Abstract
Free full text

Differential protein-protein interactions of LRRK1 and LRRK2 indicate roles in distinct cellular signaling pathways
Abstract
Genetic studies show that LRRK2, and not its closest paralogue LRRK1, is linked to Parkinson’s disease. To gain insight into the molecular and cellular basis of this discrepancy, we searched for LRRK1- and LRRK2-specific cellular processes by identifying their distinct interacting proteins. A protein microarray-based interaction screen was performed with recombinant 3xFlag-LRRK1 and 3xFlag-LRRK2 and, in parallel, co-immunoprecipitation followed by mass spectrometry was performed from SH-SY5Y neuroblastoma cell lines stably expressing 3xFlag-LRRK1 or 3xFlag-LRRK2. We identified a set of LRRK1- and LRRK2-specific as well as common interactors. One of our most prominent findings was that both screens pointed to epidermal growth factor receptor (EGF-R) as a LRRK1-specific interactor, while 14-3-3 proteins were LRRK2-specific. This is consistent with phosphosite mapping of LRRK1, revealing phosphosites outside of 14-3-3 consensus binding motifs. To assess the functional relevance of these interactions, SH-SY5Y-LRRK1 and -LRRK2 cell lines were treated with LRRK2 kinase inhibitors that disrupt 14-3-3 binding, or with EGF, an EGF-R agonist. Redistribution of LRRK2, not LRRK1, from diffuse cytoplasmic to filamentous aggregates was observed after inhibitor treatment. Similarly, EGF induced translocation of LRRK1, but not of LRRK2, to endosomes. Our study confirms that LRRK1 and LRRK2 can carry out distinct functions by interacting with different cellular proteins.
Introduction
Since the identification of mutations in leucine-rich repeat kinase 2 (LRRK2) linked to familial forms of Parkinson’s disease (PD) (Paisan-Ruiz et al., 2004; Zimprich et al., 2004), LRRK2 has been shown to be an important contributor to PD pathogenesis. Mutations in LRRK2 are a common cause of familial forms of PD where the phenotype is clinically similar to that of sporadic PD (Haugarvoll et al., 2008; Healy et al., 2008). In addition, genetic variation at the LRRK2 locus is also associated with sporadic PD (Satake et al., 2009; Simón-Sánchez et al., 2009; Ross et al., 2011). However, the precise cellular processes regulated by LRRK2 and the exact nature of its dysfunction in the etiology of disease is incompletely understood.
LRRK2 is a member of the ROCO family of multidomain proteins which are characterized by the presence of a Ras of complex (ROC) protein domain and an adjacent C-terminal of ROC domain (COR) (Lewis, 2009). LRRK2’s closest paralogue is LRRK1, which displays a similar domain organization: besides the signature ROC-COR domains both proteins also encode a serine-threonine kinase domain C-terminal of ROC-COR, and N-terminal leucine-rich and ankyrin-like repeats (Marín, 2006; 2008; Civiero et al., 2012). Differences between the two proteins are that LRRK2 additionally displays an N-terminal segment encoding armadillo-like repeats, while the WD40 domain present in the LRRK2 C-terminus is absent in the equivalent section of LRRK1 (Marín, 2006; 2008; Civiero et al., 2012) (Figure S1B). There is no genetic support for the involvement of LRRK1 in the pathogenesis of PD, suggesting that LRRK2 has unique properties that are relevant to the disease process (Haugarvoll et al., 2007; Taylor et al., 2007). We have previously shown that the detrimental effects of mutations in LRRK2 are not reproduced by introducing equivalent mutations in LRRK1 (Greggio et al., 2007). Collectively, these observations suggest that although the two proteins have a similar modular organization, LRRK1 and LRRK2 have distinct properties which may be important for disease pathogenesis.
However, LRRK1 has also been shown to interact with LRRK2 (Paisan-Ruiz et al., 2004; Zimprich et al., 2004; Klein et al., 2009; Dächsel et al., 2010). This interaction, and the high degree of homology between the two proteins, indicates that LRRK1 may be involved in the LRRK2 signaling network. In order to better understand the relationship between cellular signaling functions of LRRK1 and LRRK2, we have screened for protein-protein interaction partners of LRRK1 and LRRK2. These screens pointed to a LRRK1-specific interaction with EGF-R and a LRRK2-specific interaction with 14-3-3 proteins, confirming previous reports (Haugarvoll et al., 2008; Healy et al., 2008; Nichols et al., 2010; Hanafusa et al., 2011). In this study, we further characterize these two interactions, in particular the crosstalk between the two pathways. We show that these interactions are specific for each LRRK and that they mediate specific, independently occurring cellular translocation processes for each LRRK. In turn, these results point to segregated functional complexes in the LRRK1 and LRRK2 signaling networks in mammalian cells.
