Abstract
Free full text

Smad-independent transforming growth factor-ß signaling in fibroblasts via c-Abl and Egr-1: selective modulation by imatinib mesylate
Abstract
The non-receptor protein tyrosine kinase c-Abl regulates cell proliferation and survival. Recent studies provide evidence that implicate c-Abl as a mediator for fibrotic responses induced by Transforming growth factor-ß (TGF-ß), but the precise mechanisms underlying this novel oncogene function are unknown. Here we report that when expressed in normal fibroblasts, a constitutively active mutant of Abl mutant that causes chronic myelogenous leukemia stimulated the expression and transcriptional activity of the early growth response factor Egr-1. Mouse embryonic fibroblasts lacking c-Abl were resistant to TGF-ß. Sensitivity of these cells to TGF-ß could be rescued by wildtype c-Abl, but not by a kinase-deficient mutant form of c-Abl. Furthermore, Abl kinase activity was necessary for the induction of Egr-1 by TGF-ß in normal fibroblasts, and Egr-1 was required for stimulation of collagen by Bcr-Abl. Lesional skin fibroblasts in mice with bleomycin-induced scleroderma displayed evidence of c-Abl activation in situ, and elevated phospho-c-Abl correlated with increased local expression of Egr-1. Collectively, these results position Egr-1 downstream of c-Abl in the fibrotic response, delineate a novel Egr-1-dependent intracellular signaling mechanism that underlies the involvement of c-Abl in TGF-ß responses, and identify Egr-1 as a target of inhibition by imatinib. Furthermore, the findings demonstrate in situ activation of c-Abl paralleling the up-regulation tissue expression of Egr-1 in fibrosis. Pharmacological targeting of c-Abl and its downstream effector pathways may therefore represent a novel therapeutic approach to blocking TGF-ß-dependent fibrotic processes.
Introduction
Systemic sclerosis (SSc), a chronic disease of unknown etiology, is characterized by vascular injury, autoimmune inflammatory responses and tissue fibrosis (Jiminez and Derk, 2004). Multiple cytokines and growth factors that can induce fibroblast activation have been implicated in the pathogenesis of fibrosis (Varga and Abraham, 2007). Of these, transforming growth factor- ß (TGF-ß) plays a pivotal role by driving collagen synthesis, epithelial-mesenchymal transition, and myofibroblasts transdifferentiation (Varga and Trojanowska, 2008). In fibroblasts, the Smad pathway serves as the most important signal transduction mechanism for TGF-ß. Canonical Smad signaling is activated upon TGF-ß binding to the type 2 transmembrane receptors (TßRII), leading to phosphorylation of type 1 receptor (TßRI) and activation of cytoplasmic Smad2/3. Activated Smad2/3 then forms a complex with Smad4 and accumulates within the nucleus where it binds to consensus Smad binding element (SBE) sequences to activate transcription (Massague et al., 2005). Recent studies also provide substantial evidence for the important roles of non-Smad signaling pathways in mediating TGF-ß responses (Moustakas et al., 2005). These include MAP kinases, TAK1, as well as early growth response-1 (Egr-1) (Pannu and Trojanowska, 2004; Javelaud and Mauviel, 2005; Wenner and Yan, 2003; Bhattacharyya et al., AJP). Establishing the link between distinct signal transduction pathways with particular TGF-ß responses is important for gaining further insights into TGF-ß biology, and for the development of anti-TGF-ß therapies selectively targeting profibrotic responses.
The c-Abelson (c-Abl) oncogene, a member of the Src family of non-receptor protein tyrosine kinases, regulates cell proliferation, cytoskeletal remodeling and apoptosis in response to DNA damage and stress (Kharbanda et al; 1997, 1995 and 1995). Transforming mutants of c-Abl are found in 95% of patients with chronic myelogenous leukemia (CML) (Groffen et al., 1984). The Philadelphia chromosome, a hallmark of CML, represents reciprocal translocation of the Abl gene from chromosome 9 to the breakpoint cluster region of chromosome 22 t(9;22). This translocation results in the fusion protein Bcr-Abl (breakpoint cluster region Abelson leukemia) which has constitutive tyrosine kinase activity and is directly implicated in causing leukemic transformation, presumably via phosphorylation of multiple protein targets, resulting in the activation of mitogenic pathways (Rowley, 1973; Lugo 1990). Imatinib mesylate (Gleevec), a small molecule kinase inhibitor active against c-Abl as well as Bcr-Abl, suppresses the growth of Bcr-Ab-expressing progenitor cells, and is highly effective in the treatment of CML, with > 70% stable cytogenetic remission (Druker et al., 1996, La Rosee et al., 2002, Goldman and Melo, 2003; Deininger et al., 1997). Recent studies exploring the function of c-Abl in non-myeloid cell types indicate that in mesenchymal cells but not epithelial cells, c-Abl integrates TGF-ß-induced serine/threonine receptor kinase signaling with tyrosine kinase pathways (Wilkes et al., 2006). Imatinib mesylate was shown to abrogate the stimulation of collagen gene expression in fibroblasts in vitro, and to prevent the development of tissue fibrosis in vivo (Daniels et al., 2004; Wilkes et al., 2006, Distler et al., 2007, Wang et al., 2005). It has been proposed that the ablation of TGF-ß signaling by imatinib and related inhibitors that block c-abl tyrosine kinase might usher in a promising new era in the treatment of fibrosis (Rosenbloom and Jimenez, 2008). Despite the emerging evidence implicating c-Abl in the stimulation of collagen synthesis and fibrotic responses, the underlying molecular basis, and the mediators downstream of c-Abl, are not well understood.
The present studies were therefore undertaken in order to characterize the involvement of c-Abl in TGF-ß-mediated stimulation of collagen synthesis, focusing on the potential role of Egr-1. Here we report that constitutively active Abl not only stimulated collagen gene expression in normal fibroblasts, but also directly induced Egr-1 expression and transcriptional activity in the absence of added TGF-ß. Induction of Egr-1 by Abl occurred via an extracellular regulated kinase (ERK)-dependent signaling mechanism, and was necessary for the stimulation of collagen gene expression. Bleomycin-induced scleroderma in the mouse was associated with accumulation of phosphorylated c-Abl within fibrotic dermis, and correlated with increased expression of Egr-1 in situ. These results lead us to propose a novel model for fibrogenesis in which TGF-ß-induced activation of c-Abl results in enhanced Egr-1 expression and consequent fibrotic cellular responses.
Materials and Methods
Cell culture and reagents
Primary cultures of human dermal fibroblasts were established by explantation from neonatal foreskin and studied at early passages (<8) (Mori et al., 2004). Murine NIH3T3 fibroblasts were obtained from the American Type Culture Collection (Mansassas, VA). Wildtype and Egr-1-null (Egr-1−/−) fibroblasts were established by explantation from neonatal mouse skin and studied at early passages. Abl-null/Arg-null (Abl−/−Arg−/−) mouse embryonic fibroblasts (MEFs) stably expressing wildtype c-Abl or a kinase-inactive form of c-Abl were a gift from Pamela Woodring (Woodring et al., 2002). Fibroblasts were maintained in modified Eagle's medium (EMEM) or Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum (FCS) (Gibco BRL, Grand Island, NY), 1% vitamin, and 2 mM L-glutamine. Culture media and tissue culture reagents were from Biowhittaker (Walkersville, MD). In selected experiments, fibroblasts were incubated in serum-free media supplemented with 0.1% bovine serum albumin (BSA) for 24 h before treatment. The kinase inhibitors imatinib mesylate (Novartis Pharmaceuticals, St. Louis, MO) or U0126 (Cell Signaling, Beverly, MA) were added to the cultures for 30 min, followed by TGF-ß1 (12.5 ng/ml) (PeproTech, Rocky Hill, NJ) for up to 48 h. Cellular toxicity was evaluated by Trypan blue dye exclusion.
Quantitative real-time PCR
Total RNA was isolated from fibroblasts using Trizol reagent (Life Technologies, Grand Island, NY). For quantitative real-time PCR (qPCR), 1 μg of total RNA was reverse-transcribed to cDNA using Reverse Transcription System (Promega, Madison, WI), and products (50 ng) were amplified using SYBR Green PCR Master Mix (Applied Biosytems, Foster City, CA) on the Applied Biosystems 7500 Prism Sequence Detection System (Bhattacharyya et al, AJP). The primers used are shown in Table 1.
Table 1
Sequences Used for Real-Time Quantitative PCR Gene cDNA sequence
| Human | |
| Bcr-Abl | Forward: 5’- CATTCCGCTGACCATCAATAAG-3’ |
| Reverse: 5’- GATGCTACTGGCCGCTGAAG-3’ | |
| Egr-1 | Forward: 5’-TGCGGCAGAAGGACAAGAAAGC-3’ |
| Reverse: 5’-TGAGGAAGGGAAGCTGCTGACC-3’ | |
| COL1A1 | Forward: 5’- CCAGAAGAACTGGTACATCAGCA-3’ |
| Reverse: 5’- CGCCATACTCGAACTGGGAAT-5’ | |
| COL1A2 | Forward: 5’- GATGTTGAACTTGTTGCTGAGG-3’ |
| Reverse: 5’- TCTTTCCCCATTCATTTGTCTT-3’ | |
| Actin | Forward: 5’-AATGTCGCGGAGGACTTTGAT-3’ |
| Reverse: 5’-AGGATGGCAAGGGACTTCCTG-3’ | |
| Mouse | |
| COL1A1 | Forward: 5’- CCTGAGTCAGCAGATTGAGAA-3’ |
| Reverse: 5’ACTGAACTTGACCGTACACCAGTACTCTCCGCTCTTCAA-3’ | |
| COL1A2 | Forward: 5’-CCGTGCTTCTCAGAACATCA-3’ |
| Reverse: 5’-CTTGCCCCATTCATTTGTCT-3’ | |
| GAPDH | Forward: 5’-GTCGTGGATCTGACGTGCC-3’ |
| Reverse: 3’-GATGCCTGCTT CACCACCTT-3’ |
Western analysis
At the end of the experiments, whole cell lysates or nuclear and cytoplasmic extracts were prepared, and subjected to immunoblot analysis, as described previously (Mori et al, Exp Cell Res, 2000). In brief, equal amounts of proteins (20-50 μg/lane) were separated by SDS polyacrylamide gel electrophoresis (SDS-PAGE) in 4-15% gels, proteins were transferred to Immobilon-P membranes (Millipore, Billerica, MA ) and immunoblotted overnight with primary antibodies specific for Type I collagen (Southern Biotech, Birmingham, AL), α-smooth muscle actin and α-tubulin (both from Sigma-Aldrich, St. Louis, MO), c-Abl and phospho-cAbl, p44/42 and phospho-p44/42, phospho-Smad1, Smad1, phospho-Smad2 and phospho-Smad3 (all from Cell Signaling, Beverly, MA), Egr-1(C19), or β-actin (C-2) (all from Santa Cruz Biotechnology, Santa Cruz, CA). Membranes were then incubated with appropriate secondary antibodies and subjected to enhanced chemiluminescence detection using ECL Reagent (Amersham-Pharmacia, Piscataway, NJ).
