Abstract
Free full text

Autophagy gone awry in neurodegenerative diseases
Abstract
Autophagy is essential for neuronal homeostasis and its dysfunction has been directly linked to a growing number of neurodegenerative disorders. The reasons behind autophagic failure in degenerating neurons can be very diverse because of the different steps required for autophagy and the characterization of the molecular players involved in each of them. Understanding the step(s) affected in the autophagic process in each disorder could explain differences in the course of these pathologies and will be essential to develop targeted therapeutic approaches for each disease based on modulation of autophagy. In this review we present examples of different types of autophagic dysfunction described in common neurodegenerative disorders, and discuss the prospect of exploring some of the recently identified autophagic variants and the interactions among autophagic and non-autophagic proteolytic systems as possible future therapeutic targets.
Although autophagy – the degradation of cytosolic components in lysosomes – has been known for more than 5 decades, its importance in the central nervous system, and in particular, in neurons, has only recently been demonstrated1–4. The wealth of information explosion in the autophagic field3 is leading to a better understanding of classic neuronal disorders, in particular, those dealing with protein mishandling and problems in cellular quality control.
As the field advances, some chapters in our understanding of autophagy are finally reaching closure, such as the initial controversy of whether or not autophagy even occurred in neurons—neuronal accumulation of autophagosomes has been described in multiple brain disorders (reviewed in 1,5,6), and it is clear that neurons have the machinery and molecular components required for carrying out autophagy. Neurodegeneration and protein inclusions have been described in mouse models incompetent to perform autophagy in neuronal tissues7,8, making a strong case for a critical role of autophagy in maintenance of neuronal homeostasis and protein quality control in neurons. More recent studies using similar genetic approaches have now confirmed an essential function of autophagy in neuronal development and remodeling9–12.
In contrast to other topics, such as the nature of the autophagic defect in different neurodegenerative disorders, are now making headlines, and numerous studies and resources are dedicated to their detailed dissection. This review will focus on the different types of autophagic dysfunction in neurodegeneration and the importance of identifying the autophagic step(s) altered in each particular disorder for therapeutic purposes.
Autophagic pathways in neurons
Cellular quality control through autophagy is particularly relevant in neurons, where the total content of altered proteins and damaged organelles cannot be reduced by redistribution to daughter cells via cell division. The neuronal surveillance mechanisms need to identify these malfunctioning structures and assure their autophagic degradation before their intracellular buildup give rises to neurotoxicity5,6. Delivery of autophagic subcellular components to the damaged structures has to accommodate unique neuronal architecture where the cytoplasm can extend to long distances through the many projections from the cellular body, and accommodate the dynamic traffic to and from polarized neuronal projections. Besides neuronal homeostasis, autophagy is also utilized for the continuous remodeling of the neuronal terminals that is required to support neuronal plasticity9–12. Based on these prior observations, it would seem unsurprising that alterations in the autophagic system would be intimately linked to different neuronal diseases where the integrity of cellular machineries may be compromised.
The first clue of altered autophagy in different neurodegenerative settings comes from abnormal amount of autophagosomes in the affected neurons13–15. However expansion of this autophagic compartment could come from any impairment in the multiple steps leading up to autophagy, and only provides information on macroautophagy, one of the subtypes of autophagy. In fact, the term autophagy refers to the degradation of cytosolic components in lysosomes independently of the mechanism by how the degraded cargo is delivered to the lysosomal compartment. In most mammalian cells, delivery occurs by one of three ways that distinguishes the subtypes of autophagy: macroautophagy, microautophagy and chaperone-mediated autophagy. The characteristics, regulation and main molecular components of these autophagic pathways have been reviewed in detail elsewhere1–3. Briefly, macroautophagy and microautophagy involve the direct sequestration of whole areas of the cytosol by invaginations at the lysosomal membrane (in the case of microautophagy), or by a membrane that seals to form a double membrane vesicle or autophagosome (in macroautophagy). Microautophagic vesicles at the lysosomal membrane “pinch off” into the lysosomal lumen and cargo is degraded by the lysosomal hydrolases upon digestion of the vesicles’ limiting membrane16. In the case of macroautophagy, fusion between autophagosomes and lysosomes mediates the delivery of the autophagic cargo into the lysosomal lumen1,2. In the third common type of autophagy, chaperone-mediated autophagy (CMA), cargo is not sequestered but is instead selectively recognized by a complex of cytosolic chaperones which mediates its delivery to a receptor/translocation unit at the lysosomal membrane17,18. Cargo gains access to the lysosomal lumen through the translocation complex, thus limiting CMA to soluble proteins that can undergo complete unfolding. All three autophagic pathways usually coexist in the same cell and alterations in both macroautophagy and CMA have recently been associated to specific neurodegenerative disorders17.