Methods
Constructs and antibodies
Eukaryotic expression constructs of 3xFlag-tagged LRRK1 (pCHMWS-3xFlag-LRRK1) and 3xFlag-tagged LRRK2 (pCHMWS-3xFlag-LRRK2) have been previously described (Daniëls et al., 2010; Civiero et al., 2012). To generate eGFP-tagged versions of these constructs, the 3xFlag tag was excised (using restriction sites NheI and BamHI) and an eGFP sequence (excised from the peGFP-C1 plasmid using restriction sites NheI and BglII) was ligated in the place, yielding the pCHMWS-eGFP-LRRK1 and pCHMWS-eGFP-LRRK2 constructs.
For constructs encoding 2xMyc-tagged 14-3-3 isoforms (pCMV-3B-2xMyc-14-3-3-zeta and pCMV-3B-2xMyc-14-3-3-beta), 14-3-3 beta and zeta were amplified from pEBG-6P1 14-3-3 beta and EBG2T 14-3-3 zeta vectors (kindly provided by Prof. Dario Alessi, University of Dundee, UK) using Pfu polymerase (Promega, Madison, WI, USA), cloned into pCR8GW/TOPO (Life Technologies, Grand Island, NY, USA), and subcloned into 2xMyc gateway destination vector. The PCR primers used are: Zeta-FOR: ATGGATAAAAATGAGCTGGTTCAG, Zeta-REV: TTAATTTTCCCCTCCTTCTCCTGC, Beta-FOR: ATGACAATGGATAAAAGTGAGCTG, Beta-REV: TTAGTTCTCTCCCTCCCCAGCGTC.
Myc-tagged EGF-R (pLPCX-EGF-R-Myc) was kindly provided by Dr. Guha (University of Toronto, Canada) (Gajadhar and Guha, 2010).
To generate knockdown constructs, short hairpin sequences targeting LRRK1 (reference sequence NM_024652) or LRRK2 (reference sequence NM_198578.3) were cloned into microRNA sequences in the 3’UTR region of a blasticidin resistance gene under control of the SFFV promoter in lentiviral vector transfer plasmids (see Supplementary Table 3 for sequences of cloning oligonucleotides), yielding the following constructs: LV_miR_LRRK1_6734-sh1, LV_miR_LRRK1_4366-sh2, LV_miR_LRRK1_3351-sh3, LV_miR_LRRK1_6091-sh4, LV_miR_LRRK2_7814-sh1, LV_miR_LRRK2_6251-sh3, LV_miR_LRRK2_2384-sh5, (note that the number immediately following the gene name refers to the position on the reference sequence of the first base of the short hairpin sequence). Controls for the knockdown vectors included empty vector control as well as vectors encoding short hairpin sequences targeting firefly luciferase (Osório et al., 2013).
Antibodies used in this study are: monoclonal mouse anti-Myc antibody (9e10, Roche, Basel, Switzerland), monoclonal mouse anti-Flag antibody (M2, Sigma Aldrich, St. Louis, MO, USA), polyclonal rabbit anti-EGFR antibody (Cell Signaling Technology, Beverly, MA, USA), rabbit polyclonal pan 14-3-3 antibody (Santa Cruz Biotechnology, Dallas, TX, USA), rabbit monoclonal Hsp90 antibody (Cell Signaling Technology), rat monoclonal Hsc70 antibody (Abcam, Cambridge, UK), rabbit polyclonal BAG5 antibody (Sigma Aldrich) and monoclonal mouse anti-early endosome antigen 1 (EEA1, BD Biosciences, Erembodegem, Belgium). Polyclonal sheep anti-LRRK1 was obtained from Prof. Dario Alessi (University of Dundee, Dundee, UK). Anti-LRRK2 antibodies used were the rabbit monoclonal antibodies C41-2 and UDD3 (antibody produced by Epitomics with the support of the Michael J. Fox Foundation) as well as the mouse monoclonal antibody N138/6 (antibody produced by Neuromab with the support of the Michael J. Fox Foundation) (Davies et al., 2013).