DNA affinity precipitation assays
Nuclear extracts were prepared from confluent fibroblasts incubated with imatinib (10 μM) and TGF-ß1 for 90 min, and subjected to DNA affinity precipitation assays, as described (Chen et al., 2006). Briefly, synthetic double-stranded oligonucleotides corresponding to the consensus SBE sequence were biotinylated at the 5’ end, and incubated with nuclear extracts in a binding mixture containing Streptavidin-agarose bead, followed by centrifugation. The bound proteins were separated by 4–20% SDS-PAGE, transferred to Immobilon-P membranes, and immunoblotted with antibodies specific for phospho-Smad2 or phospho-Smad3 (Cell Signaling), followed by visualization using ECL reagent.
Chromatin immunoprecipitation (ChIP) assays
Chromatin immunoprecipitation assays were performed using Ez-Magna CHIP ™ G (Millipore, Billerca, MA) according to the manufacturer's protocol. Briefly, confluent cultures of skin fibroblasts transfected with Bcr-Abl or empty vector were incubated with imatinib in the presence of TGF-ß1 (10 ng/ml) for 60 min. Formaldehyde was added to the cultures (final concentration 1%) to cross-link chromatin. Nuclear extracts were then prepared and sonicated on ice to generate chromatin DNA fragments with an average length of 500-1000 bp. Aliquots of lysates were immunoprecipitated with antibodies to Egr-1 (588, Santa Cruz). Immunoprecipitated chromatin was recovered, and PCR amplification of the captured DNA sequences was performed using primers complementary to the COL1A2 promoter region harboring the Egr-1-binding sites −141 to −133 bp and distal −303 to −295 bp. The primer sequences were forward primer, 5’-CTACAGGGCACAGGTGAGG- 3’, and reverse primer, 5’-AAAGCCCGGATCTGCCCTA-3’, to generate a 422-bp amplification product (Chen SJ, JBC). DNA samples were analyzed by electrophoresis in 2% agarose gels.
Transient Transfection Assays
The reporter plasmids 772COL1A2-CAT (containing the –772/+58 bp fragment of the human pro α 2 (I) collagen gene) (Ihn et al., 1997) and plasmids harboring 1.2 kb of the mouse Egr-1 gene promoter fused to luciferase gene (Sukhatme et al., 1988) were used for transient transfection assays. Subconfluent cultures of foreskin fibroblasts, NIH3T3 fibroblasts, or MEFs in media containing 0.1% FBS or 0.1% BSA and no serum were transfected using Superfect reagent (Qiagen, Valencia, CA). In each experiment, fibroblasts were cotransfected with Renilla luciferase pRL-TK plasmids (Promega, Madison, WI) as control for transfection efficiency. Fibroblasts were pretreated with imatinib (10 μM) or U0126 (10 μM) for 30 min, followed by incubation with TGF-ß1 (10 ng/ml). At the end of the experiments, cultures were harvested and cell lysates were assayed for their luciferase or chloramphenicol acetyltransferase (CAT) activities (Chen et al., 1999). Experiments were performed in triplicates and repeated at least two times.
c-Abl kinase assays
The regulation of cellular c-Abl activity was determined by in vitro kinase assays (Wilkes et al., 2006). Briefly, cultures incubated with TGF-ß1 for indicated periods were lysed in kinase buffer (Wilkes, JBC, 2004). Cellular c-Abl was immunoprecipitated using antibody (K12; Santa Cruz Biotechnology), immune complexes were collected with protein A-Sepharose, washed twice in kinase buffer and then incubated in kinase buffer containing 0.5μci [γ 32P] ATP. The reaction was allowed to proceed for 5 min, then was stopped and the complexes were subjected to SDS-PAGE, followed by autoradiography to examine the amount of phosphorylated GST-Crk substrate. Total c-Abl protein was detected by Western analysis.
Immunofluorescence confocal microscopy
Endogenous Smad localization was examined by immunofluorescence laser scanning microscopy (Mori et al., 2000). Briefly, subconfluent foreskin fibroblasts or MEFs in media with 0.1% FBS were pretreated with indicated concentrations of imatinib, followed by TGF-ß1 (2.5 ng/ml) for 30 min or 24 h. Cultures were then fixed with 100% methanol and incubated with primary antibodies to Type I collagen (Southern Biotech), or to Smad1/2/3 or Smad4 (both from Santa Cruz), followed by FITC-labeled secondary antibodies. Nuclei were identified by 4,6-diamidino-2-phenylindone (DAPI). Subcellular distribution of fluorescence was evaluated by laser scanning confocal microscopy using a Zeiss LSHS10 microscope, and nuclear accumulation was determined by quantitating the colocalization of Smad2/3 with nuclear DAPI plotted as two-dimensional scatterplots (Mori Y, Exp cell res 2008).
Expression of phospho-cAbl in vivo
To evaluate activation of c-Abl in vivo, six week-old female BALB/c mice (five mice per group) weighing 20 g received daily subcutaneous injections of bleomycin (Nihon Kayoku, Tokyo, Japan) or phosphate-buffered saline (PBS) (Lakos et al., 2004). Twenty eight days after initiation of injections, mice were sacrificed, and lesional skin was harvested and processed for analysis (Takagawa et al., 2003). Briefly, four μm sections were deparaffinized, rehydrated and incubated with peroxidase blocking solution (DAKO Corporation, Carpinteria, CA) followed by rabbit polyclonal antibodies to phospho-cAbl (Cell Signaling) at a dilution 1:100, and donkey anti-rabbit IgG (Promega) as secondary antibodies at a dilution of 1:1000. Bound antibodies were detected using DAKO Envision + System following the manufacturer's instructions. Substitution of the primary antibody with isotype-matched irrelevant IgG (Invitrogen, Carlsbad, CA) at the same concentration served as negative control in every case. After counterstaining with hematoxylin, sections were mounted with Permount (Fisher Scientific, Pittsburgh, PA) and viewed under an Olympus BH-2 microscope. Images were obtained by digital capture. Fibroblasts were identified by their spindle-shaped morphology. The number of immunopositive cells/field was determined at 400x magnification by two blinded observers, and the ratio of positive cells/total cells in each field was calculated. Animal protocols were institutionally approved, and were in accordance with the guidelines of the NIH/Association for Assessment and Accreditation of Laboratory Animal Care.
Statistical analysis
For comparisons of the means in control and treated fibroblasts, statistical significance was determined using the unpaired Student's t-test. A p value < 0.05 was considered significant.
Results
Abl activation is sufficient and necessary for enhanced collagen gene expression
In immortalized cell lines, c-Abl has been shown previously to mediate the induction of fibrotic responses, such as stimulation of the synthesis of collagen and fibronectin (Daniels et al., 2004; Wilkes et al., 2006; Distler et al., 2007). It was important to examine the role of c-Abl in the regulation of collagen synthesis in primary cultures of fibroblasts. Transfection of foreskin fibroblasts with expression plasmids for Bcr-Abl, a fusion protein with a constitutively active Abl tyrosine kinase domain resulted in a marked increase in Type I collagen levels with a magnitude comparable to TGF-ß (Fig. 1A, upper panel). In contrast, wildtype c-Abl induced only a modest stimulation in absence of TGF-ß (Fig. 1A, lower panel). Furthermore, transient transfection assays showed that ectopic Bcr-Abl was by itself able to stimulate the activity of the COL1A2 promoter (Fig. 1B), and stimulation was further enhanced in the presence of TGF-ß. Blockade of c-Abl kinase by imatinib abrogated TGF-ß induced stimulation of Type I collagen synthesis (Fig. 2A, left and data not shown). There was no effect on cellular viability at the concentrations of imatinib used (data not shown). The stimulation of COL1A1 mRNA expression (Fig. 2B) and of COL1A2 CAT activity (Fig. 2C) were similarly abrogated. Parallel experiments indicated that imatinib also abrogated the stimulation of Type I collagen synthesis (Fig. 2E) and COL1A1 and COL1A2 mRNA expression (Fig. 2D) in Bcr-Abl-expressing fibroblasts.
