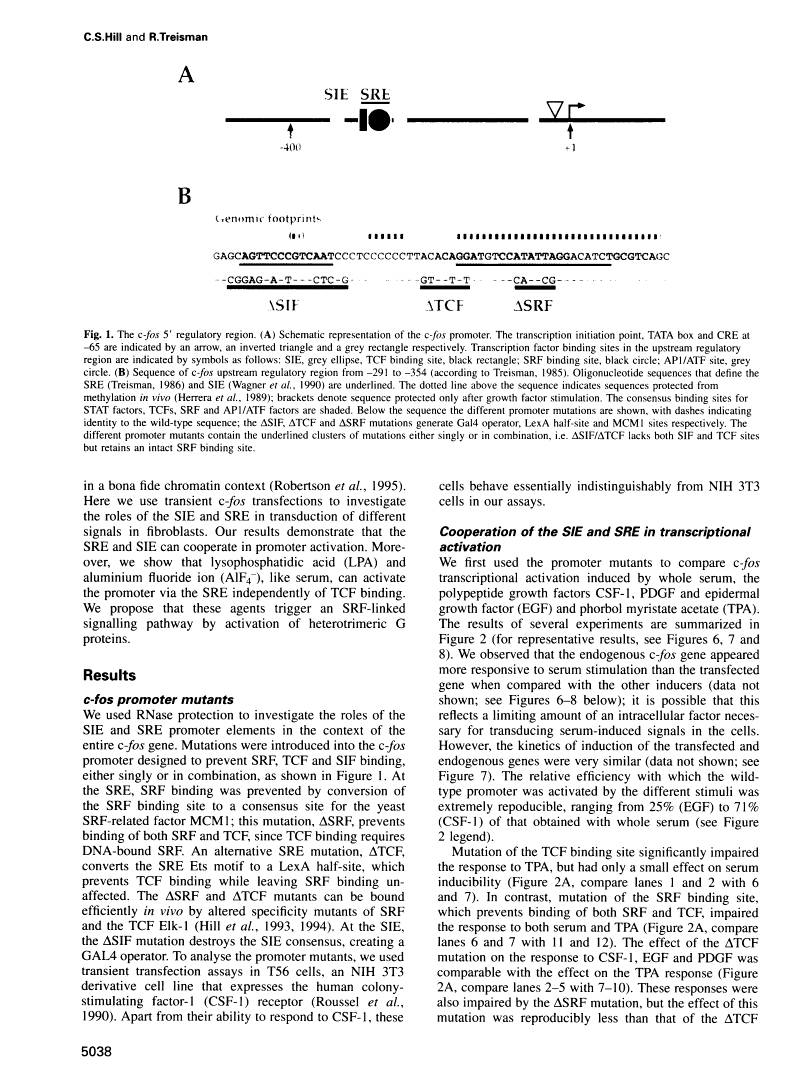
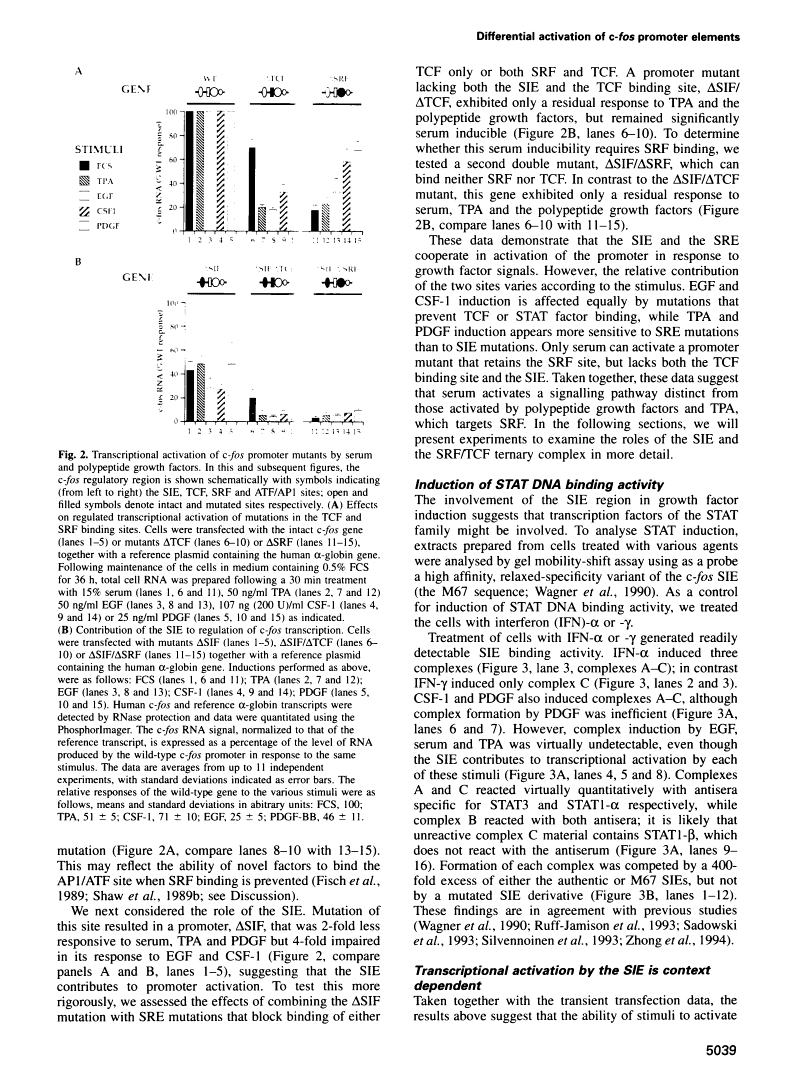
