Abstract
Free full text

Type II Interferon Promotes Differentiation of Myeloid-Biased Hematopoietic Stem Cells
Associated Data
Abstract
Interferon gamma (IFNγ) promotes cell division of hematopoietic stem cells (HSCs) without affecting the total HSC number. We postulated that IFNγ stimulates differentiation of HSCs as part of the innate immune response. Here we report that type II interferon signaling is required, both at baseline and during an animal model of LCMV infection, to maintain normal myeloid development. By separately evaluating myeloid-biased and lymphoid-biased HSC subtypes, we found that myeloid-biased HSCs express higher levels of IFNγ receptor and are specifically activated to divide after recombinant IFNγ exposure in vivo. HSCs show increased expression of the transcription factor C/EBPβ after infection. Furthermore, myeloid-biased HSCs are transiently depleted from the marrow during the Type II interferon-mediated immune response to Mycobacterium avium infection, as measured both functionally and phenotypically. These findings indicate that IFNγ selectively promotes differentiation of myeloid-biased HSCs during an innate immune response to infection. This represents the first report of a context and a mechanism for discriminate utilization of the alternate HSC subtypes. Terminal differentiation, at the expense of self-renewal, may compromise HSC populations during states of chronic inflammation.
INTRODUCTION
A number of disease states highlight the significant effects that interferons can have on hematopoiesis. Viral bone marrow suppression can be mediated by Type I interferons1, and patients with severe aplastic anemia frequently have high Type II interferon levels2. Interferons may also be used therapeutically in some hematologic malignancies3–5.
Despite these observations, little is known about the effects of interferons on the hematopoietic stem cell (HSC). HSCs are adult stem cells with the unique dual capacity to produce all of the cell types of the peripheral blood and self-renew throughout life. Since abnormal control of HSC quiescence may lead to bone marrow failure or leukemia, understanding the factors, both genetic and environmental, that control HSC function is of significant clinical importance.
Recently, it was discovered that Type I and Type II interferon can promote cell division within the usually quiescent pool of HSCs in vivo6, 7. These findings are notable not only because there are relatively few secreted factors that cell autonomously stimulate HSC cell division, but also because of the very common nature of interferons. Interferons are released systemically to activate the inflammatory response to infections such as viruses (generally Type I interferon) and intracellular pathogens (generally Type II interferon). Although HSCs are mostly quiescent, they can be induced to divide by systemic infection or by introduction of the viral mimetic polyinosinic:polycytidylic acid (PI:C)6, 7. This observation leads to a number of important questions regarding the role of interferon signaling to HSCs in the immune response, and the effect of such signaling on risk for cancer or bone marrow failure.
In an effort to begin to address these questions, we previously noted that the total pool of HSCs does not increase despite increased cell divisions. Furthermore, following in vivo interferon exposure, HSCs have a defective repopulation capacity upon transplantation6, 8. Collectively, these findings suggest that while HSCs progress through the cell cycle more frequently in the presence of interferon, most of those cell divisions result in production of other cell types, not more HSCs. In other words, interferon-mediated cell divisions likely contribute to differentiation rather than self-renewal events. This observation leads to the intriguing possibility that direct cell autonomous interferon signaling to HSCs contributes to generation of differentiated immune cells as part of an acute immune response.
Recent work suggests that distinct HSC subtypes exist in the marrow; “myeloid-biased” or “lymphoid-deficient” HSCs produce relatively more myeloid progeny whereas “lymphoid-biased” or “balanced” HSCs produce relatively more lymphoid progeny9–11. Myeloid-biased HSCs (My-HSCs) express higher levels of CD150, have increased Hoechst dye efflux ability, increased quiescence, and a greater self-renewal capacity compared to lymphoid-biased HSCs (Ly-HSC)9, 11, 12. While the relative proportion of My-HSCs appears to increase with age, a functional role for these HSC subtypes has not yet been demonstrated13. Furthermore, the relationship between interferon stimulation and HSC subtypes has not been examined previously.
In this study, we have used genetic approaches and in vivo infection to study consequences of interferon-mediated cell division in HSCs. We report that IFNγ is required for normal myeloid development from the level of the stem cell, and that it acts specifically on myeloid-biased HSCs to promote myeloid differentiation during infection. This represents the first report of a functional role for distinct HSC subtypes during an inflammatory response and suggests that terminal differentiation could be a mechanism for HSC depletion after extensive interferon exposure. These findings lend clarity to our understanding of the molecular basis of inflammation-related disorders of hematopoiesis.
MATERIALS AND METHODS
Mice
Wild-type C57Bl/6 (CD45.2) and C57Bl/6.SJL (CD45.1) mice 6–12 weeks of age were used. C57Bl/6 Ifngr1−/− (Stock #3288) mice were obtained from Jackson Laboratories (Bar Harbor, ME)14. C57Bl/6 Ifnar1−/− mice were obtained from Christian Schindler (Columbia)15. C57Bl/6 Stat1−/− mice were obtained from David Levy (NYU)16. Ifngr1−/− CD45.1 mice were obtained by crossing C57Bl/6 Ifngr1−/− and C57Bl/6.SJL (CD45.1) mice. All mouse strains were maintained at an AALAC-accredited, specific-pathogen-free animal facility at Baylor College of Medicine. Genotypes were confirmed by PCR of genomic DNA.
Microbial Infections
Lymphochoriomeningitis virus subtype Armstrong was obtained from Michael Jordan (U. Cincinnati). LCMV propagated in BHK21 cells was titered using a standard plaque assay17. Mice were infected by intraperitoneal injection of 1 × 105 plaque-forming units and bone marrow was harvested after 6 days unless otherwise noted. For mycobacterial infections, mice were infected i.v. with 2 × 106 colony-forming units of M. avium as described18 and bone marrow was harvested after 1 month unless otherwise noted.