Cell culture and transfection
HEK293T cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Life Technologies) supplemented with 8% Fetal Bovine Serum (FBS), Harlan Sera-Lab Ltd., Indianapolis, USA) and 50 μg/ml gentamycin (Life Technologies) at 37°C in a humidified atmosphere containing 5% CO2. SH-SY5Y human neuroblastoma cells were cultured in DMEM supplemented with 15% FCS, 50 μg/ml gentamycin and non-essential amino acids (100X, Life Technologies) also at 37°C with 5% CO2. Transient expression was performed by transfecting plasmids with linear polyethylenimine (Polysciences Europe GmbH, Eppelheim, Germany) according to the manufacturer’s protocol for 24-48 h.
Protein microarray screen for LRRK1 and LRRK2 interaction partners
Detailed methodology for the protein microarray experiments has been described previously (Beilina et al., 2014). For the current analysis, protoarrays® version 5.30 (Life Technologies) were probed with 6 μg purified 3xFlag-GFP, 3xFlag-LRRK1 or 3xFlag-LRRK2 in buffer alone. Arrays were probed with antibodies against the Flag tag and scanned.
To understand the specific interactions for each protein, we first filtered Z-scores, which represent the number of standard deviations for the signal of each protein against background on that array, for LRRK1 or LRRK2 against 3xFlag-eGFP, thus excluding common false-positive interactors. We then took the filtered lists of Z-scores and performed direct comparisons for both proteins. The raw data for LRRK1 and LRRK2 is included in the supporting information.
Affinity purification of LRRK complexes followed by mass spectrometry identification of complex components
HEK293T cells stably expressing 3xFlag-LRRK1 or 3xFlag-LRRK2 were expanded up to twelve T150 tissue culture flasks (Sigma Aldrich) per condition. When the flasks reached 100% confluency, cells were trypsinized and collected in a 50 ml falcon tube per condition. After rinsing two times with PBS, lysis was performed for 20 minutes on ice in 10 ml Lysis buffer (25 mM Tris/HCl pH 7.4, 150 mM NaCl, 5 mM MgCl2, 0.1% (v/v) Triton X-100 and protease inhibitor cocktail (Roche)). Lysates were centrifuged at 4°C for 10 minutes at 14000xg and cleared lysates were incubated overnight with anti-Flag M2 agarose beads (Sigma Aldrich) at 4°C on a rotator. Beads were washed four times with Lysis buffer. After washing, immunoprecipitates were eluted in elution solution (50% (v/v) acetonitrile, 1% (v/v) Formic acid) and supernatants were dried in a speedvac. The dried pellets were dissolved in SDS loading buffer and the isolated protein complexes were fractionated with SDS-PAGE. Coomassie brilliant blue staining was performed to visualize proteins and sample lanes were cut into five equal gel pieces, followed by an overnight in-gel trypsin digestion at 37°C. Extracted peptides were further purified using C18 matrix and analyzed by a 4800 MALDI-TOF/TOF Mass Analyzer (AB SCIEX, Framingham, MA, USA). List of identified proteins were compiled based on a protein identification probability of at least 80% as determined by Scaffold™ software v3.6.3 (Proteome software, Portland, OR, USA).
Co-immunoprecipitation and protein purification
For co-immunoprecipitation on overexpressed proteins, HEK293T cells stably expressing 3xFlag-LRRK1 or LRRK2 were transfected with pCMV-3B-2xMyc-14-3-3-zeta and pLPCX-EGF-R-Myc with polyethyleneimine and lysed after 48-72 hours in Co-IP buffer (10 mM Tris/HCl pH 7.4, 150 mM NaCl, 10 mM MgCl2, 0.5% Triton X-100 and protease inhibitor cocktail (Roche)). Lysates were centrifuged at 4°C for 10 minutes at 14000×g. Cleared lysates were incubated for 3 to 18 hours with anti-Flag M2 agarose beads at 4°C on a rotator. Beads were washed 4 times with Co-IP buffer. After washing, immunoprecipitates were eluted by addition of 2x SDS loading buffer.