The “when” and “where” of the macroautophagic halt in neurodegeneration
The detailed molecular characterization of macroautophagy and the development of probes to track and methods modulate this process have been instrumental in our current understanding of the physiological functions of this pathway3. These advances have facilitated the identification of autophagic digressions in numerous human disorders (a complete description of the pathophysiology of macroautophagy can be found in1,19,20), including a growing number of neurological disorders such as Alzheimer’s Disease (AD), Parkinson’s Disease (PD), Huntington’s Disease (HD) and Amyotrophic Lateral Sclerosis (ALS)13,14,21–26. Different findings in recent years have helped to consolidate a connection between macroautophagy and neurodegenerative disorders and propelled the current interest in this topic. For example, aggregates formed by a number of pathogenic proteins have proven to be amenable for degradation by macroautophagy22,27. In addition, pharmacological upregulation of macroautophagy has been shown effective in reducing neuronal aggregates and slowing down the progression of neurological symptoms in flies and mouse models of HD28. These findings have generated a justifiable level of optimism and have led to an idea that upregulation of macroautophagy might represent a plausible therapeutic intervention in these disorders. However, recent studies have put a note of caution on the applicability of macroautophagy upregulation as a generalized treatment. For example, inhibition, rather than stimulation, of macroautophagy increases neuronal survival in some pathological conditions displaying high content of neuronal autophagic vacuoles such as ischemic stroke15,29–31. How can blocking macroautophagy be beneficial when it is the only pathway that can eliminate the pathogenic proteins once they form aggregates? The main reason is that an increase in autophagosomes is not always indicative of “more” autophagy – at least not more degradation via autophagy. Cells could display a higher number of autophagosomes when macroautophagy is upregulated (more formation of autophagosomes) but also when clearance of autophagosomes is impaired (less fusion/degradation of autophagosomes by lysosomes)21,32. Understanding the nature of the changes in the autophagic pathway leading to autophagic malfunction has now become a priority.
Because autophagic degradation involves multiple steps, we discuss the consequences of alterations in each of the different steps of macroautophagy in the context of different neurodegenerative disorders (Fig. 1).
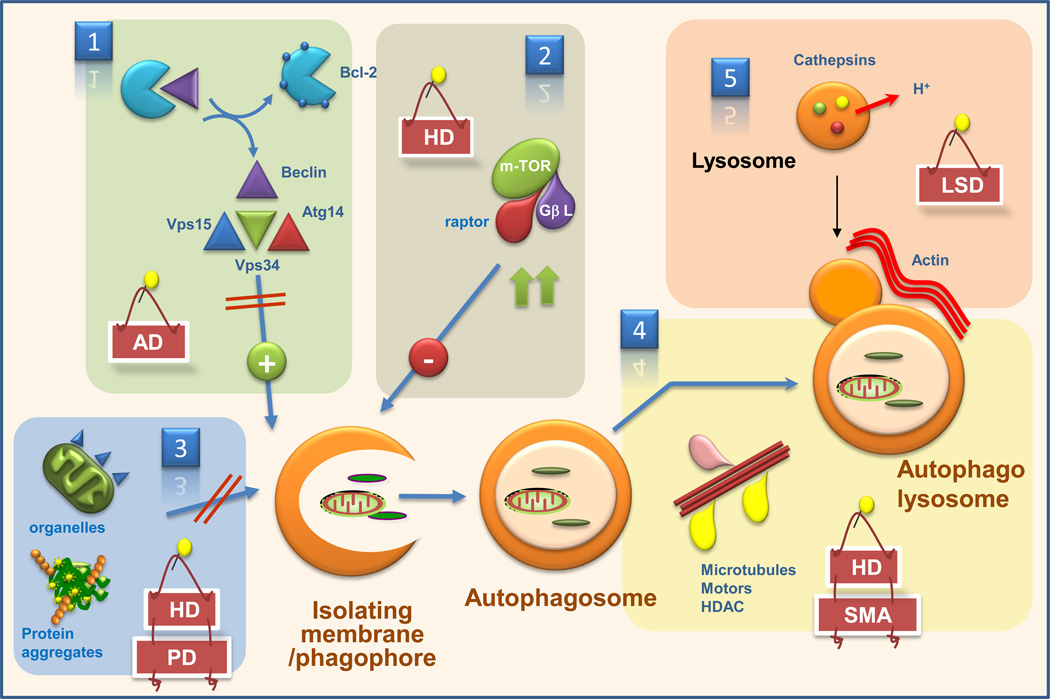
The possible defects that could be behind macroautophagy malfunctioning in different neurodegenerative disorders are depicted: 1. Reduced autophagy induction; 2. Enhanced autophagy repression; 3. Altered cargo recognition; 4. Inefficient autophagosome/lysosome fusion, and 5. Inefficient degradation of the autophagic cargo in lysosomes. Examples of neurodegenerative diseases for which alterations in each autophagic step have been described are shown. Atg: autophagy-related proteins; Vps: vesicular protein secretion protein; HDAC: histone deacetylase; AD: Alzheimer’s disease; HD: Huntington’s disease; PD: Parkinson’s diease; LSD: lysosomal storage disorders; SMA: spinal muscular atrophy.