Bone marrow transplantation
For bone marrow transplants 250 donor HSCs from 8–12 week old CD45.2 treated or mutant mice or wild-type controls (C57Bl/6) were admixed with 2 × 105 CD45.1 rescue whole bone marrow (WBM) cells and retroorbitally injected into 6–8 week old CD45.1 wild-type C57Bl/6 recipients that had been lethally irradiated with a split dose of 10 Gy. Engraftment was measured at 4, 8, 12, and 16 weeks post-transplant by staining of WBCs from peripheral blood with anti-CD45.1 and anti-CD45.2 antibodies. Lineage distribution was determined using the following antibody cocktail: PeCy7 anti-Gr1, PECy7 anti-Mac1, PeCy7 anti-B220, PacBlue anti-B220, PacBlue anti-CD4, PacBlue anti-CD8. Ifngr1−/− versus Ifnar1−/− HSC transplants were conducted by crossing Ifngr1−/− mice into a CD45.1 background. HSCs from Ifngr1−/− CD45.1 mice and Ifnar1−/− CD45.2 mice were transplanted into lethally irradiated CD45.1/CD45.2 heterozygous WT recipient mice.
BrdU and Ki67 labeling
For BrdU labeling, mice received an intraperitoneal injection of 4 mg BrdU (Sigma) 1–3 days prior to sacrifice and analysis of cells. For experiments in which BrdU was injected 3 days prior to sacrifice, BrdU was also added at 1 mg/mL to the drinking water. For My- and Ly-HSC BrdU experiments, HSCs were sorted and mixed with carrier B220+ splenocytes prior to BrdU staining, as previously described18. Samples were analyzed for BrdU incorporation using the BrdU Flow Kit (BD PharMingen). For Ki67 labeling, samples were fixed and permeabilized prior to staining with anti-Ki67 antibody (eBioscience).
Cytokine administration and detection
For in vivo IFNγ experiments, recombinant IFNγ (eBioscience) was injected intravenously at 10µg per mouse. IFNγ and IFNα levels were detected with a mouse interferon ELISA (BD [Franklin Lakes, NJ]).
Flow cytometry and peripheral blood analysis
Peripheral blood analysis was conducted using an Advia 120 hematology system (Siemens). MoFlo, LSRII, and FACS-Scan instruments were used for flow cytometric analysis and cell sorting. Unless otherwise specified, HSCs were identified as Lin–, c-kit+, Sca1+, CD150+, CD48– as previously described11, 19. Lineage markers included Ly-6G (Gr1), CD11b (Mac1), B220, CD4, CD8, and Ter11920. Myeloid-biased HSCs were identified as the lower part of the SP distribution among CD150+ SPKLS cells whereas lymphoid-biased HSCs were isolated from the upper part of the SP distribution as previously described11.
Methylcellulose assays
1 × 104 whole bone marrow cells were suspended in methylcellulose containing cytokines (Stem Cell Technologies) to support growth of mouse hematopoietic progenitors. Colonies were counted after 12 days and identified by morphology.
RNA purification and realtime PCR
RNA was isolated from 1.5–6 × 104 My- and Ly-HSCs (SPLKSCD150+, upper versus lower SP) (pooled from several mice), using the RNAqueous kit (Ambion). LCMV, Ifnar1, Ifngr1, and Cebpb Taqman probes were used with Taqman PCR Mastermix and a 7900HT Fast Real-Time PCR system. Samples run in triplicate were normalized to internal 18S controls (Applied Biosystems [Foster City, CA]). Testing for LCMV in sorted HSCs was done by qPCR according to the method of McCausland and Crotty21. Testing for M. avium DNA was done by the method of Park et al22.
Statistics
Mean values ± SEM are shown. Student’s t-test or 2-way ANOVA were used for comparisons (GraphPad Prism Version 5.0).
RESULTS
Interferons (specifically IFNα and IFNγ) are powerful inducers of cell cycle activity in HSCs in vivo6, 7. To investigate the importance of IFNα/β and IFNγ in baseline number and function of HSCs, we analyzed the bone marrow of mice lacking interferon alpha receptor 1 (Ifnar1), interferon gamma receptor 1 (Ifngr1), or signal transducers and activators of transcription 1 (Stat1), a major transcription factor for both IFNα/β and IFNγ signaling. Using flow cytometry, we characterized the number of HSCs in the bone marrow. The percentage of long-term HSCs in all genotypes was similar (Figure 1A). The absolute number of HSCs per bone was also similar (data not shown).
