxidant properties, vitamin E has many biological functions, including regulation of enzymatic reactions, effects on gene expression, and in memory (35–42). The complexity of vitamin E action raises an important question about the mechanism by which vitamin E diminishes 7-DHC derived oxysterol formation. Theoretically, vitamin E can either prevent formation or accelerate clearance of already formed oxysterols. Our current experiments suggest that antioxidant-mediated decrease is primarily observed for oxysterols formed by lipid peroxidation, including DHCEO, THCEO, and 7-kDHC (20, 22, 24). No significant changes were observed for the levels of 4α-OH-7-DHC and 4β-OH-7-DHC, but these two oxysterols are likely formed from enzymatic oxidation of 7-DHC as they were not identified as products of free radical peroxidation of 7-DHC (18). These results suggest that the vitamin E action is preventing the formation and cellular build-up of oxysterols derived from 7-DHC peroxidation. In this case, vitamin E triggered increase in lipid transcripts Dhcr7, Fasn, Fads1 and Scd1 would be an adaptive, secondary, and indirect result of reduced oxysterol levels, and not due to the direct effect of vitamin E on transcription. However, we cannot exclude the possibility that vitamin E triggers de novo transcription, which might also contribute to the decreased oxysterol levels, as both of these two mechanisms would be consistent with known antioxidant and transcription-inducing properties of vitamin E (40, 42). Furthermore, it is possible that these two mechanisms work in concert, and the balance between these mechanisms might be tissue-specific.
Brain and liver tissue have different molecular and cellular properties, and generate different 7-DHC-derived oxysterol profiles. While the brain generates high levels of DHCEO, the liver tissue of mutant mice shows increased levels of 4α-OH-DHC and 4β-OH-DHC, with approximately the same levels of 7-kDHC and THCEO in both tissues. Importantly, the vitamin E enriched diet was effective in decreasing oxysterol levels in both liver and brain of newborn Dhcr7 knockout mice, suggesting that the effect is systemic, yet tissue-specific at the same time. While a wide range of concentrations (10 nM to 10 μM) have been effective in cell culture experiments in reducing oxysterol formation in SLOS fibroblast cells, only the high concentration of vitamin E and AOX (500 nM) were effective in reversing gene expression changes in cultures between the two concentrations tested (data not shown for 50 nM). It is difficult to directly translate concentrations used in cell cultures to mouse feeding regimen. Therefore for practical reasons, this specific vitamin E rich mouse diet was selected because it has been tested in another mouse model where it has been shown to decrease oxidative stress (32).
Our experiments provide conclusive evidence that antioxidants and vitamin E reduce oxysterol levels in Dhcr7 mutant mice and human fibroblasts from SLOS patients. Yet, the clinical importance of these findings remains unknown at the current time, and our findings do not warrant antioxidant/vitamin E supplementation in SLOS patients. Vitamin E, a lipophilic compound, in high concentration can produce unwanted and potentially serious side effects, including fatigue, weakness, headaches, nausea, diarrhea and increased risk of bleeding (43–46). Furthermore, mouse physiology is different from human physiology in many aspects, and previous studies suggest that vitamin E is poorly transported across the placenta in humans (47–49). In addition, in this initial, conceptual study we have chosen Dhcr7-mutant mice with an extreme phenotype that die shortly after birth, and the antioxidant/vitamin E supplementation through maternal diet was insufficient for a full rescue of a lethal phenotype. Thus, to further evaluate the potential effects and putative benefits of antioxidants, a number of other experiments must be undertaken on both in vitro and in vivo model systems. Future studies will have to include 1) a comprehensive evaluation of 7-DHC derived oxysterols of fibroblasts from SLOS patients with various Dhcr7 mutations, as well as their response to antioxidants, 2) studies of Dhcr7-mutant mice with a milder phenotype that survive to adulthood, which would allow the assessment of antioxidants on postnatal development, neuronal morphology, gene expression changes, and behavior, 3) testing the effectiveness of a combined cholesterol supplementation, inhibition of 7-DHC synthesis by statins and antioxidant supplementation in both in vitro and in vivo SLOS models, and 4) identifying novel, non-toxic compounds with antioxidant properties that could decrease the formation of 7-DHC-derived oxysterols.