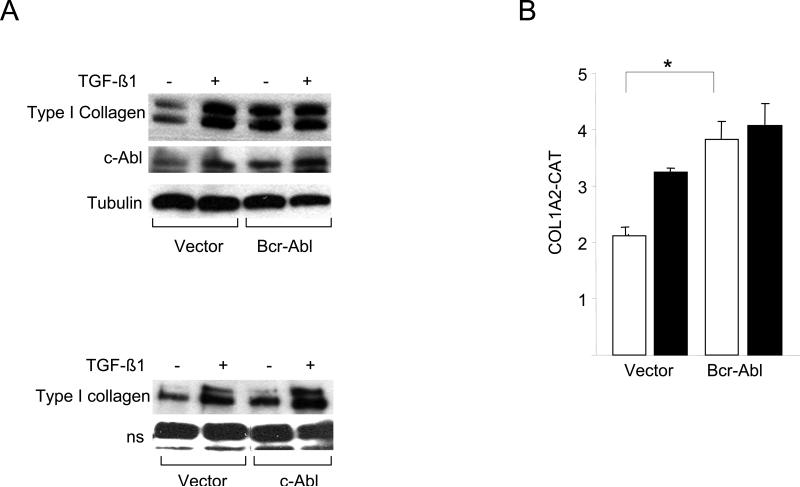
Confluent foreskin fibroblasts were transiently transfected with Bcr-Abl (A, top panel; B) or wildtype c-Abl (A, lower panel) and incubated with TGF-β for 24 h. A. Whole cell lysates were subjected to Western analysis. Representative immunoblots are shown. B. Fibroblasts were cotransfected with 772COL1A2-CAT. Cell lysates were assayed for their CAT activities. Results, normalized with Renilla luciferase, are expressed as means ± S.D. of triplicate determinations. Open boxes, untreated fibroblasts; closed boxes, TGF-ß-treated fibroblasts. *p<0.005.
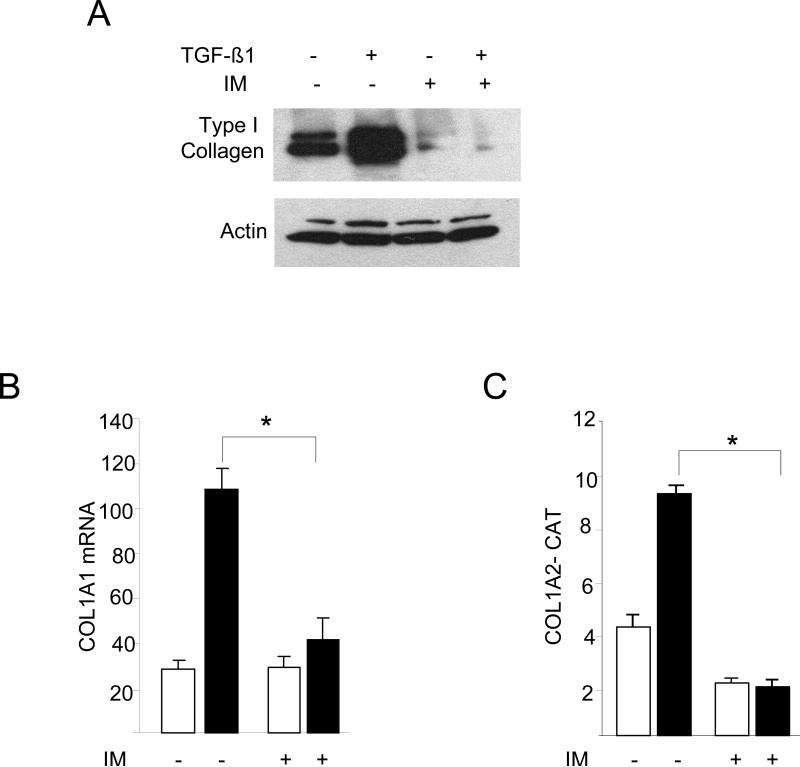
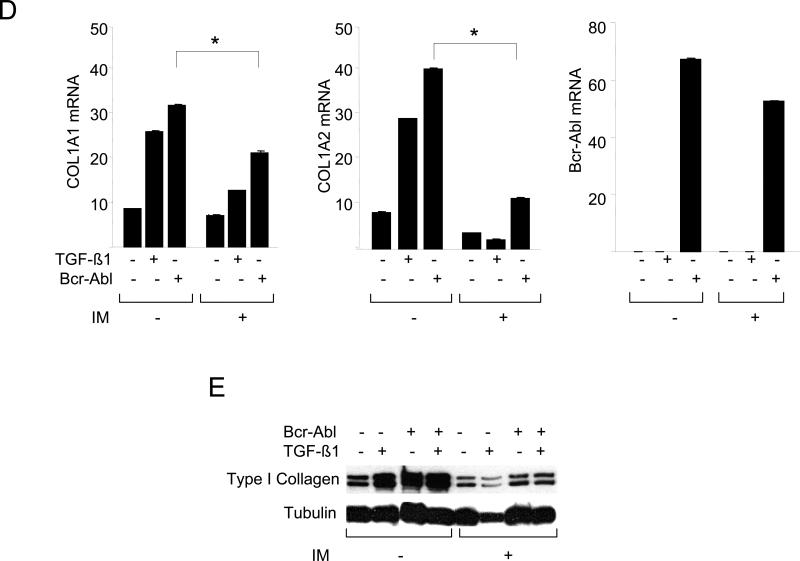
Foreskin fibroblasts were pretreated with 10 μM imatinib (IM) for 30 min, followed by TGF-ß1 for a further 24 h. A. Whole cell lysates were subjected to Western analysis. Representative immunoblots. B. Total RNA was examined by real-time qPCR. Results normalized with actin are shown as means ± SD from triplicate determinations. Open boxes, untreated fibroblasts; closed boxes, TGF-ß-treated fibroblasts. *p<0.005. C. Fibroblasts were transiently transfected with 772COL1A2-CAT. Cell lysates were assayed for their CAT activities. The results are the means ± S.D. of triplicate determinations. Open boxes, untreated fibroblasts; closed boxes, TGF-ß-treated fibroblasts. D. Fibroblasts were transfected with Bcr-Abl and total RNA was subjected to realtime qPCR analysis. The results are representative of two independent experiments. * p<0.005. E. Whole cell lysates were subjected to Western analysis. Representative immunoblots are shown. IM, imatinib mesylate.
These studies were complemented by a loss-of-function approach using MEFs null for c-Abl and Arg (Abl−/−Arg−/−). The results of Western analysis (Fig. 3A) and metabolic labeling with [14C]-proline (Fig. 3B) showed that TGF-ß failed to stimulate Type I collagen synthesis in Abl−/−Arg−/− MEFs. Similar effects were seen on the stimulation of fibronectin synthesis. Real-time qPCR analysis confirmed the attenuated TGF-ß response in Abl−/−Arg−/− MEFs (Fig. 3C). Furthermore, both basal COL1A2 promoter activity, as well as TGF-ß-induced stimulation, were significantly attenuated in Abl−/−/Arg−/− MEFs (Fig. 3D). Rescue with wildtype c-Abl not only enhanced the basal levels of collagen synthesis and mRNA expression, but also restored TGF-ß-induced stimulation in these cells (Figs. 4A and B). In marked contrast, a kinase-inactive mutant c-Abl failed to restore TGF-ß sensitivity. Collectively, these gain-of-function and loss-of-function experiments implicate Abl as both sufficient and necessary for stimulation of collagen gene expression in primary fibroblasts, firmly establishing a functional role for tyrosine kinase signaling in this TGF-ß response. As observed previously in NIH3T3 and IMR90 fibroblastic line (Daniels et al., 2002), stimulation of primary fibroblasts by TGF-ß induced a marked increase in c-Abl kinase activity within 15 min and peaking at 60 min (Fig. 5A). Furthermore, cellular c-Abl was phosphorylated on Tyr-245 in parallel with phosphorylation of Smad2 and p44/42 (Fig. 5B). c-Abl is thus a direct target of TGF-ß that is necessary and sufficient for stimulation of collagen synthesis in primary fibroblasts.
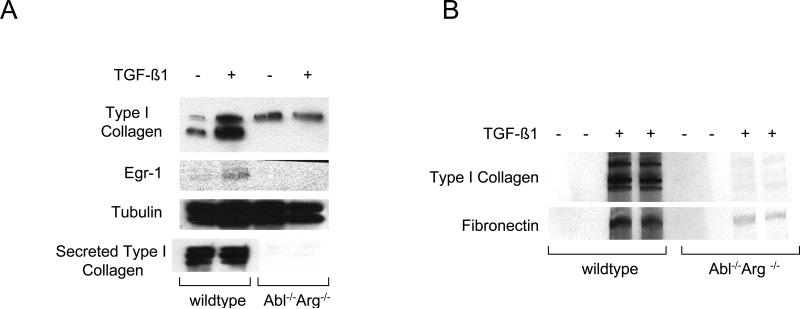
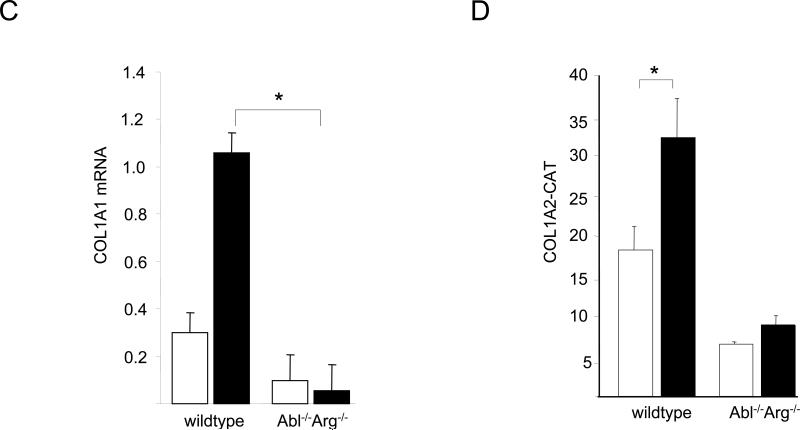
Fibroblasts from Abl −/−Arg −/− and wildtype mouse embryos in parallel were incubated with TGF-ß for 24 h. A. Whole cell lysates were subjected to Western analysis. Representative immunoblots. B. Following radiolabeling of cultures with [14C]-proline for 24 h, conditioned media were harvested and subjected to SDS-PAGE. Representative autoradiographs. C. Total RNA was subjected to real-time qPCR analysis. Results, expressed relative to actin, are shown as the means ± S.D.of triplicate determinations from a representative experiment. Open boxes, untreated fibroblasts; closed boxes, TGF-ß-treated fibroblasts. *p<0.005. D. Fibroblasts were transiently transfected with 772COL1A2-CAT. Following incubation with TGF-ß for 24 h, cell lysates were assayed for their CAT activities. The results are shown as the means ± S.D. of triplicate determinations. Open boxes, untreated fibroblasts; closed boxes, TGF-ß-treated fibroblasts. *p<0.005.