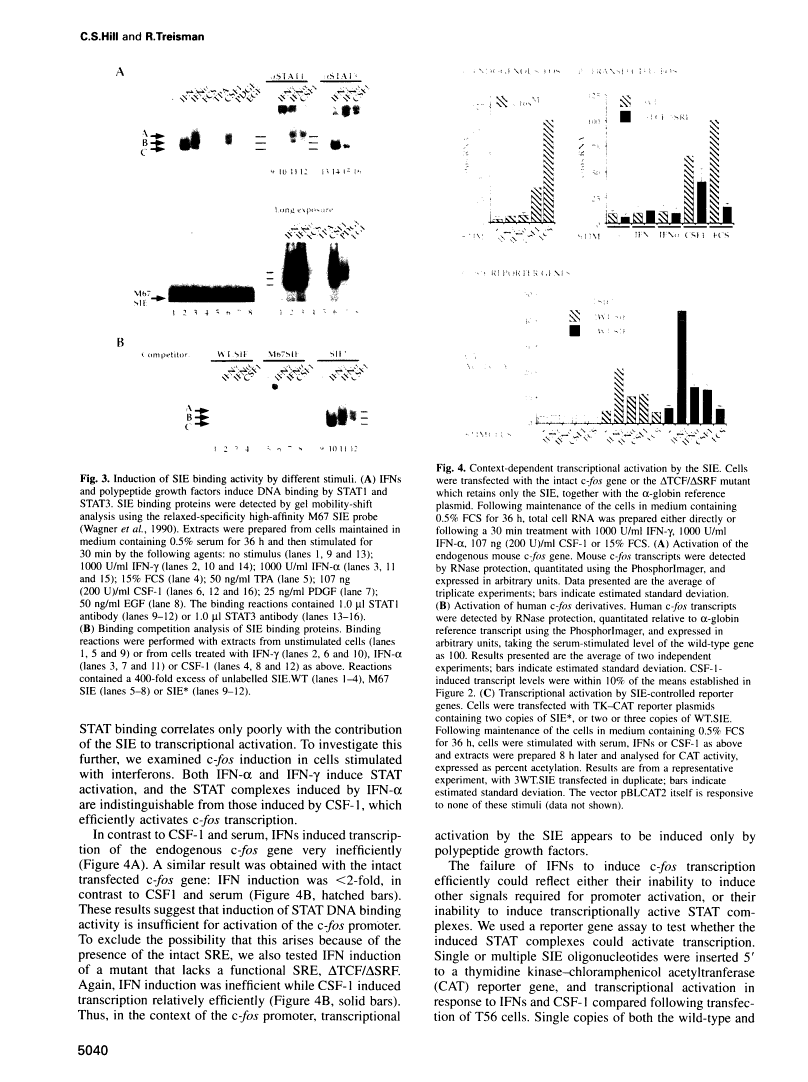
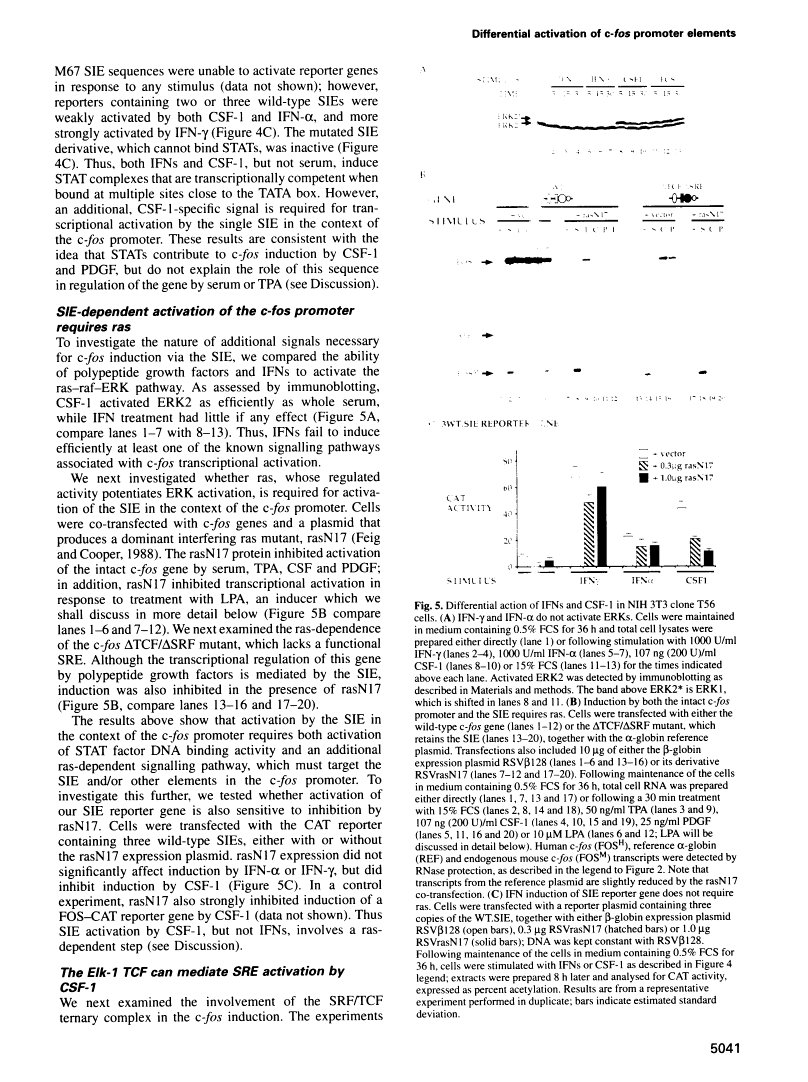
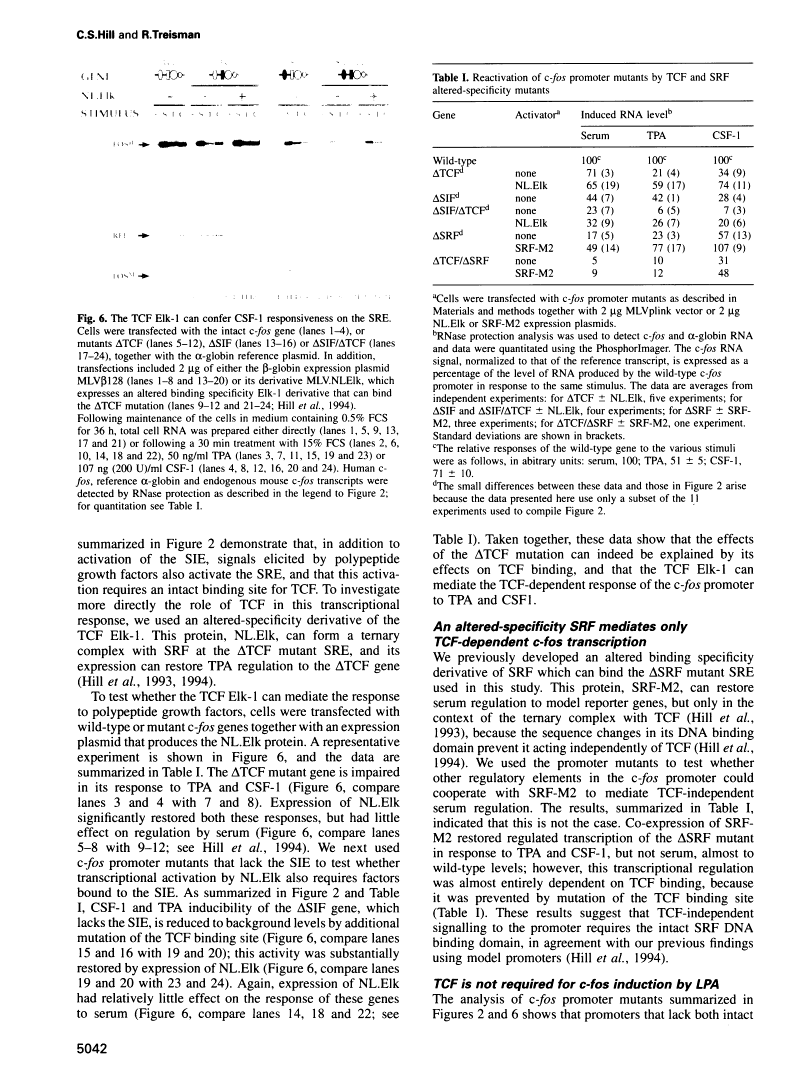