Induction of autophagy
Formation of the isolation membrane/phagophore of the autophagosome is the earliest event in macroautophagy. Discrete regions in the endoplasmic reticulum (the omegasomes) may serve as the nucleation site for the formation of autophagosomes in mammalian cells33 where components required for the formation of the isolation membrane (Atg or autophagy-related proteins) are recruited. For the most part, Atgs that participate in the formation of the isolation membrane – the Atg5-12-16 complex, the LC3-phosphatidyl-ethanol-amine protein-to-lipid conjugation complex and their corresponding conjugating enzymes34 – do not seem to exist in limiting amounts inside cells. Although knock-outs and knock-downs of components such as Atg5 or Atg7 have been extensively used to suppress macroautophagy7,8, pathological conditions arising by depletion of these factors in mammals have yet to be identified. However, decreased level of effector Atgs has been reported in the brain of aging flies, and restoration of proteins to their youthful levels delays neurodegeneration and extends their life-span35. More limiting seems to be the class III phosphatidyl-inositol-3-kinase complex (PI3K) that mediates the nucleation of the phagophore. Three proteins – Vps15, Vps34 and beclin-1 – are essential components of this complex, and their recruitment to the phagophore initiates the nucleation process36,37 (Fig. 1, panel 1). Cellular levels of beclin-1 have often been correlated with autophagic activity, and heterozygous deletion of beclin-1 leads to neurodegeneration9. In contrast, the increased levels of beclin-1 described in different neurodegenerative disorders often reflect neuronal upregulation of macroautophagy in response to pathogenic proteins or neuronal injury38. The limiting nature of beclin-1 could be behind the aggravating effect of aging in neurodegeneration as lower levels of beclin-1 have been reported in brains from old individuals39. However, cellular availability of beclin-1, rather than just the total cellular level, might hold the key to defective autophagy in different pathologies. Integration of beclin-1 into the nucleation complex is negatively regulated by its binding to Bcl-240, and this itself is modulated through posttranslational modifications of beclin-141. It is thus conceivable that changes in the enzymes that mediate these posttranslational modifications or in the cellular subcompartmentalization of beclin-1 could underlie the basis for autophagic failure in some neurodegenerative settings12,37,40,41.
Macroautophagy is negatively regulated by a second major kinase complex, the serine/threonine protein kinase mTOR (mammalian target of rapamycin)42 (Figure 1, panel 2). Chemical inhibition of mTOR, often used to activate macroautophagy, was indeed the first autophagic manipulation shown to slow down the progress of neurodegeneration28 and sequestration of mTOR in protein aggregates has been proposed to mediate upregulation of macroautophagy in the animal models of HD28. However, whether or not changes in the autophagic targets downstream of mTOR43 occur in neurodegeneration requires further investigation.
Cargo sequestration
Although macroautophagy was previously considered an “in-bulk” process, overwhelming evidence now supports selectivity in the sequestration of autophagic cargo44,45 (Fig. 1, panel 3). Recognition of certain posttranslational modifications, often polyubiquitination, by molecules that bind both cargo and components of the autophagic machinery mediates this selectivity45,46. P62, the first cargo-recognizing molecule identified, binds preferentially to a particular type of ubiquitin linkage (K63) on the surface of protein aggregates and brings autophagosome formation to these aggregates through its interaction with LC347,48. P62 has turned out to be a complex molecule that not only participates in autophagic clearance of aggregates but also modulates aggregate formation and regulates stress-response genes. These other functions of p62 could explain in part why deletion of p62 ameliorates hepatic injury in animals deficient for macroautophagy in liver49. This effect is however organ-specific, because deletion of p62 did not suppress neurodegeneration in neuronal macroautophagy deficient mice49. Cargo recognition by p62 is not limited to protein aggregates but it also includes organelles and even pathogens50,51. Ubiquitin is also the recognition signal for NBR1 and NDP52, novel p62-like molecules. The targeted cargo in the case of NBR1 is limited to proteins52 whereas NDP52 recognizes ubiquitin-coated bacteria inside human cells53.