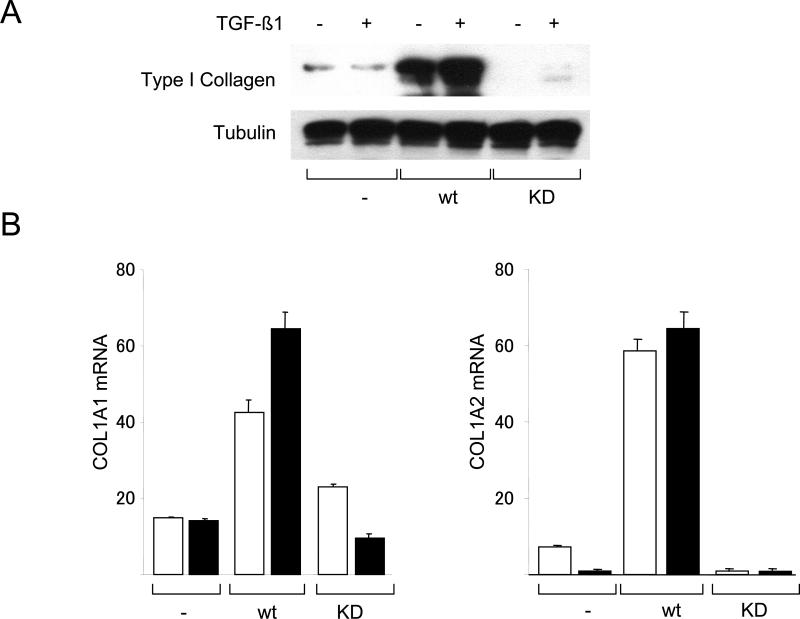
Abl −/−Arg −/− MEFs stably expressing c-Abl or a kinase-inactive mutant form of cAbl (KD) in parallel were incubated with TGF-ß for 24 h. A. Whole cell lysates were subjected to Western analysis. Representative immunoblots. B. Total RNA was subjected to real-time qPCR analysis. Results, expressed relative to actin are means ± S.D. from triplicate determinations from a representative experiment. Open boxes, untreated fibroblasts; closed boxes, TGF-ß-treated fibroblasts. *p<0.005.
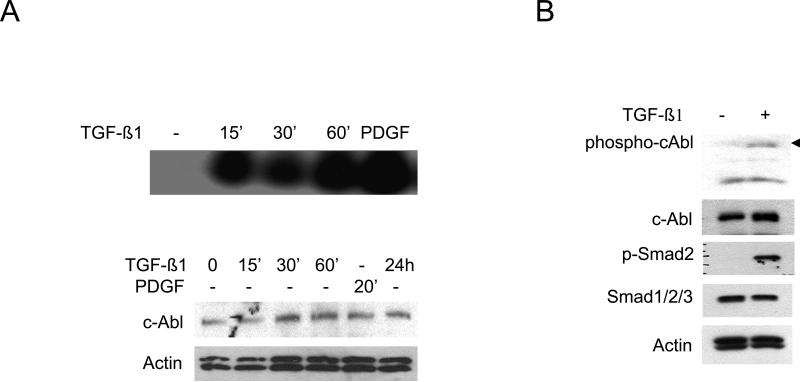
A. Confluent fibroblasts were incubated with TGF-ß1 (10 ng/ml) or PDGF (25 ng/ml) for indicated periods. Whole cell lysates were immunoprecipitated with antibodies to c-Abl and kinase activity was assayed using GST-Crk as substrate (upper panel). Western analysis of whole cell lysates (lower panel). B. Fibroblasts were incubated with TGF-β1 for 30 min and whole cell lysates were subjected to Western analysis.
Smad2/3-independent inhibition of TGF-ß signaling by imatinib mesylate
As downstream targets for activated ALK5 in fibroblasts, Smad2 and Smad3 serve as the principal signal transducers for TGF-ß stimulation of collagen and related profibrotic genes (Chen et al., 1999). Interstingly, the inhibitory effect of imatinib on collagen synthesis was independent of TGF-ß-induced phosphorylation and nuclear accumulation of Smad3 or Smad4 (Figs. 6A upper panel and 6C, and data not shown), or of binding of activated Smad2/3 to SBE sequences (Fig. 6B). Furthermore, Smad2/3 nuclear accumulation was comparable in Abl−/−/Arg−/− MEFs and in wildtype control MEFs treated with TGF-ß (Fig. 6D). A recent report indicated that imatinib blocked the phosphorylation of Smad1 induced by TGF-ß (Pannu et al., 2008). Smad1 activation by TGF-ß was originally described in endothelial cells, where the response involved both the ALK5 and ALK1 TGF-ß receptors (Goumans et al., 2002). Subsequent reports have established that Smad1 can be activated in mesenchymal cells, and may play a role in fibrosis (Takahashi et al., 2004; Pannu et al., 2007; Pannu et al., 2008). While in the present studies TGF-ß induced phosphorylation of Smad1 in normal fibroblasts, the levels of phospho-Smad1 in TGF-ß-stimulated fibroblasts were unaffected by pretreatment with imatinib (Fig. 6A, lowel panel).
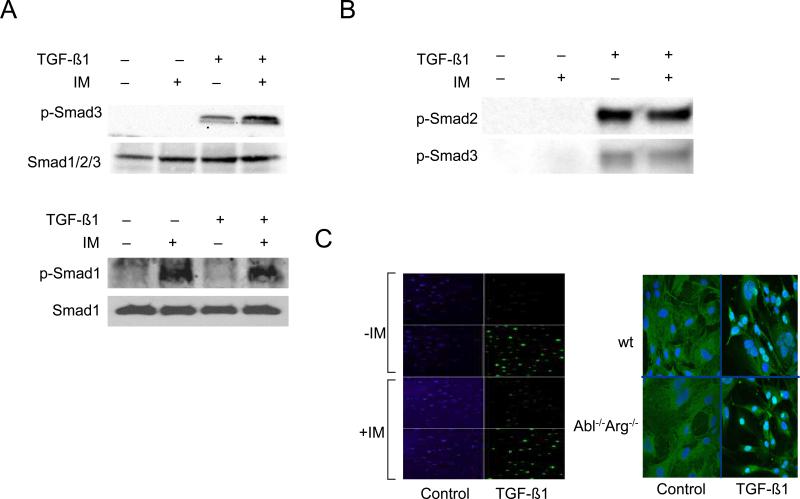
A-C. Confluent foreskin fibroblasts were pretreated for 30 min with imatinib (10 μM) followed by incubation with TGF-ß1 for a further 30 min. A. Whole cell lysates were subjected to Western analysis. Representative immunoblots. B. Nuclear extracts were subjected to DNA affinity precipitation assays using biotin end-labeled oligonucleotides harboring the SBE sequence as described under “Materials and Methods.” A representative Western blot. C . Fibroblasts were immunostained with antibodies to Smad4, and examined by immunofluorescence confocal microscopy. Representative images. IM, Imatinib mesylate. D. Abl −/−/Arg −/− MEFs and wildtype MEFs in parallel were incubated with TGF-ß1 (30 min) and Smad2/3 localization was examined by confocal microscopy. Representative images (left panel). The nuclear localization of Smad2/3 was quantitated as described under methods (right panel). Results represents the means ± S.D. from separate fields. Open boxes, untreated fibroblasts; closed boxes, TGF-ß-treated fibroblasts. *p<0.005.
Egr-1 is downstream of c-Abl in the TGF- ß signal transduction pathway
These results indicate that Abl mediates the stimulation of collagen gene expression, and inhibition of Abl kinase by imatinib blocks this response. However, since the phosphorylation of Smad2/3, the principal mediator of TGF-ß responses, appeared to be unaffected by imatinib, the identity of the mediators downstream of Abl remain unclear. Bcr-Abl is known to effect the function of multiple intracellular signaling pathways. Reported downstream effectors of Bcr-Abl include the extracellular signal-regulated kinases, c-jun N-terminal kinase (JNK), p38 mitogen activated kinase, PI3K/Akt and Stat5 (Raitan et al., 1995; Che et al., 2001; Sánchez-Arévalo Lobo V J. et al., 2005; Skorski et al., 1997; Danial et al., 2000). Because we have shown in previous work that the early response transcription factor Egr-1 is rapidly induced by TGF-ß in normal fibroblasts, and plays an indispensable direct role in the stimulation of collagen synthesis (Chen et al., 2006, Bhattacharyya et al., 2008), here, we examined the regulation of Egr-1 by Abl. The results showed that ectopic Bcr-Abl stimulated Egr-1 protein synthesis and mRNA expression (Fig. 7). This response was abrogated by pretreatment of the fibroblasts imatinib, indicating that the stimulatory effect was directly dependent on Abl kinase activity. To explore the functional contribution of Abl in TGF-ß regulation of Egr-1, NIH3T3 fibroblasts were cotransfected with Egr-1 promoter-luc reporter constructs along with Bcr-Abl. Constitutively active Abl induced a ~2-fold increase in Egr-1 promoter activity (Fig. 8A). The stimulation of Egr-1 by TGF-ß is mediated via ERK1/2 and MEK1 (Bhattacharyya et al., 2008). Because MEK1 kinase can be activated by c-Abl (Kharbanda et al., 2000), we examined its role in the Egr-1 response using the MEK1 inhibitor U0126. Inhibition of MEK1 activity markedly attenuated the stimulation of Egr-1 induced by Bcr-Abl (Fig. 8A). As expected, U0126 also prevented stimulation induced by TGF-ß. These results indicate that Abl is both sufficient and necessary for MEK1/ERK1/2-mediated induction of Egr-1 in fibroblasts.