For co-immunoprecipitation of endogenous Hsc70, Hsp90 and BAG5, HEK293T cells were transfected with 2 μg of 3xFlag-LRRK1, 3xFlag-LRRK2 or GUS-3xFlag with lipofectamine. Twenty-four hours after transfection cells were lysed for 30 minutes on a rotator in Co-IP buffer 2 (20 mM Tris pH 7.5, 150 mM NaCl, 1 mM EDTA, 0,3 % Triton X-100, 10 % Glycerol, Protease (EDTA free) and phosphatase inhibitors). Lysates were centrifuged at 4°C for 10 minutes at 14000xg. Subsequently, 20 μg Protein G beads were added to each tube and mixed for 30 minutes at 4°C followed by the addition of 20 μl flag beads. Beads were washed with Co-IP buffer 3 (same as Co-IP buffer 2 except Triton X-100 concentration is 0,1 % instead of 0,3 % and 0,1 mM nucleotide is added) and proteins were eluted with 30 μl of 3xFlag peptide in Tris 25 mM pH 7.5, 150 mM NaCl, MgCl2 10 mM, dithiothreitol (DTT) 2 mM, beta-glycerophosphate 5 mM, Na3VO4 0.1 mM and 0.02% Triton.
Samples were resolved on 3–8% Tris-Acetate or 10% Bis-Tris gels (Life Technologies) and subsequently blotted onto PVDF membranes (Bio-Rad, Hercules, CA, USA). Blots were probed with rabbit polyclonal 14-3-3 antibody (Santa Cruz Biotechnology, 1:500), rabbit EGF-R antibody (Cell Signaling Technology, Danvers, Massachusetts, USA, 1:2000), rat Hsc70 (Abcam, 1:2000), rabbit Hsp90 (Cell Signaling Technology, 1:2000) and rabbit BAG5 (Sigma Aldrich, 1:2000). Immunoreactive proteins were visualized using the enhanced chemiluminescence plus (ECL plus) technique (GE Healthcare, Little Chalfont, England).
Assessment of LRRK2 kinase inhibitor- and EGF-induced subcellular translocation
To test translocation of LRRK1 or LRRK2, cells stably overexpressing eGFP-LRRK1 or eGFP-LRRK2 were plated out on 12 mm coverslips in a 24-well plate or in 8-well chamber slides. To test LRRK2 kinase inhibitor-induced translocation, cells were treated with 1 μM of the LRRK2 kinase inhibitor IN1 (obtained from Division for Signal Transduction, University of Dundee, with support from the Michael J. Fox Foundation) by diluting the compound into the cell culture medium to its end concentration and incubating the cells for 90 minutes at 37°C. Cells were fixed and mounted onto cover slips as described in supporting information. Cells were imaged using a confocal laser scanning microscope (Fluoview 1000, Olympus, Shinjuku, Tokyo, Japan) and manually scored for translocation to filamentous structures, based on the presence of one or more filamentous accumulations of eGFP-LRRK1 or eGFP-LRRK2.
To test translocation of LRRK1 or LRRK2 under influence of EGF, cells were placed on serum-free DMEM for 12-16 hours, then treated with 100 ng/ml of EGF or 100 ng/ml EGF-Rh and incubated at 37°C for the time points indicated in the results section (0-30 minutes). Cells were then washed two times with PBS, fixed in formaldehyde 4% w/v in PBS and mounted onto cover slips as described in supporting information. Cells were imaged using a confocal laser scanning microscope (Fluoview 1000, Olympus) and manually scored for the presence of endosome-like accumulations positive for LRRK1 or LRRK2. Cells were scored positive based on the presence of at least five endosomal accumulations of eGFP-LRRK1 or eGFP-LRRK2 per cell. For experiments using fluorescently labeled EGF, cells displaying eGFP-LRRK1 or eGFP-LRRK2 and EGF positive endosomes (at least 5 LRRK/EGF double positive endosomes per cell) were scored positive. For each condition tested, at least 3 wells with 20-100 cells per well were scored. Results were expressed as the percentage of positively scored cells. All conditions were tested and quantified in triplicate or more.
Statistical analysis
All quantitative data are expressed as means ± SEM. Significance of differences was assessed by Student’s t test or by one-way ANOVA followed by a Dunnett post-test comparing each experimental group to the LRRK2 control group. For EGF-induced translocation, a two-way ANOVA was performed using time and treatment as parameters followed by Bonferroni post tests comparing each experimental group to the LRRK2 control group. Results were considered statistically significant if p<0.05.
More supporting methods are available in Supporting Information.