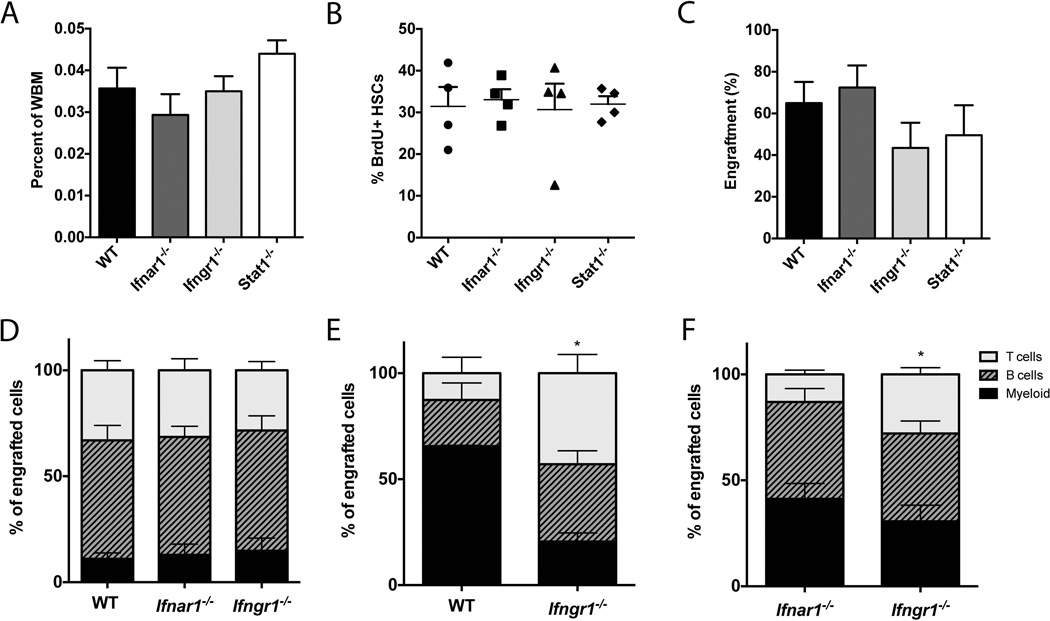
(A) The baseline percentage of HSCs (Lin–, Sca1+, cKit+ (LSK), CD150+, CD48–) is not significantly different in WT, Ifnar1−/−, Ifngr1−/−, or Stat1−/−, mice all on B6 background. n=3. (B) BrdU incorporation in HSCs (SPLSK CD150+) after 3 days of BrdU exposure was not different among genotypes. n=4. Data represent a single experiment. (C) Engraftment of 250 HSCs (SPLSK) was also similar among genotypes. n=5–6. (D) HSCs (SPLSK) from CD45.2 WT, Ifnar1−/−, or Ifngr1−/− mice were transplanted into CD45.1 WT recipients and lineage composition of peripheral CD45.2 cells was assessed at 16 weeks post-transplant. n=3–5. (E) HSCs (SPLSK CD150+) from CD45.2 Ifngr1−/− mice were secondarily transplanted into CD45.1 WT recipients and lineage composition of peripheral CD45.2 cells was assessed at 16 weeks post-transplant. n=2–4. Data represent a single experiment. (F) CD45.2 Ifnar1−/− and CD45.1 Ifngr1−/− HSCs (SPLSK CD150+) were co-transplanted into CD45.1/CD45.2 heterozygous recipients, and lineage composition of peripheral CD45.2 or CD45.1 cells was measured at 16 weeks post-transplant. n=14. Bars represent average and SEM. Unless otherwise noted, each graph is representative of 2–3 experiments. *p<0.05 by 2-way ANOVA.
Next we measured division of HSCs from WT, Ifnar1−/−, Ifngr1−/−, and Stat1−/− mice. BrdU incorporation in HSCs was similar in the various genetic backgrounds (Figure 1B). Furthermore, engraftment of 250 HSCs (side population, lineage− Sca1+ cKit+ abbreviated as SPLSK) was similar for all genotypes (Figure 1C). Of note, 96.6% of SPLSK cells are also EPCR+ and CD48-, indicating a highly select population of long-term HSCs (Supplementary Figure 1). These findings indicate that stem cells from all of the genotypes are competent to engraft and sustain the hematopoietic system.
We further investigated whether HSCs lacking Ifnar1 or Ifngr1 show a difference in differentiation potential by assessing the peripheral blood output of HSCs following transplantation. The lineage distribution of cells from transplanted Ifnar1−/− HSCs and Ifngr1−/− HSCs was normal upon primary transplant (Figure 1D and Supplementary Figure 2a). However, upon secondary transplant, Ifngr1−/− HSCs (SPLSK CD150+) produced fewer myeloid cells when compared to WT HSCs, with just 20.6% (SEM 4.1%) of engrafted myeloid cells being donor-derived compared to 65.6% (SEM 0.43%) in the WT control at 16 weeks post transplant. Meanwhile, the percentages of Ifngr1−/− donor-derived B and T cells were greater at 36.5% (SEM 6.3%) and 43.0% (SEM 8.9%) respectively, versus 21.8% (SEM 2.7%) B cells and 12.6% (SEM 7.6%) T cells for the WT donor-derived cells (Figure 1E). In terms of donor contribution, Ifngr1−/− HSCs appeared capable of producing all the various cell types, but tended to produce more lymphoid cells relative to WT (Supplementary Figure 2B).
A difference in differentiation pattern was also evident when Ifngr1−/− HSCs were co-transplanted with Ifnar1−/− HSCs, with 30.6% myeloid (SEM 5.9%) among the Ifngr1−/−-derived cells versus 41.3% myeloid (SEM 7.2%) among the Ifnar1−/−-derived cells (Fig 1F). Thus, by direct comparison, Ifngr1−/− HSCs tended to produce more lymphoid and less myeloid cells than Ifnar1−/− HSCs, and this was also borne out when examining the contribution of donor cells to each cell type (Supplementary Figure 2C). Since these data were generated from animals after long-term (16 weeks) engraftment of transplanted stem cells, they suggest that IFNγ could contribute to lineage specification at very early stages of hematopoietic differentiation, thus affecting the overall myeloid/lymphoid ratio. In summary, adoptive transfer experiments indicate that Ifngr1−/− HSCs have a subtle bias towards lymphoid differentiation when compared to WT HSCs upon secondary transplantation, suggesting that baseline IFNγ signaling normally participates in myeloid differentiation at the level of the stem cell.