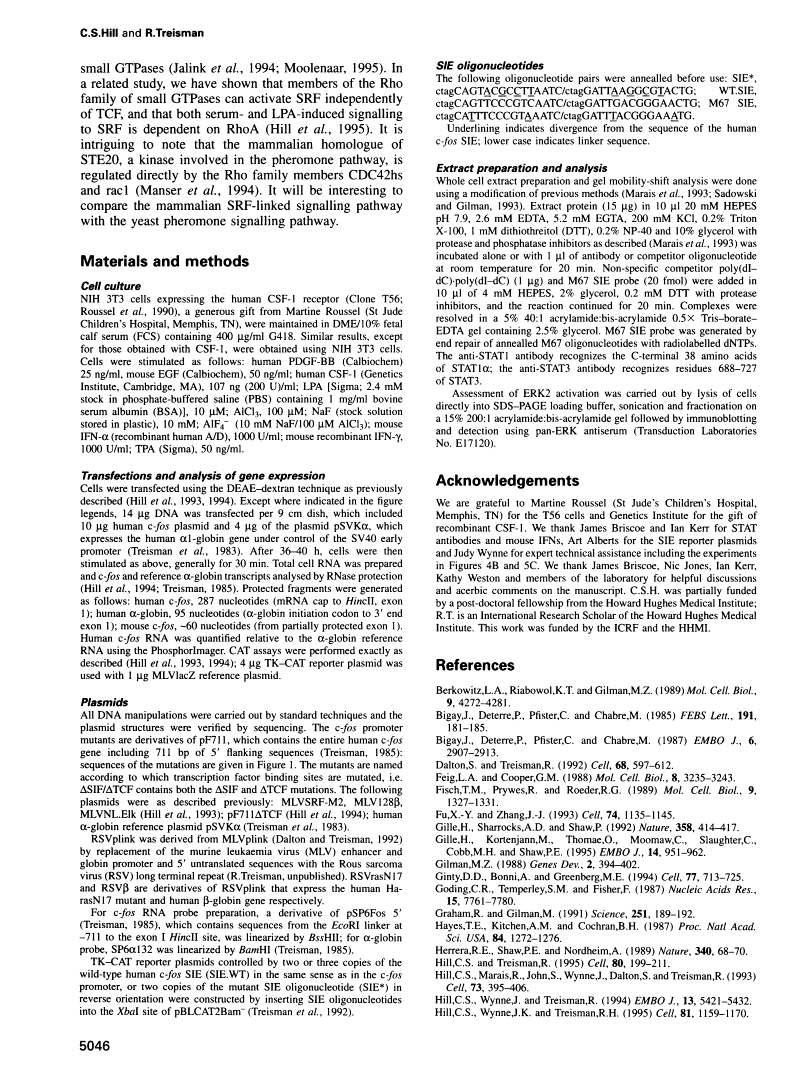
Abstract
Free full text

Differential activation of c-fos promoter elements by serum, lysophosphatidic acid, G proteins and polypeptide growth factors.