Results
Protein-protein interaction (PPI) screens for LRRK1 and LRRK2 using protein microarray and Affinity Purification - Mass Spectrometry (AP-MS)
To identify direct interaction partners of LRRK1 and LRRK2, we probed protein microarrays containing ~9000 human proteins with soluble full-length 3xFlag-LRRK1, 3xFlag-LRRK2 and as a negative control 3xFlag-GFP, prepared as previously described (Civiero et al., 2012). Proteins were prepared, probed to protein microarrays and further processed in parallel to obtain comparable binding conditions for all proteins. Interaction strengths were ranked by Z-score (Figure 1A) and false-positive non-specific interactors were filtered out by removing proteins that bound to 3xFlag-tagged eGFP. In this analysis, we found the two proteins to have distinct interacting partners (Figure 1A). Using a Z-score cutoff of 3, we identified 30 candidate proteins unique for LRRK1 (Supporting Table 1A), 57 proteins unique for LRRK2 (Supporting Table 1B) and 16 shared proteins (Supporting Table 1C). All raw data files are included as supplemental material. Some of the unique interacting proteins included EGF-R for LRRK1 and 14-3-3 proteins for LRRK2 (Figure 1A).
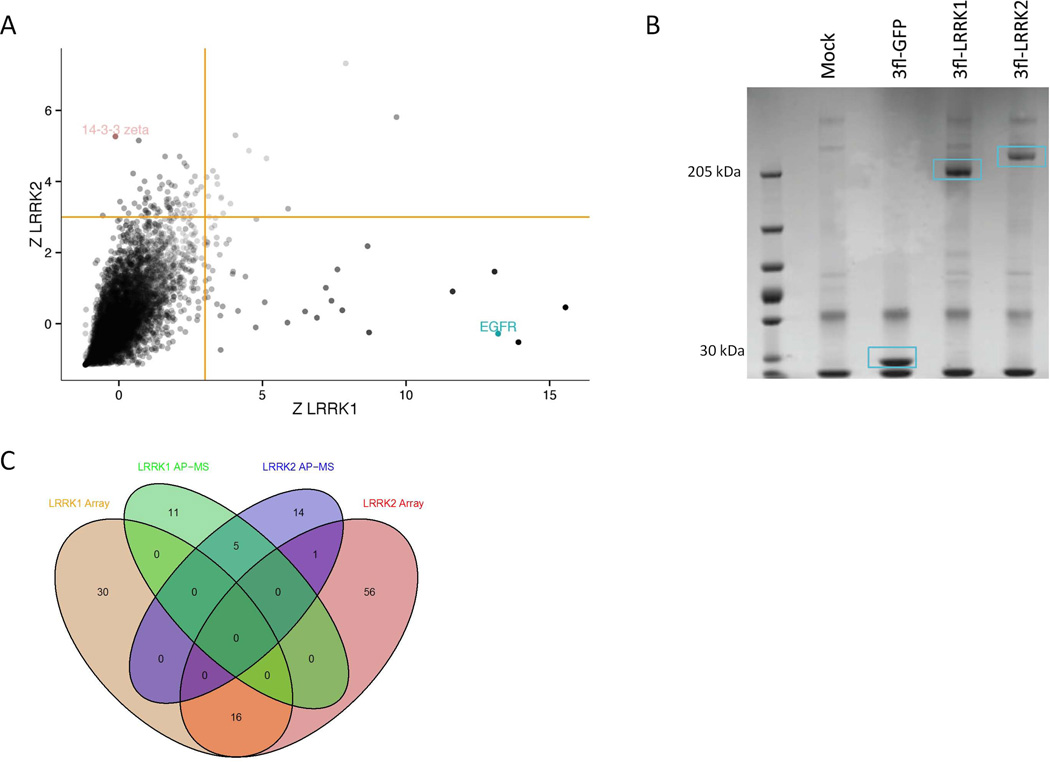
A. Protein microarray screen for LRRK1/2 interactors. Recombinant 3xFlag-eGFP or 3xFlag-LRRK1/2 generated as previously described (Civiero et al., 2012) was used to probe protein microarrays containing ~9000 proteins. Z-binding score was determined for each protein as described in the materials and methods. Depicted here is a plot of the Z-scores of each protein for binding to LRRK1 (x-axis) and LRRK2 (y-axis). Putative LRRK1/2 binding proteins are those that score high for LRRK1/2 binding (Z-scoreLRRK1 > 3) and low for eGFP binding (Z-scoreeGFP < 3). Yellow lines indicate the cut-off Z-score values of 3 (Beilina et al., 2014).