We hypothesized that interferon-related effects on HSCs would be more dramatic in the context of an infection. Therefore we utilized a lymphochoriomeningitis virus (LCMV) subtype Armstrong mouse model of acute viral infection. Introduction of 1 × 105 pfu viral particles by intraperitoneal injection elicited a sublethal acute infection with an associated combined Type I and Type II interferon response. Following infection, there was an early high peak of IFNα followed by a lower but more sustained release of IFNγ (Figure 2A). Wild type mice demonstrated clear changes in the bone marrow, with a 25–36% decrease in total bone marrow cellularity (data not shown) and an expansion in the progenitor fraction (SP, Lin-) that was maximal at day 6 following infection, just after the appearance of IFNγ (Figure 2B). Consistent with this expansion, the number of colony-forming units from the marrow of LCMV infected animals was increased at day 6 following infection (Figure 2C). Colony formation in methylcellulose largely reflects myeloid progenitor abundance23. Of note, there was no increase in colony forming units when LCMV infections were conducted in an Ifngr1-deficient background, suggesting that IFNγ is necessary for this phenotype (Figure 2C). There was also a dramatic increase in peripheral monocytes at 6 days post infection, while the absolute lymphocyte count was not statistically different (Figure 2D). Indeed, peripheral blood analysis revealed that white blood cells peaked at 8 days post infection, logically after the expansion of progenitors and CFUs in the marrow (Figure 2E). The absolute number of granulocytes and monocytes both peaked at 8 days post infection (Supplementary Figure 3). These findings are consistent with induction of the innate immune response early in LCMV infection and demonstrate an expansion of bone marrow progenitors and colony forming units just prior to a peak in peripheral leukocytosis24.
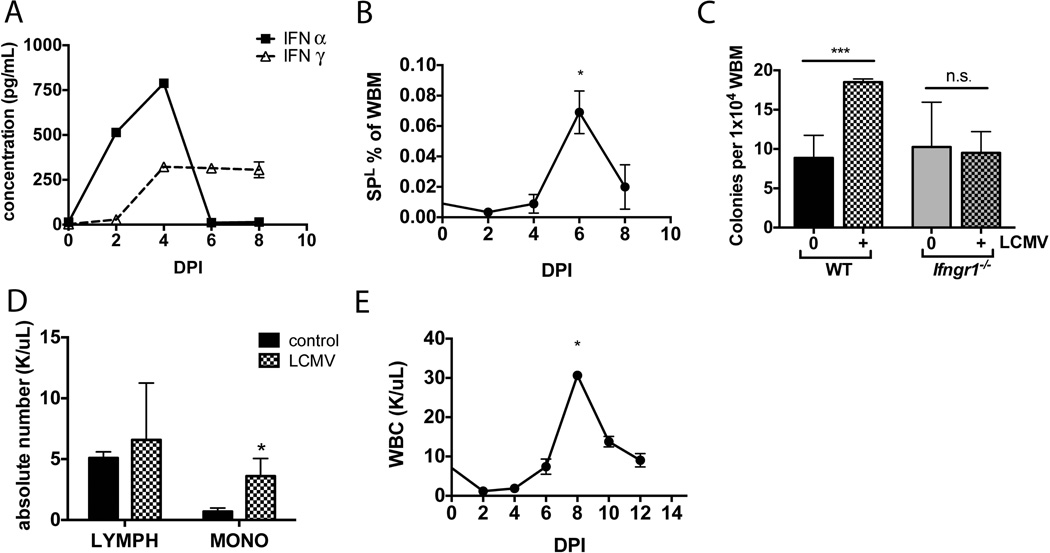
Wild type animals were infected by IP injection with 1 × 105 pfu LCMV Armstrong. (A) The levels of IFNα and IFNγ in serum were measured by ELISA at 2-day intervals over the first 8 days following infection. n=3 per time point. (B) Hematopoietic stem and progenitor cells (SP, Lin−) increased in marrow of infected WT animals, peaking at 6 days following LCMV infection in WT animals. n=3 per time point. (C) 1 × 104 WBM cells from naïve or LCMV infected animals were cultured in methylcellulose media for 12 days. Bars show numbers of total colonies. n=6. (D) Complete blood counts for peripheral blood of naïve or LCMV infected WT animals were compared on day 6 post-infection. The monocyte count was significantly increased. n=3 (E) After LCMV infection, WBC count peaked at 8 days post-infection, just after the increase seen in progenitors and CFUs. Each graph is representative of 2–3 experiments. *p<0.05, *** p<0.001, n.s. not significant.
Given the interferon released during LCMV infection, we expected to find increased cell cycle activity in the HSC compartment. Indeed, BrdU incorporation in the LT-HSC (LK CD150-hi, CD48-, EPCR+) population was markedly elevated at 6 days post infection with LCMV (Figure 3A), indicating increased DNA synthesis. The Sca1 marker was intentionally excluded from these analyses because it can be aberrantly expressed during LCMV infection25. Furthermore, we evaluated cell cycle activity by Ki67-staining and found that the LCMV-mediated increase in HSC cell cycle activity was not evident in Ifngr1−/− mice, indicating an IFNγ-dependent response (Figure 3B). Notably, the number of long-term HSCs (LK CD150-hi, CD48-, EPCR+), whether described as a percentage of whole bone marrow (Figure 3C) or the absolute number, did not change following infection (Figure 3D). Similar results were obtained if HSCs were identified by Hoechst dye efflux (SPLK CD150+, CD48-, EPCR+) (Supplementary Figure 4). The increase in cell cycle activity without a change in absolute number of HSCs suggested that most cell divisions contribute to differentiation or mobilization rather than self-renewal events during LCMV infection. We postulated that the dramatic increase in cell cycling of HSCs during this time could represent a response to escalated peripheral demand26, 27.