Abstract
The upstream regulatory region of the c-fos promoter contains two growth factor-regulated promoter elements: the serum response element, which binds a ternary complex comprising serum response factor (SRF) and a ternary complex factor (TCF); and the sis-inducible element (SIE) which binds STAT transcription factors. We used transient transfection of c-fos promoter mutants in NIH 3T3 cells to assess the contributions of these elements to activation by different extracellular stimuli. Colony-stimulating factor-1, platelet-derived growth factor and epidermal growth factor activate the c-fos promoter via cooperation of the SIE and the SRE; however, mutants that can bind SRF but not STATs or TCF remain inducible by whole serum. Activation by the SIE is context-dependent: interferons activate STAT DNA binding activity and transcription of SIE reporter genes, but not the c-fos promoter, which requires an additional ras-dependent signal. SRE activation by receptor tyrosine kinases requires TCF binding, and can be mediated by the TCF Elk-1. In contrast, SRE activation following activation of heterotrimeric G proteins by lysophosphatidic acid or aluminium fluoride ion requires SRF but is independent of TCF binding. These results suggest that heterotrimeric G proteins activate a signalling pathway distinct from those that activate the STATs and the TCFs, that controls SRF activity.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (2.6M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berkowitz LA, Riabowol KT, Gilman MZ. Multiple sequence elements of a single functional class are required for cyclic AMP responsiveness of the mouse c-fos promoter. Mol Cell Biol. 1989 Oct;9(10):4272–4281. [Europe PMC free article] [Abstract] [Google Scholar]
- Bigay J, Deterre P, Pfister C, Chabre M. Fluoroaluminates activate transducin-GDP by mimicking the gamma-phosphate of GTP in its binding site. FEBS Lett. 1985 Oct 28;191(2):181–185. [Abstract] [Google Scholar]
- Bigay J, Deterre P, Pfister C, Chabre M. Fluoride complexes of aluminium or beryllium act on G-proteins as reversibly bound analogues of the gamma phosphate of GTP. EMBO J. 1987 Oct;6(10):2907–2913. [Europe PMC free article] [Abstract] [Google Scholar]
- Dalton S, Treisman R. Characterization of SAP-1, a protein recruited by serum response factor to the c-fos serum response element. Cell. 1992 Feb 7;68(3):597–612. [Abstract] [Google Scholar]
- Feig LA, Cooper GM. Inhibition of NIH 3T3 cell proliferation by a mutant ras protein with preferential affinity for GDP. Mol Cell Biol. 1988 Aug;8(8):3235–3243. [Europe PMC free article] [Abstract] [Google Scholar]
- Fisch TM, Prywes R, Roeder RG. An AP1-binding site in the c-fos gene can mediate induction by epidermal growth factor and 12-O-tetradecanoyl phorbol-13-acetate. Mol Cell Biol. 1989 Mar;9(3):1327–1331. [Europe PMC free article] [Abstract] [Google Scholar]
- Fu XY, Zhang JJ. Transcription factor p91 interacts with the epidermal growth factor receptor and mediates activation of the c-fos gene promoter. Cell. 1993 Sep 24;74(6):1135–1145. [Abstract] [Google Scholar]
- Gille H, Sharrocks AD, Shaw PE. Phosphorylation of transcription factor p62TCF by MAP kinase stimulates ternary complex formation at c-fos promoter. Nature. 1992 Jul 30;358(6385):414–417. [Abstract] [Google Scholar]
- Gille H, Kortenjann M, Thomae O, Moomaw C, Slaughter C, Cobb MH, Shaw PE. ERK phosphorylation potentiates Elk-1-mediated ternary complex formation and transactivation. EMBO J. 1995 Mar 1;14(5):951–962. [Europe PMC free article] [Abstract] [Google Scholar]
- Gilman MZ. The c-fos serum response element responds to protein kinase C-dependent and -independent signals but not to cyclic AMP. Genes Dev. 1988 Apr;2(4):394–402. [Abstract] [Google Scholar]