Inefficient recognition of aggregate proteins by macroautophagy, which depends on the nature of the aggregate protein, has been described in an aggregate-prone experimental setting54. For example, while cytosolic inclusions of α-synuclein, synphilin-1, mutant tau or huntingtin are readily amenable to macroautophagy removal, inclusions of p38 and desmin persist in the cytosol even when macroautophagy is maximally activated.54. Surprisingly, p62 is present in both types of aggregates, suggesting that p62 is necessary but not sufficient to bring together the autophagy machinery and activate autophagic clearance. Intrinsic properties of the aggregating proteins, specific posttranslational modifications or changes in their interaction with cargo-recognizing molecules could determine amenability for autophagic clearance. In this respect, acetylation has recently shown to modulate autophagic clearance, although with different effect depending on the substrate protein. Thus, whereas acetylation of a fragment of huntingtin facilitates its autophagic clearance55, acetylation of ataxin-7 prevents its autophagy-mediated turnover56.
Changes not only in the substrates, but also in the autophagic system itself could lead to inefficient cargo recognition. In fact, we have recently found a paradoxical decrease in macroautophagy-mediated degradation in different HD models, despite proper formation and clearance of autophagosomes57. Analysis of these autophagosomes has revealed a marked decrease in their cargo content, giving the impression of “empty” autophagosomes. Because the failure to recognize cargo is not limited to a particular cytosolic component, it is plausible that a primary defect in the autophagosome membrane is behind the observed failure.
Autophagosome clearance
Degradation of the sequestered cargo only occurs when autophagosomes fuse to lytic compartments (i.e. lysosomes or endosomes). In contrast to yeast, where a subset of SNARE proteins has been shown to mediate fusion of autophagosomes to the vacuole, the components that participate in fusion of mammalian autophagosomes to lysosomes or endosomes are poorly characterized2. So far only the Rab7 GTPase and Vtilb have been shown necessary for mammalian autophagic fusion, although the participation of other Rabs and several VAMS has also been proposed2. In addition to these components in the membrane of autophagosomes and lysosomes, autophagosome clearance also involves the participation of the cellular cytoskeleton and cytosolic modulators1–4.
Alterations in autophagosome clearance have become a common theme for a growing number of neurodegenerative disorders. The distinctive characteristic of the affected neurons is an increase in number of autophagic vacuoles that do not associate with increased autophagic flux. Defects can originate from the inability to mobilize autophagosomes from their site of formation toward lysosomal/endosomal compartments, decreased fusion between their membranes or decreased proteolysis inside lysosomes (Fig. 1, panel 4). For example, changes in the properties of microtubules, motor associated proteins such as dynein, dynactin or tubulin deacetylases (e.g. HDAC6) have been described in different neurodegenerative settings with altered macroautophagy58–62. Cells defective in HDAC6 also display a primary defect in vesicular fusion that is independent of microtubules, but involves instead the actin cytoskeleton63. Formation of actin bundles at the surface of autophagosomes is required for fusion63, but interestingly, only needed for quality control autophagy and not for starvation-induced autophagy.
In some instances, autophagosome/lysosome fusion occurs but degradation of the delivered cargo is incomplete or nonexistent (Fig. 1, panel 5). Changes in the lysosomal lumen, such as reduced lysosomal acidification, accumulation of undigested byproducts and decreased content or activity of lysosomal hydrolases, have been described behind such degradative failure. In this respect, many conditions that fall into the category of lysosomal storage disorders – a group of diseases characterized by deficit or malfunctioning of specific lysosomal enzymes – have an associated deficient autophagic clearance which could explain, at least in part, the neurological symptoms often associated with these disorders64–66. A primary defect in lysosomal acidification has also been recently identified in forms of AD resulting from alterations in presenilin 167. The lower proteolytic capability of these lysosomes leads to the massive neuronal accumulation of undegraded autophagosomes observed in the AD brain at advances stages.
Consequences of the autophagic failure
Defective autophagy has different effects in cellular homeostasis depending on the autophagic step primarily affected. Failure to induce autophagosome formation results in cytosolic persistence of non-sequestered cargo which could promote aggregation of other intracellular components (aggregation “seed”) or become a source of toxic products (i.e. ROS production by damaged mitochondria). Accumulation of protein aggregates, higher content of abnormal non-functional mitochondria, deformities of the endoplasmic reticulum and an increase in the number and size of lipid droplets, have been described in the different conditional ATG knock-out mice7,8,10.
When autophagic failure originates from inefficient cargo recognition, the extent of cellular impairment depends on whether recognition problems are limited to a particular type of cargo or they affect sequestration of all intracellular components. The consequences of general failure to recognize autophagic cargo are the same as the ones described when autophagy induction fails. Because autophagosomes are still formed, however, bulk removal of randomly-sequestered soluble components is often preserved57. When only a particular type of cargo escapes targeted autophagy, the cellular consequences depend on the effects that accumulation of that cargo can cause. For example, inability to recognize mitochondria results in poor mitochondria turn-over, alterations in mitochondria dynamics, and the increase in oxidative damage associated to mitochondria malfunctioning68,69.