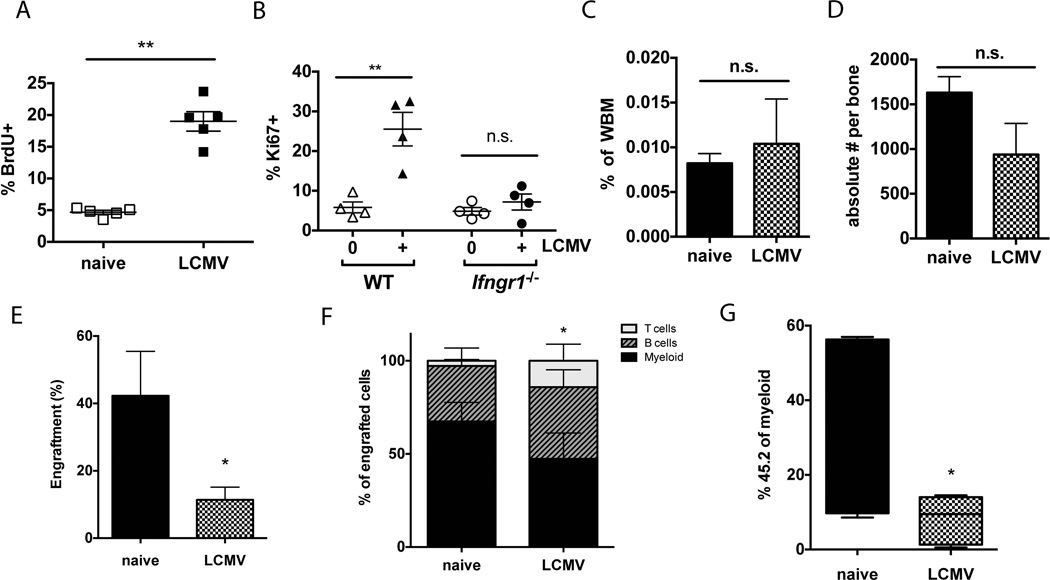
(A) Percent BrdU incorporation was measured in HSCs (Lin−, cKit+, CD150+, CD48−, EPCR+) of naïve or LCMV-infected animals 6 days following infection. BrdU was administered 24 hours before analysis. n=5. (B) HSCs (Lin−, cKit+, CD150+, CD48−, EPCR+) from WT or Ifngr1−/− mice were isolated at day 6 following LCMV infection and stained for intracellular Ki67. n=4. (C) HSCs (Lin−, cKit+, CD150+, CD48−), quantified as percent of whole bone marrow, was not significantly different 6 days after LCMV infection. n=3–4. (D) HSCs (Lin−, cKit+, CD150+, CD48−), quantified as absolute number per bone, were not significantly different 6 days after LCMV infection. n=3–4. (E) Engraftment of 250 HSCs (SPLSK CD150+) from LCMV-infected animals at 6 dpi was diminished at 16 weeks post-transplant compared to HSCs from naïve animals. (F) Upon transplantation into naïve recipients, HSCs (SPLSKCD150+) from LCMV-infected animals at 6 dpi show reduced myeloid output. Bars indicate lineage distribution of donor-derived bone marrow cells 20 weeks after transplantation. (G) The percentage of total myeloid cells derived from LCMV-treated donor HSCs was significantly diminished compared to control donor HSCs 16 weeks after transplant. Box-and-whiskers plots indicate percentage of all myeloid cells that are derived from donor HSCs. Each graph is representative of 2–3 experiments. * p<0.05; ** p<0.01; n.s. not significant.
HSC differentiation is associated with reduced self-renewal and engraftment capacity28, 29. Hence if HSCs tend to differentiate during LCMV infection, we would expect engraftment efficiency to decrease. Indeed, when we conducted adoptive transfer experiments, the peripheral blood contribution of HSCs from LCMV-infected animals was significantly diminished compared to HSCs from naïve animals (Figure 3E). We further noted that the marrow of recipient mice 20 weeks after transplantation with HSCs from LCMV-infected animals was significantly depleted in the myeloid compartment and enriched in the lymphoid compartment (Figure 3F). Of the total peripheral myeloid cells in the recipient mice at 16 weeks post transplant, a smaller fraction were derived from HSCs of LCMV-infected animals compared to HSCs from naïve animals (Figure 3G). This was not true for the B or T cell compartments, suggesting that the major effect of LCMV infection was in suppression of myeloid differentiation. These differences occurred despite lack of evidence of on-going infection in the recipient animals, including normal weight, spleen size, total bone marrow cellularity and screening of the donor HSCs and recipient blood for LCMV RNA.
Given the myeloid production defect of Ifngr1−/− HSCs as well as the myeloid deficiency of HSCs from bone marrow of LCMV-infected animals, we hypothesized that IFN signaling could influence lineage specification at the level of the HSC. We wondered whether HSC subtypes could participate in IFN-dependent differentiation. We therefore isolated Ly-HSCs and My-HSCs based on their differential Hoechst dye efflux properties and separately evaluated expression of the IFNγ and IFNα receptors by RT-PCR. We found that My-HSCs (lower SPLSK CD150+, CD48-) express 20-fold more Ifngr1 compared to Ly-HSCs (upper SPLSK CD150+, CD48-) (Figure 4A). There was no difference in expression of Ifnar1 between the two subpopulations (data not shown). We then injected mice with recombinant IFNγ and measured BrdU incorporation in separately sorted populations of My-HSCs and Ly-HSCs. While Ly-HSCs showed a baseline higher level of BrdU incorporation, only My-HSCs displayed increased BrdU incorporation in response to IFNγ stimulation (Figure 4B). These findings indicate that My-HSCs are more responsive to IFNγ stimulation compared to Ly-HSCs.