- Ginty DD, Bonni A, Greenberg ME. Nerve growth factor activates a Ras-dependent protein kinase that stimulates c-fos transcription via phosphorylation of CREB. Cell. 1994 Jun 3;77(5):713–725. [Abstract] [Google Scholar]
- Goding CR, Temperley SM, Fisher F. Multiple transcription factors interact with the adenovirus-2 EII-late promoter: evidence for a novel CCAAT recognition factor. Nucleic Acids Res. 1987 Oct 12;15(19):7761–7780. [Europe PMC free article] [Abstract] [Google Scholar]
- Graham R, Gilman M. Distinct protein targets for signals acting at the c-fos serum response element. Science. 1991 Jan 11;251(4990):189–192. [Abstract] [Google Scholar]
- Hayes TE, Kitchen AM, Cochran BH. Inducible binding of a factor to the c-fos regulatory region. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1272–1276. [Europe PMC free article] [Abstract] [Google Scholar]
- Herrera RE, Shaw PE, Nordheim A. Occupation of the c-fos serum response element in vivo by a multi-protein complex is unaltered by growth factor induction. Nature. 1989 Jul 6;340(6228):68–70. [Abstract] [Google Scholar]
- Hill CS, Treisman R. Transcriptional regulation by extracellular signals: mechanisms and specificity. Cell. 1995 Jan 27;80(2):199–211. [Abstract] [Google Scholar]
- Hill CS, Marais R, John S, Wynne J, Dalton S, Treisman R. Functional analysis of a growth factor-responsive transcription factor complex. Cell. 1993 Apr 23;73(2):395–406. [Abstract] [Google Scholar]
- Hill CS, Wynne J, Treisman R. Serum-regulated transcription by serum response factor (SRF): a novel role for the DNA binding domain. EMBO J. 1994 Nov 15;13(22):5421–5432. [Europe PMC free article] [Abstract] [Google Scholar]
- Hill CS, Wynne J, Treisman R. The Rho family GTPases RhoA, Rac1, and CDC42Hs regulate transcriptional activation by SRF. Cell. 1995 Jun 30;81(7):1159–1170. [Abstract] [Google Scholar]
- Hipskind RA, Rao VN, Mueller CG, Reddy ES, Nordheim A. Ets-related protein Elk-1 is homologous to the c-fos regulatory factor p62TCF. Nature. 1991 Dec 19;354(6354):531–534. [Abstract] [Google Scholar]
- Jalink K, Hordijk PL, Moolenaar WH. Growth factor-like effects of lysophosphatidic acid, a novel lipid mediator. Biochim Biophys Acta. 1994 Dec 30;1198(2-3):185–196. [Abstract] [Google Scholar]
- Janknecht R, Ernst WH, Nordheim A. SAP1a is a nuclear target of signaling cascades involving ERKs. Oncogene. 1995 Mar 16;10(6):1209–1216. [Abstract] [Google Scholar]
- Janknecht R, Ernst WH, Pingoud V, Nordheim A. Activation of ternary complex factor Elk-1 by MAP kinases. EMBO J. 1993 Dec 15;12(13):5097–5104. [Europe PMC free article] [Abstract] [Google Scholar]
- Johansen FE, Prywes R. Two pathways for serum regulation of the c-fos serum response element require specific sequence elements and a minimal domain of serum response factor. Mol Cell Biol. 1994 Sep;14(9):5920–5928. [Europe PMC free article] [Abstract] [Google Scholar]
- König H. Cell-type specific multiprotein complex formation over the c-fos serum response element in vivo: ternary complex formation is not required for the induction of c-fos. Nucleic Acids Res. 1991 Jul 11;19(13):3607–3611. [Europe PMC free article] [Abstract] [Google Scholar]
- Kortenjann M, Thomae O, Shaw PE. Inhibition of v-raf-dependent c-fos expression and transformation by a kinase-defective mutant of the mitogen-activated protein kinase Erk2. Mol Cell Biol. 1994 Jul;14(7):4815–4824. [Europe PMC free article] [Abstract] [Google Scholar]
- Lütticken C, Coffer P, Yuan J, Schwartz C, Caldenhoven E, Schindler C, Kruijer W, Heinrich PC, Horn F. Interleukin-6-induced serine phosphorylation of transcription factor APRF: evidence for a role in interleukin-6 target gene induction. FEBS Lett. 1995 Feb 27;360(2):137–143. [Abstract] [Google Scholar]
- Manser E, Leung T, Salihuddin H, Zhao ZS, Lim L. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature. 1994 Jan 6;367(6458):40–46. [Abstract] [Google Scholar]