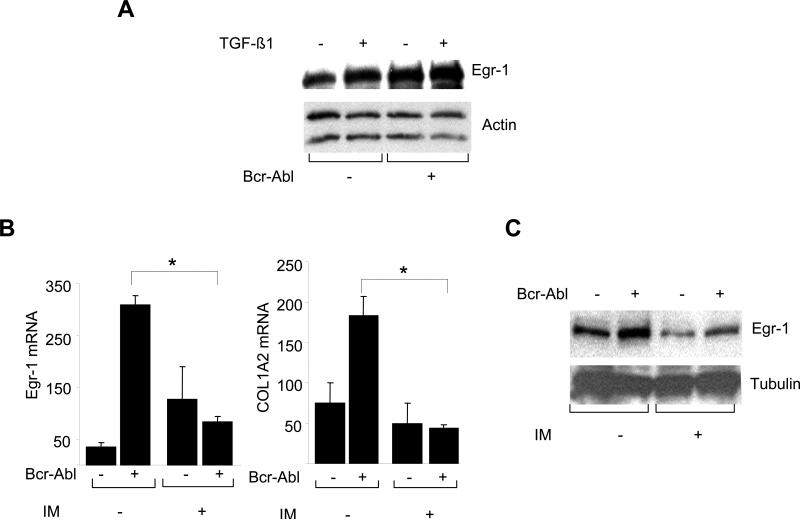
A. Confluent foreskin fibroblasts were transiently transfected with Bcr-Abl and incubated with TGF-β for 24 h. A. Whole cell lysates were subjected to Western analysis. Representative immunoblots are shown. B. Confluent fibroblasts were transiently transfected with Bcr-Abl in presence of imatinib. Total RNA normalized with actin was subjected to realtime qPCR analysis. The results are the means ± S.D. of triplicate determinations. *p<0.005. C. Whole cell lysates from confluent cultures treated with imatinib were subjected to Western analysis. Representative immunoblots are shown.
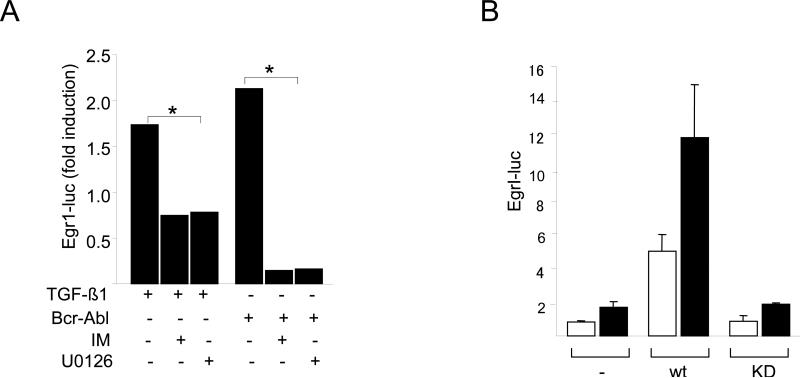
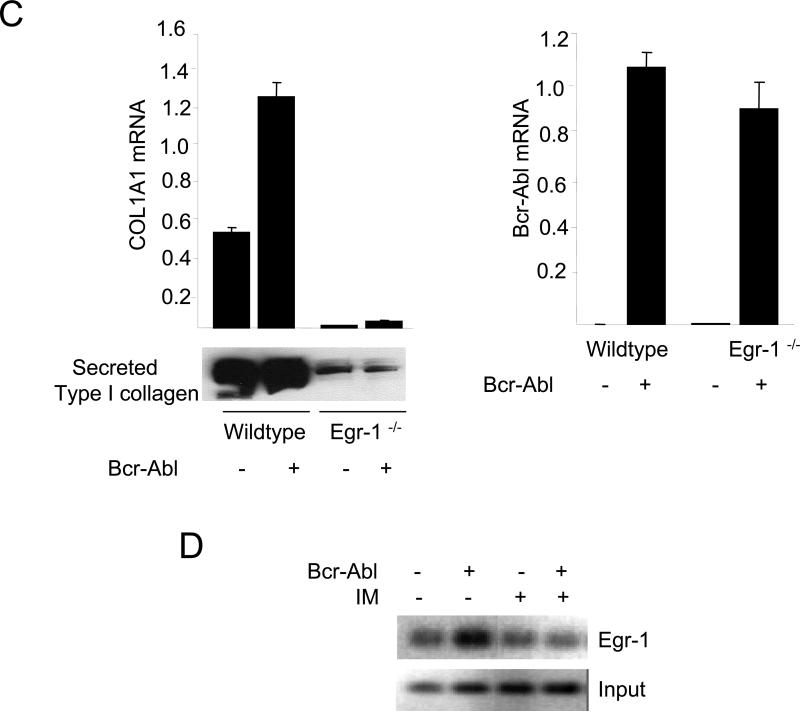
A. NIH3T3 fibroblasts were transiently cotransfected with indicated concentrations of Bcr-Abl along with Egr-1-luc. Following pretreatment with imatinib or U0126, cultures were incubated with TGF-ß1 for a further 24 h and cell lysates were assayed for their luciferase activities. The results, normalized with Renilla luciferase, are expressed as fold-induction relative to TGF-ß or Bcr-Abl. *p<0.005. B. Abl −/−Arg −/− MEFs stably expressing c-Abl or a kinase-deficient mutant form of c-Abl (KD) were transfected with Egr-1- luc, and following incubation with TGF-ß1 for 24 h, cell lysates were assayed for their luciferase activities. The results, normalized with Renilla luciferase, are expressed as means ± S.D. of triplicate determinations from two independent experiments. Open boxes, untreated MEFs; closed boxes, TGF-ß-treated MEFs. C. Wildtype and Egr-1−/− skin fibroblasts were transfected with Bcr-Abl and total RNA were subjected to realtime qPCR analysis (upper panel), and secreted Type I collagen was examined by Western blot analysis (lower panel). The results are the means ± S.D. of triplicate determinations. D. ChIP assays. Foreskin fibroblasts transfected with Bcr-Abl were cross-linked with formaledehyde, chromatin was immunoprecipitated with antibodies to Egr-1, followed by PCR amplification with COL1A2-specific primers. PCR products were analyzed by agarose gel electrophoresis. Input genomic DNA was used as positive control. IM, Imatinib mesylate.
To further examine the role of Egr-1 downstream of c-Abl in TGF-ß signaling, complementary experiments were performed using Abl −/−Arg −/− MEFs. Transient transfection assays showed that wildtype c-Abl, but not the kinase-inactive mutant form, could rescue the activation of the Egr-1 promoter by TGF-ß (Fig. 8B). The key role of Egr-1 downstream of c-Abl in TGF-ß-induced collagen stimulation was further established in skin fibroblasts lacking Egr-1. In contrast to wildtype fibroblasts, in Egr-1−/− fibroblasts ectopic Bcr-Abl failed to stimulate COL1A1 mRNA expression (Fig. 8C upper panel) or secretion of Type I collagen into the media (Fig. 8C lower panel).
Next, the effect of Bcr-Abl on Egr-1 binding to its functional recognition sites in COL1A2 promoter was examined in intact cells. Foreskin fibroblasts expressing ectopic Bcr-Abl were cross-linked with formaldehyde, and chromatin was subjected to ChIP. Whereas no Egr-1 was detected on the COL1A2 promoter in control fibroblasts expressing empty vector, Egr-1 binding to the COL1A2 promoter was increased in fibroblasts harboring ectopic Bcr-Abl, and imatinib abrogated this increase (Fig. 8D). Together, these results indicate that Abl directly induces the expression of cellular Egr-1, which in turn mediates the stimulation of collagen gene expression.
Bleomycin-induced fibrosis is associated with elevated phospho-cAbl expression in situ
Because little is known regarding Abl activation in the context of fibrosis, we examined the levels of phosphorylated c-Abl in a mouse model of scleroderma. Subcutaneous injections of bleomycin in BALB/c mice resulted in the development of scleroderma-like skin changes with dense fibrosis of the dermis. We have shown previously that TGF-ß plays a pivotal role in the pathological changes in this model of scleroderma (Takagawa et al., 2003). Dermal fibrosis was accompanied by a ~2-fold increase in the levels of phosphorylated c-Abl in lesional tissue compared to PBS-injected control mice (Fig. 9). Immunostaining was principally localized in fibroblasts in the deeper dermis, and was specific, as substitution of the primary antibody with rabbit IgG resulted in the absence of brown staining. To show a correlation between the expression of phospho-cAbl and Egr-1 in this model of fibrosis, simultaneous immunostaining for Egr-1 was performed. In contrast to PBS-treated mice that had little or no Egr-1 was detected in the dermis, a significant proportion of fibroblastic cells in lesional dermis showed strong positive Egr-1 immunostaining localized within the nucleus. The topological distribution of Egr-1-positive fibroblasts in the lesional dermis paralleled the distribution of phospho-cAbl.