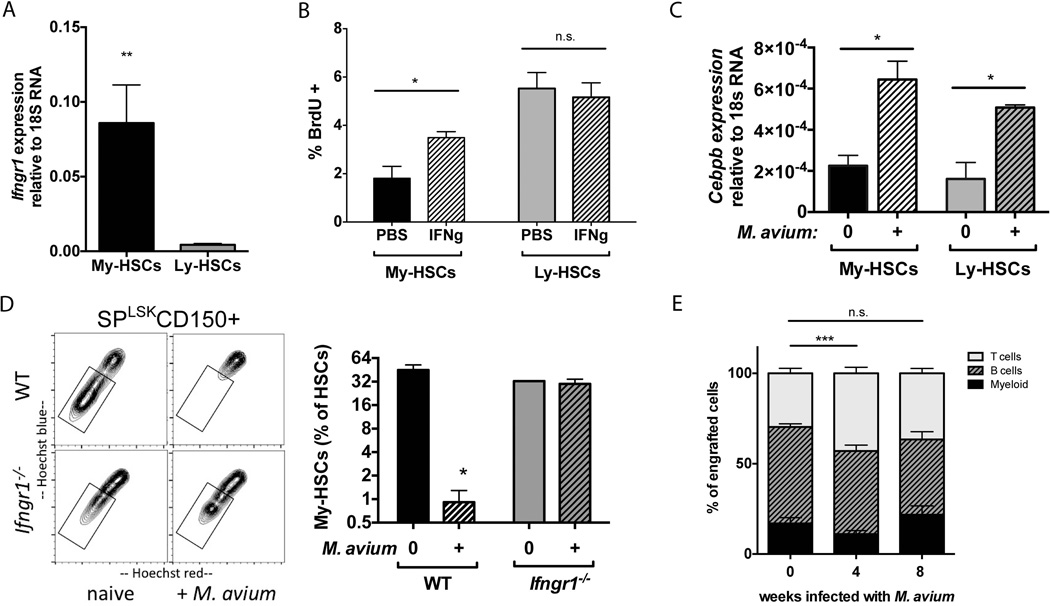
(A) Realtime PCR analysis indicates that Ifngr1 expression is greater in My-HSCs (lower SPLKS CD150+) compared to Ly-HSCs (upper SPLKS CD150+) n=3. (B) After 24-hour in vivo treatment with recombinant IFNγ, My-HSCs show increased BrdU incorporation, but BrdU incorporation is unchanged in Ly-HSCs. n=4–5. (C) Realtime PCR analysis indicates that Cebpb expression is increased in both My-HSCs and Ly-HSCs (SP, Lin−, Sca+, cKit+, CD150+, CD48−) 8 weeks after M. avium infection. (D) Representative flow plots of SPLSK CD150+ cells from naïve or M. avium-infected WT or Ifngr1−/− mice show that My-HSCs (box) are depleted from marrow during infection via an IFNγ-dependent mechanism. Aggregate data are shown in the bar graph to the right. n=3–4. (E) Recipient mice were transplanted with HSCs (SPLKS CD150+) of mice at 0, 4, or 8 weeks after M. avium infection. At 16 weeks post transplant, peripheral blood derived from HSCs of 4-week infected mice shows a relative depletion of myeloid cells. n=3–4 Each graph is representative of 2–3 experiments. *p<0.05, **<0.01, *** p<0.001, n.s. not significant.
The transcription factor C/EBPβ contributes to myeloid specification during emergency granulopoiesis30, 31. We speculated that this factor could be induced upon IFNγ exposure. Indeed, cebpb was significantly induced among HSCs after the IFNγ-mediated immune response to M. avium infection (Figure 4C). However, induction of cebpb was equally present in both My-HSCs and Ly-HSCs (Figure 4C), suggesting that cebpb stimulation and interferon-dependent cell cycle activity occur independently of each other.
In order to determine whether selection of My-HSCs by IFNγ signaling occurs during infection in vivo, we utilized Mycobacterium avium infection to elicit an IFNγ-mediated immune response in mice32, 33. Notably, the M. avium-infected animals showed a specific depletion in the proportion and total number of HSCs in the lower part of the SP distribution, characteristic of myeloid-biased HSCs. Depletion in the lower SP did not occur when M. avium infections were conducted in Ifngr1−/− mice (Figure 4D), indicating an interferon-dependent response. Similar results were obtained when assessing the frequency of My-HSCs after LCMV infection (Supplementary Figure 5). These findings suggest that My-HSCs are depleted from the marrow of M. avium-infected animals through IFNγ-dependent mobilization and/or differentiation events.
We then transplanted HSCs from M. avium infected mice and assessed the lineage distribution of peripheral blood from engrafted cells. Indeed, we found a relative depletion in myeloid output and enrichment in lymphoid cells derived from the donor HSC population 16 weeks after transplantation (Figure 4E). The relative depletion in myeloid output was also noted upon secondary transplantation of the same HSCs, indicating that changes in the HSC population were sustained through multiple rounds of transplantation (Supplementary Figure 6). This change in differentiation pattern was not observed when HSCs were derived from 8-week- M. avium infected donor mice, indicating that the composition of the stem cell population returns to baseline as the animals recover from infection (Figure 4E and Supplementary Figure 6). Of note, no infectious particles were transmitted with the transplanted HSCs, as determined by PCR of sorted cells and spleen of the recipient animals (Supplementary Figure 7). These data suggest IFNγ released during M. avium infection selectively promotes cell division among My-HSCs and depletes My-HSCs from the marrow, resulting in a transiently Ly-HSC-enriched marrow that is reflected upon transplantation.