In summary, our findings suggest that antioxidant treatment is an important mechanism by which 7-DHC derived oxysterol formation can be reduced in SLOS model systems. Preventing the formation of toxic 7-DHC derived oxysterols should be further investigated, as it can provide important insights into the molecular disturbances in SLOS, and ultimately help guide future therapeutic developments.
Acknowledgments
The National Institutes of Health (NICHD K99HD073270 to LX, NIA R01AG038739 to FEH, R01MH079299 and R01MH067234 to KM; NICHD R01HD064727 to NAP) supported this work. Zeljka Korade appreciates support from the Vanderbilt Kennedy Center for Research on Human Development. Fiona Harrison is grateful for funding from the Division of Diabetes, Endocrinology and Metabolism, of Vanderbilt University.
Footnotes
Conflict of interest: The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.biopsych.2013.06.013
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3874268?pdf=render
Citations & impact
Impact metrics
Article citations
Cholic acid increases plasma cholesterol in Smith-Lemli-Opitz syndrome: A pilot study.
Mol Genet Metab Rep, 38:101030, 28 Nov 2023
Cited by: 1 article | PMID: 38077958 | PMCID: PMC10698565
Statins for Smith-Lemli-Opitz syndrome.
Cochrane Database Syst Rev, 11:CD013521, 14 Nov 2022
Cited by: 3 articles | PMID: 36373961 | PMCID: PMC9661876
Review Free full text in Europe PMC
7-Dehydrocholesterol-derived oxysterols cause neurogenic defects in Smith-Lemli-Opitz syndrome.
Elife, 11:e67141, 16 Sep 2022
Cited by: 10 articles | PMID: 36111785 | PMCID: PMC9519149
Biochemical and Clinical Effects of Vitamin E Supplementation in Hungarian Smith-Lemli-Opitz Syndrome Patients.
Biomolecules, 11(8):1228, 17 Aug 2021
Cited by: 1 article | PMID: 34439893 | PMCID: PMC8393612
Medication effects on developmental sterol biosynthesis.
Mol Psychiatry, 27(1):490-501, 05 Apr 2021
Cited by: 14 articles | PMID: 33820938 | PMCID: PMC8490477
Review Free full text in Europe PMC
Go to all (31) article citations
Other citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
An oxysterol biomarker for 7-dehydrocholesterol oxidation in cell/mouse models for Smith-Lemli-Opitz syndrome.
J Lipid Res, 52(6):1222-1233, 14 Mar 2011
Cited by: 76 articles | PMID: 21402677 | PMCID: PMC3090243
DHCEO accumulation is a critical mediator of pathophysiology in a Smith-Lemli-Opitz syndrome model.
Neurobiol Dis, 45(3):923-929, 11 Dec 2011
Cited by: 58 articles | PMID: 22182693 | PMCID: PMC3674775
7-Dehydrocholesterol-derived oxysterols cause neurogenic defects in Smith-Lemli-Opitz syndrome.
Elife, 11:e67141, 16 Sep 2022
Cited by: 10 articles | PMID: 36111785 | PMCID: PMC9519149
Recent insights into the Smith-Lemli-Opitz syndrome.
Clin Genet, 68(5):383-391, 01 Nov 2005
Cited by: 45 articles | PMID: 16207203 | PMCID: PMC1350989
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NIA NIH HHS (2)
Grant ID: R01 AG038739
Grant ID: R01AG038739
NICHD NIH HHS (4)
Grant ID: K99 HD073270
Grant ID: K99HD073270
Grant ID: R01 HD064727
Grant ID: R01HD064727
NIEHS NIH HHS (2)
Grant ID: P01 ES013125
Grant ID: P30 ES000267
NIGMS NIH HHS (1)
Grant ID: R25 GM062459
NIMH NIH HHS (4)
Grant ID: R01 MH079299
Grant ID: R01 MH067234
Grant ID: R01MH067234
Grant ID: R01MH079299