- Marais R, Wynne J, Treisman R. The SRF accessory protein Elk-1 contains a growth factor-regulated transcriptional activation domain. Cell. 1993 Apr 23;73(2):381–393. [Abstract] [Google Scholar]
- Moolenaar WH. Lysophosphatidic acid signalling. Curr Opin Cell Biol. 1995 Apr;7(2):203–210. [Abstract] [Google Scholar]
- Norman C, Runswick M, Pollock R, Treisman R. Isolation and properties of cDNA clones encoding SRF, a transcription factor that binds to the c-fos serum response element. Cell. 1988 Dec 23;55(6):989–1003. [Abstract] [Google Scholar]
- Perkins LM, Ramirez FE, Kumar CC, Thomson FJ, Clark MA. Activation of serum response element-regulated genes by lysophosphatidic acid. Nucleic Acids Res. 1994 Feb 11;22(3):450–452. [Europe PMC free article] [Abstract] [Google Scholar]
- Piette J, Yaniv M. Two different factors bind to the alpha-domain of the polyoma virus enhancer, one of which also interacts with the SV40 and c-fos enhancers. EMBO J. 1987 May;6(5):1331–1337. [Europe PMC free article] [Abstract] [Google Scholar]
- Price MA, Rogers AE, Treisman R. Comparative analysis of the ternary complex factors Elk-1, SAP-1a and SAP-2 (ERP/NET). EMBO J. 1995 Jun 1;14(11):2589–2601. [Europe PMC free article] [Abstract] [Google Scholar]
- Robertson LM, Kerppola TK, Vendrell M, Luk D, Smeyne RJ, Bocchiaro C, Morgan JI, Curran T. Regulation of c-fos expression in transgenic mice requires multiple interdependent transcription control elements. Neuron. 1995 Feb;14(2):241–252. [Abstract] [Google Scholar]
- Roussel MF, Shurtleff SA, Downing JR, Sherr CJ. A point mutation at tyrosine-809 in the human colony-stimulating factor 1 receptor impairs mitogenesis without abrogating tyrosine kinase activity, association with phosphatidylinositol 3-kinase, or induction of c-fos and junB genes. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6738–6742. [Europe PMC free article] [Abstract] [Google Scholar]
- Ruff-Jamison S, Chen K, Cohen S. Induction by EGF and interferon-gamma of tyrosine phosphorylated DNA binding proteins in mouse liver nuclei. Science. 1993 Sep 24;261(5129):1733–1736. [Abstract] [Google Scholar]
- Sadowski HB, Gilman MZ. Cell-free activation of a DNA-binding protein by epidermal growth factor. Nature. 1993 Mar 4;362(6415):79–83. [Abstract] [Google Scholar]
- Sadowski HB, Shuai K, Darnell JE, Jr, Gilman MZ. A common nuclear signal transduction pathway activated by growth factor and cytokine receptors. Science. 1993 Sep 24;261(5129):1739–1744. [Abstract] [Google Scholar]
- Sassone-Corsi P, Visvader J, Ferland L, Mellon PL, Verma IM. Induction of proto-oncogene fos transcription through the adenylate cyclase pathway: characterization of a cAMP-responsive element. Genes Dev. 1988 Dec;2(12A):1529–1538. [Abstract] [Google Scholar]
- Schultz J, Ferguson B, Sprague GF., Jr Signal transduction and growth control in yeast. Curr Opin Genet Dev. 1995 Feb;5(1):31–37. [Abstract] [Google Scholar]
- Shaw PE, Schröter H, Nordheim A. The ability of a ternary complex to form over the serum response element correlates with serum inducibility of the human c-fos promoter. Cell. 1989 Feb 24;56(4):563–572. [Abstract] [Google Scholar]
- Shaw PE, Frasch S, Nordheim A. Repression of c-fos transcription is mediated through p67SRF bound to the SRE. EMBO J. 1989 Sep;8(9):2567–2574. [Europe PMC free article] [Abstract] [Google Scholar]
- Silvennoinen O, Schindler C, Schlessinger J, Levy DE. Ras-independent growth factor signaling by transcription factor tyrosine phosphorylation. Science. 1993 Sep 24;261(5129):1736–1739. [Abstract] [Google Scholar]
- Treisman R. Transient accumulation of c-fos RNA following serum stimulation requires a conserved 5' element and c-fos 3' sequences. Cell. 1985 Oct;42(3):889–902. [Abstract] [Google Scholar]