DISCUSSION
Interferons exert a powerful influence on the HSC population. Here we show that there is a subtle role for baseline IFNγ, but not IFNα, signaling in normal myeloid differentiation of HSCs, as evident upon secondary transplantation. Further, IFNγ signaling promotes myeloid differentiation during infection with LCMV associated with a loss of HSC self-renewal potential. Myeloid differentiation may be achieved through induction of Cebpb expression or through high Ifngr1 expression on My-HSCs, leading to specific activation of this HSC subtype. Finally, activation of My-HSCs during M. avium infection leads to transient depletion of this subtype from the marrow, reflected in temporarily reduced myeloid output upon transplantation. These findings are summarized in the accompanying model figure (Figure 5).
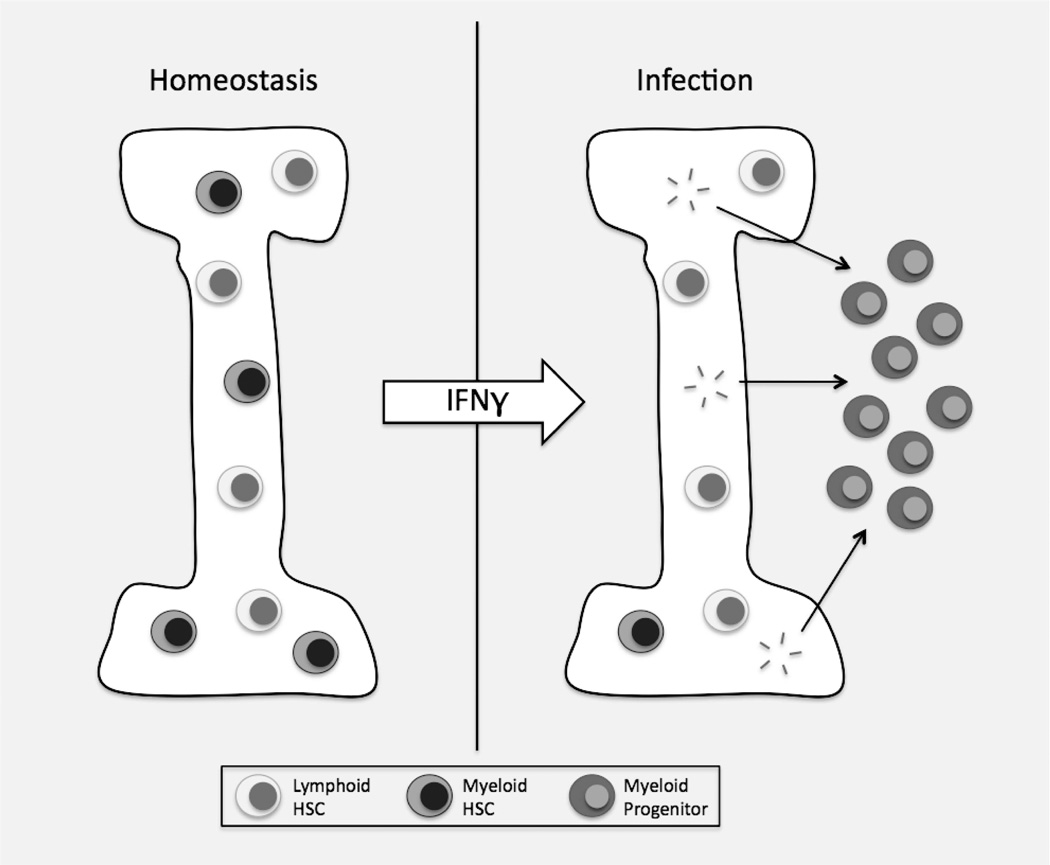
IFNγ released during infection promotes division and differentiation of HSCs, especially My-HSCs. This leads to a relative enrichment of Ly-HSCs in the marrow, reflected upon transplantation of bone marrow-derived HSCs.
Characterization of changes in hematopoietic progenitor populations in response to IFN signaling can be challenging because of a variety of technical hurdles. First, removal of HSCs from their bone marrow niche provides enough stress to the cells that specific consequences of interferon treatment may be masked during in vitro conditions34. Second, Sca1, one of the principal phenotypic markers used in the murine system, is nonspecifically induced by interferon, leading sometimes to mischaracterization of cell types25. For these reasons, in this study we avoided use of the Sca1 marker during stress conditions or did so only in the presence of multiple other selective markers and restricted ourselves to primarily in vivo experiments.
Using stringent parameters to exclude other cell types, we find that LT-HSCs (LK CD150-hi, CD48-, EPCR+) divide upon acute infection with LCMV. These divisions, as evidenced by increased BrdU uptake and increased Ki67 expression, are not evident in mice lacking Ifngr1, indicating that the response is IFNγ-dependent. Furthermore, increased cell division activity is restricted to the myeloid-biased subset of HSCs. Thus, HSC subtypes have differential sensitivity to IFNγ signaling; how such differential sensitivity affects HSC subsets after persistent or repeated inflammatory insults over time remains to be investigated.
Contribution of IFNγ signaling to myeloid development has been recognized among some hematopoietic progenitors; IFNγ promotes monopoiesis by granulocyte-macrophage progenitors (GMPs)35. Interferon-dependent mobilization of bone marrow hematopoietic progenitors has been shown to be an important contributor to immune control of some infections36. Extending those findings, our results suggest that IFNγ signaling contributes to myeloid differentiation even at the most primitive level of hematopoietic development.