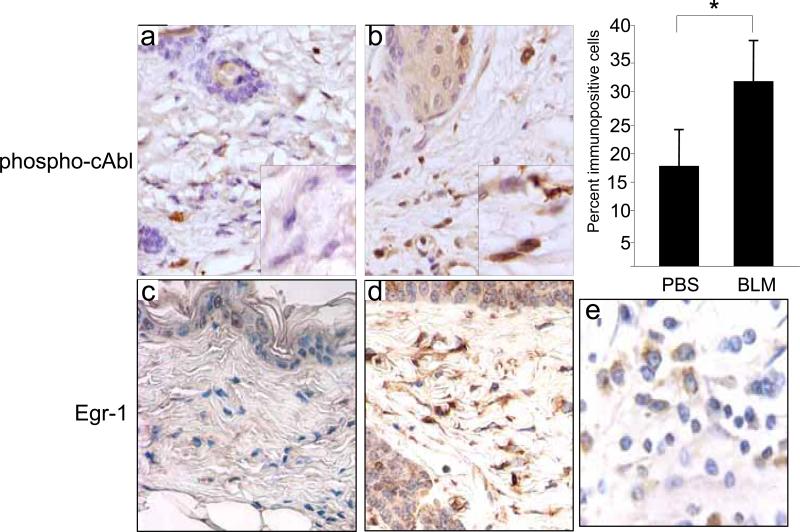
6-weeks old BALB/C mice received daily s.c. injections of bleomycin (BLM) or PBS. At day 28 after injections were started, lesional skin was examined by immunohistochemistry using antibodies against phospho-cAbl or Egr-1. Representative photomicrographs are shown. Original magnification × 400 (upper panels) or × 1000 (lower panels). The proportion of fibroblasts in the lesional dermis immunopositive for phospho-cAbl was determined. Results represents the means ± S.D. from six separate fields from at least three mice/group. Human breast carcinoma tissue was used as positive control.
DISCUSSION
Fibroblasts activation by TGF-ß, a key event in the process of fibrogenesis, involves canonical Smad as well as non-Smad signaling pathways. Previous studies implicated the oncogene c-Abl as a mediator of fibroblast activation induced by TGF-ß, but the mechanisms underlying this important function are not known. Here we show that a constitutively active Abl mutant was sufficient to induce the stimulation of both collagen and Egr-1 gene expression, replicating the response elicited by TGF-ß in normal fibroblasts. Stimulation induced by either TGF-ß or by Bcr-Abl was blocked by imatinib, indicating the critical role of Abl kinase activity, and the TGF-ß response was significantly compromised in MEFs lacking both Abl and Arg. The induction of Egr-1 by Bcr-Abl was mediated via a Smad2/3-independent MEK1-ERK1/2 signaling cascade. In turn, Egr-1 was found to be indispensable for Bcr-Abl-induced stimulation of collagen gene expression. Together, the results therefore firmly establish an essential function for Abl upstream of Egr-1 in the TGF-ß signal transduction pathway, and implicate this tyrosine kinase in the stimulation of collagen gene expression through Egr-1.
Cellular Abl regulates important processes including actin reorganization, differentiaition and apoptosis following DNA damage (Kharas M, Fruman D 2005). The biological activity of c-Abl is normally tightly controlled, and aberrant Abl kinase activity is directly responsible for the leukemic transformation of myeloid cells in CML. Genotoxic and oxidative stress, as well as PDGF, induce c-Abl activation (Schubert et al., 2007; Lewis et al., 1996; Van Etten, 1999). The present results indicate that in normal skin fibroblasts TGF-ß stimulated the kinase activity of c-Abl, and blockade of c-Abl prevented cellular responses induced by TGF-ß independent of Smad activation. These findings are in good agreement with recent reports in NIH3T3 and IMR90 cells (Daniels et al., 2004). The induction of c-Abl kinase activity in response to TGF-ß occurred in mesenchymal cells but not in epithelial cells, and was shown to involve the PI3 kinase and p21 activated kinase 2 PAK2 (Wilkes et al., 2006). Furthermore, in contrast to canonical Smad-dependent TGF-ß responses, induction of c-Abl was not dependent on internalization of the TGF-ß receptor. While the mediators upstream of c-Abl activation have been well characterized, those downstream of c-Abl in the context of fibrogenesis are not well understood.
In agreement with previous studies, we found that pretreatment of fibroblasts with imatinib did not abrogate activation of R-Smads induced by TGF-ß. In contrast, the stimulation of Egr-1 was prevented by imatinib, thereby locating Egr-1 downstream of activated c-Abl. The early-immediate Egr-1 gene product is a zinc finger transcription factor readily induced by a variety of environmental stimuli. Target genes that are directly regulated by Egr-1 include PDGF, TGF-ß, PTEN and collagen (Svaren et al., 2000; Virolle et al., 2001, Chen et al., 2006). Rapid and generally transient Egr-1 induction plays an important role in orchestrating acute tissue responses to injury (Gashler and Sukhatme, 1995, Khachigian, 2006). We have shown previously in skin and lung fibroblasts that Egr-1 was also induced by TGF-ß (Chen et al., JBC, Bhattacharyya et al., 2008). Importantly, Egr-1 in these cells was sufficient and necessary for stimulation of collagen gene expression. The mechanism for the induction of Egr-1 by TGF-ß has been only partially elucidated. We showed that TGF-ß stimulated the transcription of the Egr-1 gene, and the response was insensitive to SB431542, a selective inhibitor of ALK5-mediated Smad2/3 activation (Mori et al., 2004). Rather, TGF-ß was shown to activate the MEK1-ERK1/2 signaling cascade converging on the ternary transcription factor Elk-1 and multiple SRE elements in the Egr-1 promoter (Buchwater, 2004). The present results further delineate the mechanism of Egr-1 induction by demonstrating that in the absence of exogenously added TGF-ß, Abl was sufficient to induce Egr-1 gene expression, DNA binding and transcriptional activity. This is not the first indication that Abl regulates the expression of Egr-1. In a previous study, DNA microarray analysis of ectopic c-Abl expression in 293 epithelial cells identified Egr-1 as a novel target (Stuart et al., 2005). Stimulation of Egr-1 was attenuated by a selective inhibitor of MEK/ERK signaling. Moreover, c-Abl has been shown to directly activate MEK kinase 1 in response to DNA damage (Kharbanda et al., 2000). The essential role of the Abl-Egr-1 pathway in stimulation of collagen gene expression was confirmed with complementary experiments in Egr-1−/− fibroblasts where Bcr-Abl failed to stimulate COL1A1 mRNA expression.
In addition to leukemogenesis, Abl family kinases play important roles in the regulation of diverse cellular processes including cell proliferation, survival, differentiation and migration (Koleske et al., 2004 which one?). Accumulating evidence implicates c-Abl in the regulation of connective tissues turnover during both physiological tissue remodeling and in pathological fibrosis. The activity of c-Abl is induced not only by DNA damaging agents, but also by stimuli generated during tissue injury and repair such as PDGF as well as TGF-ß, both of which play important roles in fibrogenesis (Bonner JC, 2004, Leask A, 2008). c-Abl has been shown to directly induce the synthesis of fibronectin, collagen and connective tissue growth factor (Teckchandani et al., 2001; Pannu et al., 2008), enhance fibroblast migration and adhesion (Rajkumar V 2006; Koleske et al., 1998, Renshawa et al., 2000; Lewis et al., 1998), stimulate epithelial mesenchymal transition (He, 2006) and mediate the profibrotic activation of hepatic stellate cells in response to acetaldehyde (Ceni et al., 2006). Furthermore, pathological tissue fibrosis is associated with in situ activation of c-Abl, as seen in the lesional dermis from mice with bleomycin-induced scleroderma and from patients with early active scleroderma (Watary A&R Abstract). Inhibition of c-Abl activity might therefore explain the anti-fibrotic effects of imatinib demonstrated in mouse models of renal, lung and skin fibrosis (Wang et al., 2005; Daniels et al., 2004; Distler et al., 2007).
Whereas Egr-1 is normally low to undetectable in most tissues, its expression is up-regulated in lesional skin and lung tissues from mice with bleomycin-induced scleroderma (Bhattacharyya et al., 2008). Furthermore, lesional skin and lungs, as well as explanted dermal fibroblasts, from patients with active scleroderma show elevated Egr-1 expression (Bhattacharyya et al., 2008). The present results show that bleomycin-induced scleroderma was accompanied by elevated Abl phosphorylation in lesional dermal fibroblasts that correlated with elevated Egr-2 expression. Therefore, sustained in situ c-Abl activation might be a mechanism for inducing local Egr-1 expression or activity that contributes to the fibrotic process.
In summary, the present results demonstrate that the oncogene c-Abl is activated by TGF-ß in fibroblasts, and is in turn is both necessary and sufficient for induction of Egr-1. Whereas TGF-ß-mediated induction of Egr-1 is important for the stimulation of collagen gene expression, and was shown previously to involve a canonical ERK1/2 signal transduction mechanism associated with stress regulation of Egr-1, the events upstream of MEK1/ERK1/2 in this pathway were hitherto unknown. Here we show that c-Abl is upstream of Egr-1 in the stimulation of collagen gene expression by TGF-ß. Activation of the c-Abl-Egr-1 pathway, presumably via PI3 kinase and PAK2, thus represents an important novel mechanism for mediating TGF-ß responses in fibroblasts. The present findings also indicate that diverse stimuli eliciting the induction of Egr-1, including stress and TGF-ß, converge on c-Abl. Importantly, c-Abl-Egr-1 mediated stimulation of collagen gene expression appears to be independent of the Smad pathway, and occurs only in mesenchymal cells. Unchecked TGF-ß activity in pathological tissue repair might be associated with aberrantly sustained c-Abl activation and ERK1/2-dependent up-regulation and persistent expression of Egr-1 in fibroblasts. Sustained expression of Egr-1 in target tissues in turn would induce or perpetuate fibrotic responses. Precise delineation of the components of this signaling mechanism are likely to yield further insights into the pathogenesis of fibrosis, and lead to the identification of novel targets for therapy.
Acknowledgements
We are grateful to Anthony Koleske (Yale University, New Haven, CT) and Pamela Woodring (The Salk Institute, La Jolla, CA) for Abl-null/Arg-null and Abl-reconstituted mouse embryonic fibroblasts, and Leonidas Platanias (Northwestern University Feinberg School of Medicine) for Bcr-Abl plasmids and helpful discussions. Supported by grants from the National Institutes of Health (AR-42309).