In circumstances when the autophagic defect originates from poor clearance of autophagosomes, accumulation of autophagosomes inside cells can also be dreadful. Although autophagosome formation would at least prevent the undesirable effects of non-sequestered cytosolic cargo, this expansion of the autophagic compartment can interfere with intracellular trafficking70. Furthermore, autophagosomes can become a source of cytotoxic products. For example, in cellular and animals models of AD, the presence of the amyloid precursor protein (APP) in the accumulating autophagosomes along with the protease complex responsible for its cleavage into the pathogenic peptide β1–42 converts autophagosomes into an endogenous source for this pathogenic product70. Lastly, autophagic compartments that persist longer than usual in the cytosol can become leaky, and if leakage occurs post lysosomal fusion, the release of lysosomal enzymes often activates cell death71.
Looking for another way out: compensatory cross-talks between autophagy and other proteolytic systems
Current pharmacological options to modulate autophagy in vivo by directly acting on autophagic components are still very limited. Further expansion of the therapeutic options could be attained through a better understanding of the compensatory mechanisms and autophagic alternatives that are activated by cells when autophagy fails. In recent years, it has become evident that macroautophagy acts in a coordinated manner with other cellular proteolytic mechanisms72,73. The first insights of this coordinated function were obtained by analyzing the consequences of blocking other proteolytic systems on macroautophagy and vice versa (Fig. 2) where cells respond to blockage of CMA by activating macroautophagy in a constitutive manner72. Although both pathways are not redundant, compensatory activation of macroautophagy in basal conditions preserves homeostasis in cells with compromised CMA72. Likewise, CMA is upregulated in response to macroautophagy blockage73. Cross-talk between these pathways is of particular interest in neurodegeneration because primary blockage of CMA has been identified in PD models and certain tauopathies74–76. Pathogenic variants of alpha-synuclein and truncated forms of Tau interfere with normal functioning of the CMA translocation complex, thus reducing degradation of other CMA substrates that accumulate in the cytosol (damaged and misfolded cytosolic proteins) and compromising neuronal function74–76. The activation of macroautophagy observed in PD24 may be secondary to CMA blockage and could help alleviate these conditions.
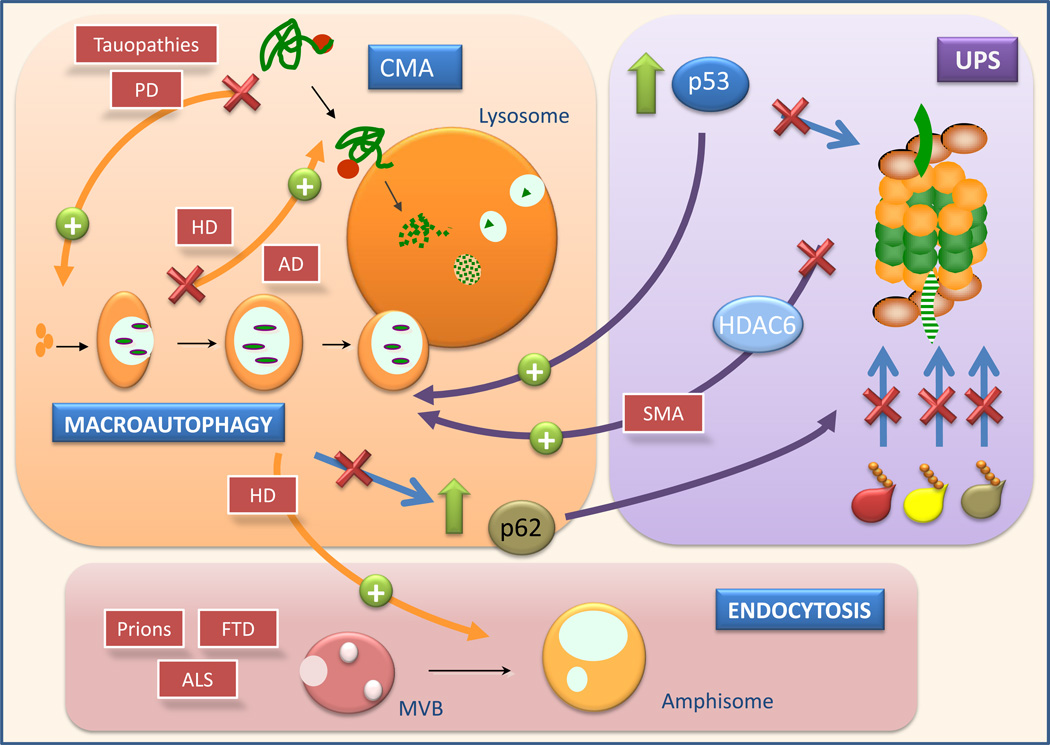
The consequences of macroautophagic blockage on the activity of other autophagic pathways, endocytosis and on the ubiquitin proteasome system (UPS) and the consequences of changes in these pathways on macroautophagy are depicted. Examples of neurodegenerative disorders for which this crosstalk has been shown to be relevant are indicated in the red boxes and are discussed in more detail in the text. MVB: multivesicular bodies; CMA: chaperone-mediated autophagy; UPS: ubiquitin proteasome system; AD: Alzheimer’s disease; HD: Huntington’s disease; PD: Parkinson’s diease; FTP:frontotemporal dementia; ALS: amyotrophic lateral sclerosis; SMA: spinal muscular atrophy.