B. Affinity purification followed by mass spectrometry identification (AP-MS) of LRRK1 and LRRK2 interaction partners. Untransduced SH-SY5Y cells or SH-SY5Y cells stably expressing 3xFlag-eGFP, 3xFlag-LRRK1 or 3xFlag-LRRK2 (see materials and methods) were used to purify Flag immunoreactive cellular complexes using Flag-M2 agarose beads. (B) Isolated complexes were resolved on SDS-PAGE and visualized by coomassie brilliant blue staining. Bait proteins are indicated by blue boxes. (C) Venn diagram of the number of identified LRRK interactors and the overlap of hits between both proteins and both screening approaches. For a more detailed list of interactors see Supporting Tables 1A-C and 2A-C.
To identify additional protein complexes and to potentially confirm some of the protein microarray hits, we used a second unbiased approach, namely affinity purification of 3xFlag-tagged LRRK1 or LRRK2 from stable SH-SY5Y cell lines followed by liquid chromatography tandem mass spectrometry (LC-MS/MS) (Figure 1B). Using this approach, we identified 16 potential LRRK1 interacting proteins (Supporting Table 2A), of which 5 were shared with LRRK2 (Supporting Table 2C). We also identified 15 LRRK2-specific interaction partners (Supporting Table 2B). Interacting proteins identified in these experiments included the EGF-R adaptor protein Grb2 for LRRK1 and 14-3-3 proteins for LRRK2, confirming the protein microarray findings. Venn diagrams summarizing the overlap of identified proteins between both LRRKs for each protein-protein interaction screen or between both screens for each LRRK are given in Figure 1C.
Taken together, our two different screening approaches led to the identification of several LRRK1/2-specific as well as common interactors. Using the protein microarray approach, the number of potential interactors identified is higher compared to the number identified with the AP-MS approach (Figure 1C, Supporting Tables 1A-C and 2A-C). Because protein microarrays use recombinant proteins, interactions can be recovered even for proteins with low abundance in cells. In contrast, AP-MS is dependent on the proteome of the cellular system used and will more likely recover interactions with abundant proteins only. There is only minor overlap between the hits identified in the two different approaches, likely due to these differences in coverage. This implies that all results obtained using these techniques should be carefully validated.
It should be noted that sometimes, known interactors are not always recovered. For instance, in the experimental runs performed in this study, we identified Bcl-2 athanogene (BAG) proteins in the LRRK1 probed arrays and not in the LRRK2 probed arrays, while in the recent study of Beilina et al. BAGs were also picked up when arrays were probed with LRRK2 (Beilina et al., 2014). This particular discrepancy may be explained by the reduced strength of the LRRK2:BAG interaction relative to the LRRK1:BAG interaction (see Figure 2, below). However, this also implies a significant false-negative rate for the assays and highlights the need for specific validation of each hit. For the AP/MS technique, false-positive and false-negative hits have begun to be characterized (Mellacheruvu et al., 2013). Together, the two screening methods nominate LRRK1:EGF-R and LRRK2:14-3-3 as robust specific interactions.