HSC subtypes have been described by several groups; and while it has been demonstrated that one subtype can generate the other (through transplantation of single cells), there is increasing evidence that subtype differences tend to be retained as stable properties of individual cells9, 12. In this study, we find that consumption of myeloid-biased HSCs during some infections leads to an apparent lymphoid bias in the progeny of transplanted HSCs that persists even after transplantation into a naïve recipient, one that has never experienced an infection itself. These findings argue that myeloid versus lymphoid bias is a stable property of each HSC subset, and that changing the stoichiometric ratio of Ly-HSCs to My-HSCs can alter the bone marrow output following transplantation. Thus, selection of stable HSC subtypes is a mechanism for controlling patterns of differentiation and, importantly, provides a biological context for how HSC subtypes can be selectively utilized in the setting of an infection to coordinate the innate immune response.
Finally, the idea that IFNγ provides a specific stimulus for My-HSCs to differentiate suggests that naturally occurring infections impose a natural selection pressure on HSCs. Each time a host is infected, a subset of HSCs is called forth from the marrow, leaving behind relatively differentiation-resistant clones that must then replenish the HSC supply in the marrow. A logical extension of this finding is, then, that the chance of uncovering particularly differentiation-deficient clones is increased upon repeated rounds of inflammation. This mechanism may contribute in part to the well-known association between interferons and cancer37.
CONCLUSION
In summary, this study demonstrates that IFNγ released during common viral and bacterial infections promotes differentiation of My-HSCs, thereby affecting the composition of the HSC population. This observation implies that naturally occurring infections provide a selection pressure on HSCs by requiring rounds of differentiation, depletion, and replenishment in the marrow. Differential responses to this selection pressure could contribute to changes in HSC and immune cell populations with age.
ACKNOWLEDGEMENTS
We would like to thank Margaret A. Goodell and members of her lab for their support of this work. We thank Yayun Zheng and Allison Rosen for technical assistance, Christian Schindler for sharing Ifnar1−/− mice, Michael Jordan at the University of Cincinnati for generously providing LCMV, and Vivien Sheehan for use of the Advia machine. This project was supported by the Cytometry and Cell Sorting Core at Baylor College of Medicine with funding from the NIH (NIAID P30AI036211, NCI P30CA125123, and NCRR S10RR024574) and the assistance of Joel M. Sederstrom. This work was supported by grants from the NHLBI K08HL098898 (KYK) and the NIDDK R00DK084259 (GAC) of the NIH, and the Department of Defense IDEA award in bone marrow failure research (10505346).
Footnotes
Author contribution summary: All authors performed experiments; GAC performed data analysis and helped design the project; KYK designed the study, performed data analysis and wrote the paper.
DISCLOSURES
The authors have no conflicts of interest to report.
REFERENCES
Full text links
Read article at publisher's site: https://doi.org/10.1002/stem.1799
Read article for free, from open access legal sources, via Unpaywall:
https://academic.oup.com/stmcls/article-pdf/32/11/3023/41985595/stmcls_32_11_3023.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1002/stem.1799
Article citations
Clonal hematopoiesis in people with advanced HIV and associated inflammatory syndromes.
JCI Insight, 9(9):e174783, 02 Apr 2024
Cited by: 0 articles | PMID: 38564303 | PMCID: PMC11141903
Forged in the fire: Lasting impacts of inflammation on hematopoietic progenitors.
Exp Hematol, 134:104215, 04 Apr 2024
Cited by: 0 articles | PMID: 38580008
Review
STAT3 protects hematopoietic stem cells by preventing activation of a deleterious autocrine type-I interferon response.
Leukemia, 38(5):1143-1155, 11 Mar 2024
Cited by: 0 articles | PMID: 38467768 | PMCID: PMC11283865
The impact of gut microbial signals on hematopoietic stem cells and the bone marrow microenvironment.
Front Immunol, 15:1338178, 13 Feb 2024
Cited by: 1 article | PMID: 38415259 | PMCID: PMC10896826
Review Free full text in Europe PMC
Single-cell time series analysis reveals the dynamics of HSPC response to inflammation.
Life Sci Alliance, 7(3):e202302309, 18 Dec 2023
Cited by: 3 articles | PMID: 38110222 | PMCID: PMC10728485
Go to all (77) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Macrophage-Lineage Cells Negatively Regulate the Hematopoietic Stem Cell Pool in Response to Interferon Gamma at Steady State and During Infection.
Stem Cells, 33(7):2294-2305, 22 May 2015
Cited by: 43 articles | PMID: 25880153 | PMCID: PMC4693298
Improved hematopoietic stem cell transplantation upon inhibition of natural killer cell-derived interferon-gamma.
Stem Cell Reports, 16(8):1999-2013, 08 Jul 2021
Cited by: 5 articles | PMID: 34242616 | PMCID: PMC8365098
A new mechanism for the aging of hematopoietic stem cells: aging changes the clonal composition of the stem cell compartment but not individual stem cells.
Blood, 111(12):5553-5561, 15 Apr 2008
Cited by: 217 articles | PMID: 18413859 | PMCID: PMC2424153
The Regulatory Role of IFN-γ on the Proliferation and Differentiation of Hematopoietic Stem and Progenitor Cells.
Stem Cell Rev Rep, 13(6):705-712, 01 Dec 2017
Cited by: 24 articles | PMID: 28852997
Review
Funding
Funders who supported this work.
NCI NIH HHS (2)
Grant ID: P30CA125123
Grant ID: P30 CA125123
NCRR NIH HHS (2)
Grant ID: S10 RR024574
Grant ID: S10RR024574
NHLBI NIH HHS (2)
Grant ID: K08HL098898
Grant ID: K08 HL098898
NIAID NIH HHS (2)
Grant ID: P30 AI036211
Grant ID: P30AI036211
NIDDK NIH HHS (3)
Grant ID: T32 DK060445
Grant ID: R00 DK084259
Grant ID: R00DK084259
NIH (3)
Grant ID: NCRR-S10RR024574
Grant ID: NCI-P30CA125123
Grant ID: NIAID-P30AI036211