Of increasing interest are also the connections between macroautophagy and other non-autophagic lysosomal pathways such as endocytosis (Fig. 2). In fact, disrupted formation of multivesicular bodies due to ESCRT-III dysfunction in the membrane of late endosomes leads to reduced autophagic flux and autophagosome accumulation in models of frontotemporal dementia77,78. Additional genetic studies have revealed that other components essential for endosome biogenesis (i.e. ESCRT-I, -II, their regulatory ATPase Vps4 and the endosomal kinase Fab1) are all required for autophagy78. Disruption of this endosomal proteins leads to accumulation of cytosolic polyubiquitinated pathogenic proteins such as huntingtin or TDP-43 (component of ALS protein inclusions), as expected from autophagic failure79,80. Functional endosomes are important for autophagosome clearance, likely through the fusion between both compartments to form amphisomes. Amphisomes are hybrid vesicular compartments that arise from the fusion of an autophagosome with endosomes, instead lysosomes. Enhanced formation of amphisomes has been demonstrated when autophagosome/lysosome fusion is compromised81, which in turn accommodates an augmented formation of autophagosomes82 (Fig. 2)
These interactions between the autophagic and endocytic pathways could be especially important in the case of prion diseases, because endocytosis is a major route of cellular entry for pathogenic forms of prion proteins (PrPsc)83. Furthermore, endocytic compartments, specifically multivesicular bodies (MVBs), can also mediate transmission of the pathogenic protein in between cells. Upon fusion of the endosome and plasma membrane, the PrPsc located in the luminal vesicles of MVB gains access to the extracellular media in the form of exosomes83. Similar interactions with the endocytic system have been proposed for other pathogenic proteins involved in non-infectious neurodegenerative disorders such as amyloid-β, α-synuclein and tau proteins84. In theory, conditions that favor endosomal degradation versus endosomal recycling should facilitate elimination of the pathogenic proteins by the lysosomal system. In this scenario, enhanced fusion of autophagosomes with endosomes may reroute the endosomal compartments toward lysosomes. Further investigation is necessary to determine whether or not this is the mechanism behind the lower intracellular levels of PrPsc and reduced PrPsc propagation observed upon upregulation of macroautophagy with trehalose and lithium85.
The cellular connections of macroautophagy expand beyond the lysosomal system to other proteolytic systems. Special attention has been paid to the interplay between macroautophagy and the ubiquitin proteasome system (UPS) (Fig. 2) (reviewed in86). Cells respond to acute proteasome blockage by upregulating macroautophagy27,87 whereas persistent chronic blockage of this protease leads to constitutively upregulated macroautophagy, but failure to further activate macroautophagy in response to stress88. Chemical upregulation of macroautophagy in mice protects them from the neurodegeneration induced upon proteasome inhibition89, reinforcing the possible therapeutic implications of this cross-talk. The fact that genetic blockage of macroautophagy resulted in massive accumulation of polyubiquitinated aggregates7,8 indicates that polyubiquitinated proteins, initially considered exclusive cargo of the UPS, are also substrates for the autophagic system. However, it remains controversial whether macroautophagy only engulfs these proteins when in aggregates or also degrades soluble polyubiquitinated proteins in a selective manner. Differences in the types of ubiquitin linkage may determine delivery to one or other degradative pathway; whereas ubiquitination of lysine 48 (K48) leads preferentially to UPS degradation, there are growing evidence that lysine 63 (K63)-ubiquinated proteins may be rerouted to macroautophagy for degradation48,90. A promising possible modulator of the macroautophagy and UPS is p53, a well characterized UPS substrate that has recently shown to upregulate macroautophagy91,92. Failure to degrade p53 by the UPS will increase its cytosolic levels leading to macroautophagy activation. In return, increased autophagy should facilitate p53 clearance and prevent engagement of the mitochondrial apoptotic pathways downstream of p5392. The microtubule-associated deacetylase HDAC6 also links polyubiquitinated proteins and autophagy as it has been shown to be essential to rescue the degeneration associated with proteasome failure in an autophagy-dependent manner87. Interestingly, blockage of macroautophagy does not enhance UPS activity but instead compromises its function93. This effect seems mediated by p62, putative substrate of both systems94, that when accumulates in the cytosol due to impaired macroautophagy, competes with other ubiquitinated proteins for delivery to the proteasome93 (Fig. 2).