- Treisman R. Identification of a protein-binding site that mediates transcriptional response of the c-fos gene to serum factors. Cell. 1986 Aug 15;46(4):567–574. [Abstract] [Google Scholar]
- Treisman R. The SRE: a growth factor responsive transcriptional regulator. Semin Cancer Biol. 1990 Feb;1(1):47–58. [Abstract] [Google Scholar]
- Treisman R, Green MR, Maniatis T. cis and trans activation of globin gene transcription in transient assays. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7428–7432. [Europe PMC free article] [Abstract] [Google Scholar]
- Treisman R, Marais R, Wynne J. Spatial flexibility in ternary complexes between SRF and its accessory proteins. EMBO J. 1992 Dec;11(12):4631–4640. [Europe PMC free article] [Abstract] [Google Scholar]
- Wagner BJ, Hayes TE, Hoban CJ, Cochran BH. The SIF binding element confers sis/PDGF inducibility onto the c-fos promoter. EMBO J. 1990 Dec;9(13):4477–4484. [Europe PMC free article] [Abstract] [Google Scholar]
- Yuan YO, Stroke IL, Fields S. Coupling of cell identity to signal response in yeast: interaction between the alpha 1 and STE12 proteins. Genes Dev. 1993 Aug;7(8):1584–1597. [Abstract] [Google Scholar]
- Zhang X, Blenis J, Li HC, Schindler C, Chen-Kiang S. Requirement of serine phosphorylation for formation of STAT-promoter complexes. Science. 1995 Mar 31;267(5206):1990–1994. [Abstract] [Google Scholar]
- Zhong Z, Wen Z, Darnell JE., Jr Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994 Apr 1;264(5155):95–98. [Abstract] [Google Scholar]
Associated Data
Articles from The EMBO Journal are provided here courtesy of Nature Publishing Group
Full text links
Read article at publisher's site: https://doi.org/10.1002/j.1460-2075.1995.tb00186.x
Read article for free, from open access legal sources, via Unpaywall:
https://www.onlinelibrary.wiley.com/doi/pdfdirect/10.1002/j.1460-2075.1995.tb00186.x
Citations & impact
Impact metrics
Article citations
Critical Protein-Protein Interactions Determine the Biological Activity of Elk-1, a Master Regulator of Stimulus-Induced Gene Transcription.
Molecules, 26(20):6125, 11 Oct 2021
Cited by: 5 articles | PMID: 34684708 | PMCID: PMC8541449
Review Free full text in Europe PMC
Role of RIN1 on telomerase activity driven by EGF-Ras mediated signaling in breast cancer.
Exp Cell Res, 396(2):112318, 16 Oct 2020
Cited by: 3 articles | PMID: 33069695 | PMCID: PMC8194098
Transcription factor Fra-1 targets arginase-1 to enhance macrophage-mediated inflammation in arthritis.
J Clin Invest, 129(7):2669-2684, 16 Apr 2019
Cited by: 40 articles | PMID: 30990796 | PMCID: PMC6597220
Druggable targets in the Rho pathway and their promise for therapeutic control of blood pressure.
Pharmacol Ther, 193:121-134, 04 Sep 2018
Cited by: 8 articles | PMID: 30189292 | PMCID: PMC7235948
Review Free full text in Europe PMC
Optical functionalization of human Class A orphan G-protein-coupled receptors.
Nat Commun, 9(1):1950, 16 May 2018
Cited by: 22 articles | PMID: 29769519 | PMCID: PMC5956105
Go to all (123) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Integration of growth factor signals at the c-fos serum response element.
Philos Trans R Soc Lond B Biol Sci, 351(1339):551-559, 01 Apr 1996
Cited by: 29 articles | PMID: 8735278
Contribution of both STAT and SRF/TCF to c-fos promoter activation by granulocyte-macrophage colony-stimulating factor.
Blood, 88(8):2906-2916, 01 Oct 1996
Cited by: 34 articles | PMID: 8874187
Growth hormone regulates ternary complex factors and serum response factor associated with the c-fos serum response element.
J Biol Chem, 272(41):25951-25958, 01 Oct 1997
Cited by: 40 articles | PMID: 9325329
Interaction of serum response factor (SRF) with the Elk-1 B box inhibits RhoA-actin signaling to SRF and potentiates transcriptional activation by Elk-1.
Mol Cell Biol, 22(20):7083-7092, 01 Oct 2002
Cited by: 38 articles | PMID: 12242287 | PMCID: PMC139817