References
- Jimenez SA, Derk CT. Following the molecular pathways toward an understanding of the pathogenesis of systemic sclerosis. Ann Intern Med. 2004;140:37–45. [Abstract] [Google Scholar]
- Varga J, Abraham D. Systemic sclerosis: a prototypic multisystem fibrotic disorder. J Clin Invest. 2007;117:557–567. [Europe PMC free article] [Abstract] [Google Scholar]
- Varga JA, Trojanowska M. Fibrosis in systemic sclerosis. Rheum Dis Clin North Am. 2008;34(1):1, 115–43. [Europe PMC free article] [Abstract] [Google Scholar]
- Massagué J, Seoane J, Wotton D. Smad transcription factors. Genes Dev. 2005;19:2783–2810. [Abstract] [Google Scholar]
- Moustakas A, Heldin CH. Non-Javelaud D, Mauviel. A Crosstalk mechanisms between the mitogen-activated protein kinase pathways and Smad signaling downstream of TGF-beta: implications for carcinogenesis. Oncogene. 2005 Aug 29;24(37):5742–50. [Abstract] [Google Scholar]
- Pannu J, Trojanowska M. Recent advances in fibroblast signaling and biology in scleroderma. Curr Opin Rheumatol. 2004 Nov;16(6):739–45. [Abstract] [Google Scholar]
- Javelaud D, Mauviel A Crosstalk mechanisms between the mitogen-activated protein kinase pathways and Smad signaling downstream of TGF-beta: implications for carcinogenesis. Oncogene. 2005;24(37):5742–50. [Abstract] [Google Scholar]
- Wenner CE, Yan S. Biphasic role of TGF-beta1 in signal transduction and crosstalk. J Cell Physiol. 2003 Jul;196(1):42–50. [Abstract] [Google Scholar]
- Miyazono K. TGF-beta receptors and signal transduction. Int J Hematol. 1997;65(2):97–104. [Abstract] [Google Scholar]
- Bhattacharyya S, Chen S-J, Wu M, Warner-Blankenship M, Ning H, Lakos G, Mori Y, Chang E, Nihijima C, Takehara K, Feghali-Bostwick C, Varga J. Smad-independent TGF-β regulation of transcription factor Egr-1 and sustained expression in fibrosis: implications for scleroderma. The American Journal of Pathology. 2008;173(4) [Europe PMC free article] [Abstract] [Google Scholar]
- Kharbanda S, Pandey P, Jin S, Inoue S, Bharti A, Yuan Z-M, Weichselbaum R, Weaver D, Kufe D. Functional interaction of DNA-PK and c-Abl in response to DNA damage. Nature. 1997;386:732–735. [Abstract] [Google Scholar]
- Kharbanda S, Pandey P, Ren R, Feller S, Mayer B, Zon L, Kufe D. c-Abl activation regulates induction of the SEK1/stress activated protein kinase pathway in the cellular response to 1- -D-arabinofuranosylcytosine. J. Biol. Chem. 1995;270:30278–30281. [Abstract] [Google Scholar]
- Kharbanda S, Ren R, Pandey P, Shafman TD, Feller SM, Weichselbaum RR, Kufe DW. Activation of the c-Abl tyrosine kinase in the stress response to DNA-damaging agents. Nature. 1995;376:785–788. [Abstract] [Google Scholar]
- Groffen J, Stephenson JR, Heisterkamp N, de Klein A, Bartram CR, Grosveld G. Philadelphia chromosomal breakpoints are clustered within a limited region, bcr, on chromosome. Cell. 1984;36(1):93–9. [Abstract] [Google Scholar]
- Rowley JD. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973 Jun 1;243(5405):290–3. [Abstract] [Google Scholar]
- Lugo TG, Pendergast AM, Muller AJ, Witte ON. Tyrosine kinase activity and transformation potency of bcr-abl oncogene products. Science. 1990 Mar 2;247(4946):1079–82. [Abstract] [Google Scholar]
- Druker BJ, Tamura S, Buchdunger E, Ohno S, Segal GM, Fanning S, et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med. 1996;2:561–566. [Abstract] [Google Scholar]
- La Rosée P, Johnson K, O'Dwyer ME, Druker BJ. In vitro studies of the combination of imatinib mesylate (Gleevec) and arsenic trioxide (Trisenox) in chronic myelogenous leukemia. Exp Hematol. 2002 Jul;30(7):729–37. [Abstract] [Google Scholar]
- Goldman JM, Melo JV. Chronic myeloid leukemia--advances in biology and new approaches to treatment. N Engl J Med. 2003 Oct 9;349(15):1451–64. [Abstract] [Google Scholar]
- Deininger MW, Goldman JM, Lydon N, Melo JV. The tyrosine kinase inhibitor CGP57148B selectively inhibits the growth of BCR-ABL-positive cells. Blood. 1997;90:3691–3698. [Abstract] [Google Scholar]
- Wilkes MC, Leof EB. Transforming growth factor beta activation of c-Abl is independent of receptor internalization and regulated by phosphatidylinositol 3-kinase and PAK2 in mesenchymal cultures. J Biol Chem. 2006 Sep 22;281(38):27846–54. [Abstract] [Google Scholar]
- Daniels CE, Wilkes MC, Edens M, Kottom TJ, Murphy SJ, Limper AH, Leof EB. Imatinib mesylate inhibits the profibrogenic activity of TGF-beta and prevents bleomycin-mediated lung fibrosis. J Clin Invest. 2004 Nov;114(9):1308–16. [Europe PMC free article] [Abstract] [Google Scholar]
- Distler JH, Jüngel A, Huber LC, Schulze-Horsel U, Zwerina J, Gay RE, Michel BA, Hauser T, Schett G, Gay S, Distler O. Imatinib mesylate reduces production of extracellular matrix and prevents development of experimental dermal fibrosis. Arthritis Rheum. 2007 Jan;56(1):311–2. [Abstract] [Google Scholar]
- Wang S, Wilkes MC, Leof EB, Hirschberg R. Imatinib mesylate blocks a non-Smad TGF-beta pathway and reduces renal fibrogenesis in vivo. FASEB J. 2005;19(1):1–11. [Abstract] [Google Scholar]
- Rosenbloom J, Jiménez SA. Molecular ablation of transforming growth factor beta signaling pathways by tyrosine kinase inhibition: the coming of a promising new era in the treatment of tissue fibrosis. Arthritis Rheum. 2008;58(8):2219–24. [Europe PMC free article] [Abstract] [Google Scholar]
- Mori Y, Ishida W, Bhattacharyya S, Li Y, Platanias LC, Varga J. Selective inhibition of activin receptor-like kinase 5 signaling blocks profibrotic transforming growth factor beta responses in skin fibroblasts. Arthritis Rheum. 2004;50:4008–4021. [Abstract] [Google Scholar]
- Woodring PJ, Litwack ED, O'Leary DD, Lucero GR, Wang JY, Hunter T. Modulation of the Factin cytoskeleton by c-Abl tyrosine kinase in cell spreading and neurite extension. J. Cell Biol. 2002;156:879–892. [Europe PMC free article] [Abstract] [Google Scholar]
- Chen SJ, Ning H, Ishida W, Sodin-Semrl S, Takagawa S, Mori Y, Varga J. The early-immediate gene EGR-1 is induced by transforming growth factor-beta and mediates stimulation of collagen gene expression. J Biol Chem. 2006;281:21183–21197. [Abstract] [Google Scholar]
- Ihn H, LeRoy EC, Trojanowska M. Oncostatin M stimulates transcription of the human 2 (I) collagen gene via the Sp1/Sp3-binding site. J Biol Chem. 1997;272:24666–24672. [Abstract] [Google Scholar]
- Sukhatme VP, Cao XM, Chang LC, Tsai-Morris CH, Stamenkovich D, Ferreira PC, Cohen DR, Edwards SA, Shows TB, Curran T. A zinc finger-encoding gene coregulated with c-fos during growth and differentiation, and after cellular depolarization. Cell. 1988;53:37–43. [Abstract] [Google Scholar]
- Chen SJ, Yuan W, Mori Y, Levenson A, Trojanowska M, Varga J. Stimulation of Type I Collagen Transcription in Human Skin Fibroblasts by TGF-β: Involvement of Smad 3. J of Invest Dermatol. 1999;112:49–57. [Abstract] [Google Scholar]
- Mori Y, Chen SJ, Varga J. Modulation of endogenous Smad expression in normal skin fibroblasts by transforming growth factor-beta. Exp Cell Res. 2000;258:374–383. [Abstract] [Google Scholar]
- Mori Y, Hinchcliff M, Wu M, Warner-Blankenship M, M Lyons K, Varga J. Connective tissue growth factor/CCN2-null mouse embryonic fibroblasts retain intact transforming growth factor-beta responsiveness. Exp Cell Res. 2008;314(5):1094–104. 10. [Europe PMC free article] [Abstract] [Google Scholar]
- Lakos G, Takagawa S, Chen SJ, Ferreira AM, Han G, Masuda K, Wang XJ, DiPietro LA, Varga J. Targeted disruption of TGF-beta/Smad3 signaling modulates skin fibrosis in a mouse model of scleroderma. Am J Pathol. 2004;165:203–217. [Europe PMC free article] [Abstract] [Google Scholar]
- Takagawa S, Lakos G, Mori Y, Yamamoto T, Nishioka K, Varga J. Sustained activation of fibroblast transforming growth factor-beta/Smad signaling in a murine model of scleroderma. J Invest Dermatol. 2003;121:41–50. [Abstract] [Google Scholar]
- Pannu J, Asano Y, Nakerakanti S, Smith E, Jablonska S, Blaszczyk M, ten Dijke P, Trojanowska M. Smad1 pathway is activated in systemic sclerosis fibroblasts and is targeted by imatinib mesylate. Arthritis Rheum. 2008;58(8):2528–37. [Abstract] [Google Scholar]