Connections between macroautophagy and the UPS are not limited to the removal of cytosolic ubiquitinated proteins but also involve removal of organelles. For example, ubiquitination of constituent proteins in the membrane of peroxisomes mediates their macroautophagy51. This novel connection between ubiquitination and organelle autophagy may be particularly important in PD-affected neurons. In fact, two genes related to familial form of PD, the ubiquitin ligase parkin and the serine/threonine kinase PINK1, have recently been implicated in autophagy of dysfunctional mitochondria68. PINK1 accumulates selectively on dysfunctional mitochondria and induces translocation of parkin to the depolarized mitochondria. Subsequently, parkin-mediated ubiquitination of mitochondrial proteins by K63 and K27 linkage favors mitochondria aggregation and recruitment of p62, which brings along the autophagic machinery69. Mutant forms of these proteins disrupt mitophagy at different steps – translocation/aggregation, ubiquitination and autophagic clearance68,95.
Therapeutic considerations stemming from the different types of autophagic failure
Identification of the specific autophagic step(s) affected in the different neuronal pathologies is an important consideration for the future development of therapeutic interventions that depend on modulating autophagy to prevent neuronal degeneration. The nature of the autophagic defect, the cellular response to that defect and elapsed time into the progression of the disease, should all be taken into account during the implementation of these therapeutic approaches.
Conditions resulting from hampered macroautophagy induction should benefit from treatments that activate macroautophagy. In contrast, inhibition of autophagy should be remedial when excessive activation of autophagy leads to cytosolic depletion of essential organelles96. Autophagy activators may have a limited beneficial effect in neurodegenerative disorders arising from defective cargo recognition. In fact, activation of autophagosome formation may increase the amount of cargo randomly sequestered and degraded via macroautophagy, but the lost of selectivity recognizing the cargo is likely to decrease the efficience of the process. A better characterization of cargo-recognition molecules is necessary in order to design molecular interventions aimed at enhancing cargo recognition. Activation of autophagy can become detrimental in the context of massive accumulation of un-degraded autophagic vacuoles observed in many neurodegenerative diseases. In fact, treatments that inhibit autophagosome formation have shown to improve neuronal viability, at least temporarily, in conditions such as frontotemporal dementia, ischemic injury or AD where most of the autophagosome accumulation originates from problems in clearance21,77. The optimal treatment should enhance autophagosome clearance by the lysosomal compartment. Although pharmacological compounds with these effects are currently unavailable, remarkably good results have been observed by promoting lysosomal biogenesis by overexpression of the transcription factor EB97. The new and healthy lysosomes may mediate removal of the accumulated autophagosomes, although it still remains unclear for how long and to what extent additional formation of lysosomes can be maintained.
Lastly, an aspect that could offer considerable room for therapeutic manipulation in the future is the increasing number of autophagic variations that co-exist in a given cell (Fig. 3). It has become evident that different mechanisms can lead to formation of autophagosomes while some molecular components once thought to be essential for macroautophagy can be dispensable. Case in point, we now know about m-TOR-dependent and m-TOR-independent autophagy46,98,99, non-canonical autophagy that occurs even in the absence of beclin-1100, and autophagosome formation even in the absence of Atg5 and Atg7101 (Fig. 3). An important task in the coming years will be matching these different autophagic variants with the different conditions that result in autophagic activation. The traditional division in basal and starvation-induced macroautophagy has been revised to make room for other cellular events requiring autophagic involvement (Fig. 3). Basal in-bulk macroautophagy and starvation-induced autophagy still remain at the extremes of this scale, whereas quality control autophagy, autophagy induced by protein aggregates, in response to organelle stress or to pathogen invasion, are finding their location in this classification as their unique properties are becoming apparent. Utilizing alternative macroautophagy variants to compensate for the defective ones could be an exciting therapeutic alternative still unexplored.