- Goumans MJ, Valdimarsdottir G, Itoh S, Rosendahl A, Sideras P, ten Dijke P. Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors. EMBO J. 2002 Apr 2;21(7):1743–53. [Europe PMC free article] [Abstract] [Google Scholar]
- Takahashi T, Abe H, Arai H, Matsubara T, Nagai K, Matsuura M, Iehara N, Yokode M, Nishikawa S, Kita T, Doi T. Activation of STAT3/Smad1 is a key signaling pathway for progression to glomerulosclerosis in experimental glomerulonephritis. J Biol Chem. 2005;280(8):7100–6. [Abstract] [Google Scholar]
- Pannu J, Nakerakanti S, Smith E, ten Dijke P, Trojanowska M. Transforming growth factor-beta receptor type I-dependent fibrogenic gene program is mediated via activation of Smad1 and ERK1/2 pathways. J Biol Chem. 2007;282(14):10405–13. [Abstract] [Google Scholar]
- Raitano AB, Halpern JR, Hambuch TM, Sawyers CL. The Bcr-Abl leukemia oncogene activates Jun kinase and requires Jun for transformation. Proc. Natl. Acad. Sci. U.S.A. 1995;92:11746–1175. [Europe PMC free article] [Abstract] [Google Scholar]
- Che W, Abe J, Yoshizumi M, Huang Q, Glassman M, Ohta S, Melaragno MG, Poppa V, Yan C, Lerner-Marmarosh N, Zhang C, Wu Y, Arlinghaus R, Berk BC. p160 Bcr mediates Platelet-Derived Growth Factor Activation of Extracellular Signal-Regulated Kinase in Vascular Smooth Muscle Cells. Circulation. 2001;104:1399. [Abstract] [Google Scholar]
- Sánchez-Arévalo Lobo VJ, Aceves Luquero CI, Alvarez-Vallina L, Tipping AJ, Viniegra JG, Hernández Losa J. Modulation of the p38 MAPK (mitogen-activated protein kinase) pathway through Bcr/Abl: implications in the cellular response to Ara-C. Biochem. J. 2005;387:231–238. [Europe PMC free article] [Abstract] [Google Scholar]
- Skorski T, Bellacosa A, Nieborowska-Skorska M, Majewski M, Martinez R, Choi JK, Trotta R, Wlodarski P, Perrotti D, Chan TO, et al. Transformation of hematopoietic cells by BCR/ABL requires activation of a PI-3k/Akt-dependent pathway. EMBO J. 1997;16:6151–6161. [Europe PMC free article] [Abstract] [Google Scholar]
- Danial NN, Rothman P. JAK-STAT signaling activated by Abl oncogenes. Oncogene. 2000;19:2523–2531. [Abstract] [Google Scholar]
- Kharas MG, Fruman DA. ABL oncogenes and phosphoinositide 3-kinase: mechanism of activation and downstream effectors. Cancer Res. 2005;65:2047–53. [Abstract] [Google Scholar]
- Schubert C, Schalk-Hihi C, Struble GT, Ma HC, Petrounia IP, Brandt B, Deckman IC, Patch RJ, Player MR, Spurlino JC, Springer BA. J Biol Chem. 2007;282(6):4094–101. [Abstract] [Google Scholar]
- Lewis JM, Baskaran R, Taagepera S, Schwartz MA, Wang JY. Integrin regulation of c-Abl tyrosine kinase activity and cytoplasmic-nuclear transport. Proc Natl Acad Sci U S A. 1996;93:15174–15179. [Europe PMC free article] [Abstract] [Google Scholar]
- Van Etten RA. Cycling, stressed-out and nervous: cellular functions of c-Abl. Trends Cell Biol. 1999;9:179–186. [Abstract] [Google Scholar]
- Svaren J, Ehrig T, Abdulkadir SA, Ehrengruber MU, Watson MA, Milbrandt J. EGR1 Target Genes in Prostate Carcinoma Cells Identified by Microarray Analysis. J Biol Chem. 2000;275(49):38524–31. [Abstract] [Google Scholar]
- Virolle T, Adamson ED, Baron V, Birle D, Mercola D, Mustelin T, Belle ID. The Egr-1 transcription factor directly activates PTEN during irradiation-induced signaling. Nature Cell Biology. 2001;3:1124–1128. [Abstract] [Google Scholar]
- Gashler A, Sukhatme VP. Early growth response protein 1 (Egr-1): prototype of a zinc-finger family of transcription factors. Prog Nucleic Acid Res Mol Biol. 1995;50:191–224. [Abstract] [Google Scholar]
- Khachigian LM. Early growth response-1 in cardiovascular pathobiology. Circ Res. 2006;98:186–191. [Abstract] [Google Scholar]
- Buchwalter G, Gross C, Wasylyk B. Ets ternary complex transcription factors. Gene. 2004;324:1–14. [Abstract] [Google Scholar]
- Stuart JR, Kawai H, Tsai KK, Chuang EY, Yuan ZM. c-Abl regulates early growth response protein (EGR1) in response to oxidative stress. Oncogene. 2005;24:8085–8092. [Abstract] [Google Scholar]
- Bonner JC. Cytokine Growth factor Rev. 2004;4::255–73. [Abstract] [Google Scholar]
- Leask A. Targeting the TGFβ, endothelin-1 and CCN2 axis to combat fibrosis in scleroderma. Cellular Signalling. 2008;20:1409–1414. [Abstract] [Google Scholar]
- Teckchandani AM, Feshchenko EA, Tsygankov AY. c-Cbl facilitates fibronectin matrix production by v-Abl-transformed NIH3T3 cells via activation of small GTPases. Oncogene. 2001;20(14):1739–55. [Abstract] [Google Scholar]
- Rajkumar VS, Shiwen X, Bostrom M, Leoni P, Muddle J, Ivarsson M, Gerdin B, Denton CP, Bou-Gharios G, Black CM, Abraham DJ. Platelet-Derived Growth Factor-β Receptor Activation Is Essential for Fibroblast and Pericyte Recruitment during Cutaneous Wound Healing. American Journal of Pathology. 2006;169:2254–2265. [Europe PMC free article] [Abstract] [Google Scholar]
- Renshawa MW, Lewisa JM, Schwartz MA. The c-Abl tyrosine kinase contributes to the transient activation of MAP kinase in cells plated on fibronectin. 2000;19:3216–3219. [Abstract] [Google Scholar]
- Lewis JM, Schwartz MA. Integrins Regulate the Association and Phosphorylation of Paxillin by c-Abl. J Biol Chem. 1998 Jun 5;273(23):14225–14230. [Abstract] [Google Scholar]
- He X. Unwinding a Path to Nuclear β-Catenin. Cell. 2006;127:40–42. [Abstract] [Google Scholar]
- Ceni E, Crabb DW, Foschi M, Mello T, Taricchi M, Patussi V, Moraldil, Moretti R, Milani S, Surrenti C, Galli A. Acetaldehyde Inhibits PPAR-g via H2O2-Mediated c-Abl Activation in Human Hepatic Stellate Cells. Gastroenterology. 2006;131:1235–1252. [Abstract] [Google Scholar]
Full text links
Read article at publisher's site: https://doi.org/10.1038/onc.2008.479
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc4006376?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1038/onc.2008.479
Article citations
Early growth response-1: Key mediators of cell death and novel targets for cardiovascular disease therapy.
Front Cardiovasc Med, 10:1162662, 28 Mar 2023
Cited by: 7 articles | PMID: 37057102 | PMCID: PMC10086247
Review Free full text in Europe PMC
Cereblon contributes to the development of pulmonary fibrosis via inactivation of adenosine monophosphate-activated protein kinase α1.
Exp Mol Med, 53(5):885-893, 17 May 2021
Cited by: 2 articles | PMID: 34002012 | PMCID: PMC8178361
Specific targeting of PDGFRβ in the stroma inhibits growth and angiogenesis in tumors with high PDGF-BB expression.
Theranostics, 10(3):1122-1135, 01 Jan 2020
Cited by: 19 articles | PMID: 31938055 | PMCID: PMC6956815
Inhibition of integrin αVβ6 changes fibril thickness of stromal collagen in experimental carcinomas.
Cell Commun Signal, 16(1):36, 02 Jul 2018
Cited by: 9 articles | PMID: 29966518 | PMCID: PMC6027735
Abrogation of transforming growth factor-β-induced tissue fibrosis in TBRIcaCol1a2Cre transgenic mice by the second generation tyrosine kinase inhibitor SKI-606 (Bosutinib).
PLoS One, 13(5):e0196559, 02 May 2018
Cited by: 7 articles | PMID: 29718973 | PMCID: PMC5931634
Go to all (52) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Imatinib mesylate blocks a non-Smad TGF-beta pathway and reduces renal fibrogenesis in vivo.
FASEB J, 19(1):1-11, 01 Jan 2005
Cited by: 203 articles | PMID: 15629889
Imatinib mesylate causes genome-wide transcriptional changes in systemic sclerosis fibroblasts in vitro.
Clin Exp Rheumatol, 30(2 suppl 71):S86-96, 01 Mar 2012
Cited by: 7 articles | PMID: 22691216 | PMCID: PMC3860597
Imatinib mesylate inhibits the profibrogenic activity of TGF-beta and prevents bleomycin-mediated lung fibrosis.
J Clin Invest, 114(9):1308-1316, 01 Nov 2004
Cited by: 398 articles | PMID: 15520863 | PMCID: PMC524221
Egr-1: new conductor for the tissue repair orchestra directs harmony (regeneration) or cacophony (fibrosis).
J Pathol, 229(2):286-297, 03 Dec 2012
Cited by: 95 articles | PMID: 23132749 | PMCID: PMC3965177
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NIAMS NIH HHS (4)
Grant ID: R56 AR042309
Grant ID: R01 AR042309-08
Grant ID: R01 AR042309
Grant ID: AR-42309
NIGMS NIH HHS (1)
Grant ID: R37 GM055816