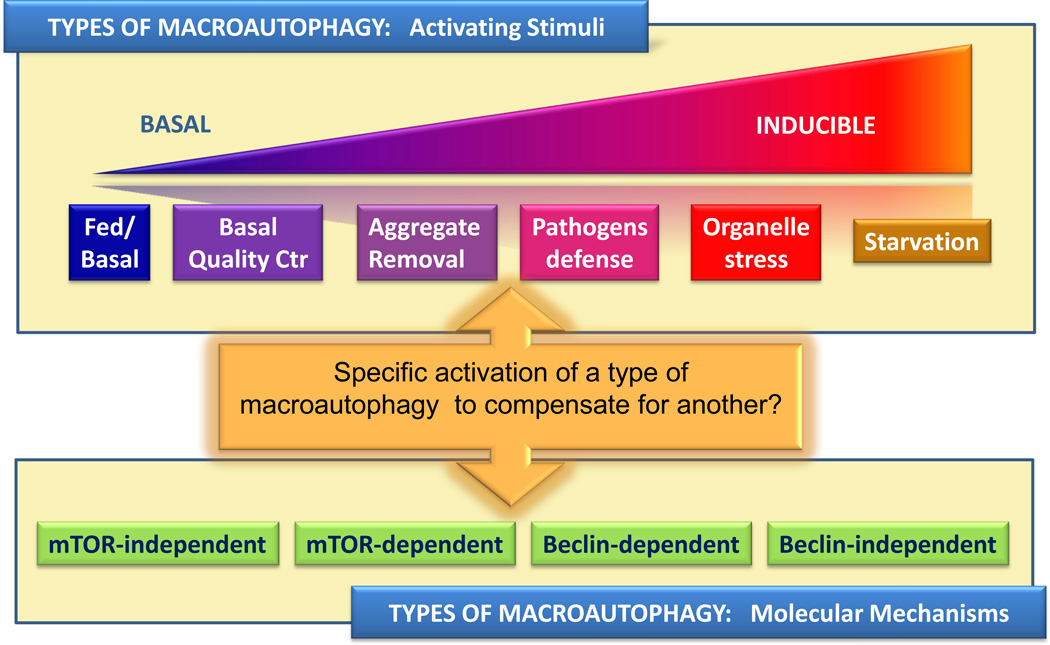
Types of macroautophagy depending on the stimuli that mediates its activation (Top) or on the molecular mechanisms involved in autophagy activation/execution (Bottom). As new understanding of these different autophagy variants is gained, it is possible that activation of one autophagic variant could be utilized to compensate for defects in other autophagy variant.
Acknowledgements
We thank numerous colleagues in the field of autophagy who through their animated discussions have helped shape this review, and Dr. Susmita Kaushik, and Ms. Samantha Orenstein for critically reading the manuscript. Work in our laboratory is supported by NIH grants from NIA (AG021904, AG031782), NIDKK (DK041918), NINDS (NS038370), a Glenn Foundation Award and a Hirsch/Weill-Caulier Career Scientist Award. E.W. is a Hereditary Disease Foundation Fellow.
References
Full text links
Read article at publisher's site: https://doi.org/10.1038/nn.2575
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4038747
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Apache is a neuronal player in autophagy required for retrograde axonal transport of autophagosomes.
Cell Mol Life Sci, 81(1):416, 05 Oct 2024
Cited by: 0 articles | PMID: 39367928 | PMCID: PMC11455771
From Brain to Muscle: The Role of Muscle Tissue in Neurodegenerative Disorders.
Biology (Basel), 13(9):719, 12 Sep 2024
Cited by: 0 articles | PMID: 39336146 | PMCID: PMC11428675
Review Free full text in Europe PMC
DBT is a metabolic switch for maintenance of proteostasis under proteasomal impairment.
Elife, 12:RP91002, 10 Sep 2024
Cited by: 0 articles | PMID: 39255192 | PMCID: PMC11386957
Reviewing the Structure-Function Paradigm in Polyglutamine Disorders: A Synergistic Perspective on Theoretical and Experimental Approaches.
Int J Mol Sci, 25(12):6789, 20 Jun 2024
Cited by: 0 articles | PMID: 38928495
Review
The marine-derived compound TAG alleviates Parkinson's disease by restoring RUBCN-mediated lipid metabolism homeostasis.
Acta Pharmacol Sin, 45(7):1366-1380, 27 Mar 2024
Cited by: 0 articles | PMID: 38538717
Go to all (591) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Autophagy and its normal and pathogenic states in the brain.
Annu Rev Neurosci, 37:55-78, 21 Apr 2014
Cited by: 129 articles | PMID: 24821313
Review
Neuronal autophagy as a mediator of life and death: contrasting roles in chronic neurodegenerative and acute neural disorders.
Neuroscientist, 18(3):224-236, 27 Apr 2011
Cited by: 53 articles | PMID: 21525331
Review
Autophagic flux control in neurodegeneration: Progress and precision targeting-Where do we stand?
Prog Neurobiol, 153:64-85, 03 Apr 2017
Cited by: 40 articles | PMID: 28385648
Review
Autophagy in the central nervous system: implications for neurodegenerative disorders.
CNS Neurol Disord Drug Targets, 9(6):701-719, 01 Dec 2010
Cited by: 64 articles | PMID: 20942791
Review
Funding
Funders who supported this work.
NIA NIH HHS (5)
Grant ID: R37 AG021904
Grant ID: AG021904
Grant ID: R01 AG021904
Grant ID: AG031782
Grant ID: P01 AG031782
NIDDK NIH HHS (2)
Grant ID: P01 DK041918
Grant ID: DK041918
NINDS NIH HHS (2)
Grant ID: P50 NS038370
Grant ID: NS038370