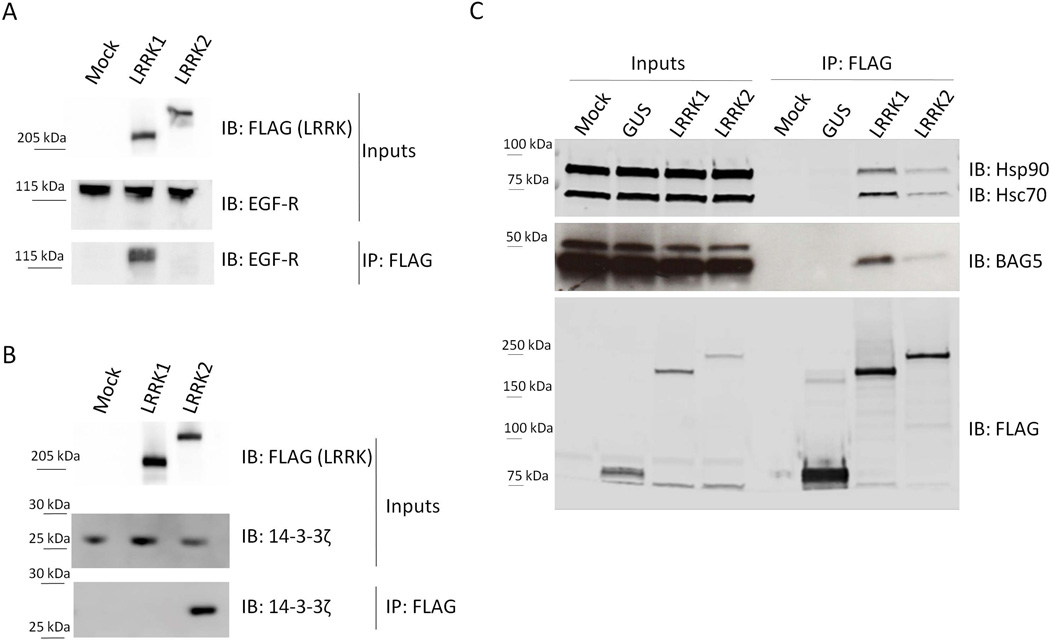
Untransduced HEK293T cells (control) and HEK293T cell lines stably expressing 3xFlag-LRRK1 or 3xFlag-LRRK2 were transfected with EGF-R or 14-3-3. IP was performed using Flag antibody followed by immunoblotting with anti-EGF-R (A, bottom panel) and anti-14-3-3 (B, bottom panel). EGF-R interacts only with LRRK1 and not with LRRK2 while 14-3-3 co-immunoprecipitates with LRRK2 but not with LRRK1. Inputs are shown in the upper panels. (C) To test three candidate common interactors of both LRRK1 and LRRK2, HEK293T cells were transfected with 3xFlag-LRRK1, 3xFlag-LRRK2 and GUS-3xFlag as a negative control. After flag immunoprecipitation, samples were blotted and probed with anti-Hsc70, -Hsp90 and - BAG5 antibodies. All three proteins interact with both LRRKs. Molecular weight markers on the right of all blots are in kilodaltons. Data are representative of at least 5 independent experiments.
Confirmation of specific interactions LRRK1:EGF-R and LRRK2:14-3-3 and common LRRK interactors Hsc70, BAG5 and HSP90
Human Embryonic Kidney (HEK) 293T cell lines stably expressing 3xFlag-LRRK1 or LRRK2 were transfected with Myc-tagged 14-3-3ζ or EGF-R. As predicted from the two initial screens, EGF-R co-immunoprecipitated with LRRK1 but not with LRRK2 (Figure 2A). Similarly, we co-immunoprecipitated 14-3-3ζ with LRRK2 but not with LRRK1 (Figure 2B). In parallel, we also tested three proteins that were identified as common interactors. HEK293T cells transfected with 3xFlag-LRRK1 and 3xFlag-LRRK2 were used to show that endogenous Hsc70, Hsp90 and BAG5 interacted with LRRK1 as well as LRRK2, confirming our protein microarray and AP-MS results (Figure 2C).
Differential protein interactions of LRRK proteins are paralleled by differential LRRK protein phosphorylation patterns
Both LRRKs are phosphorylated in mammalian cells (Greggio et al., 2007; Taymans et al., 2013), but the absence of residues in LRRK1 equivalent to LRRK2 phosphoresidues S910/S935 (Figure S1B) suggests that different residues must be phosphorylated in each protein. Furthermore, given the requirement for LRRK2-specific residues to be phosphorylated to bind 14-3-3 proteins (Nichols et al., 2010; Li et al., 2011), we hypothesized that differential phosphorylation might be important for the identified differences in protein binding seen in the screening approaches and validated above. In order to compare the phosphoresidues in both proteins, we used a phosphoproteomic approach on LRRK1 and LRRK2 affinity purified from stable HEK293T-3xFlag-LRRK1 or LRRK2 cell lines. Proteins were fractionated by SDS-PAGE and purity was assessed by Coomassie brilliant blue staining (Figure S1A). MS analysis confirmed phosphorylation of LRRK2 at several previously reported sites such as S910, S955 and S973 (Figure S1B-C) (Gloeckner et al., 2010; Nichols et al., 2010). We also identified a novel phosphorylation site at S1058, which is located in the third leucine-rich repeat of the LRR domain (Vancraenenbroeck et al., 2012).
Our analysis of LRRK1 cellular phosphorylation identified S249 at the end of the ankyrin repeat domain, S1074 and T1075 in the COR domain as well as S1241 and T1287 in the kinase domain (Figure S1B-C) as phosphorylated residues. Importantly, with the exception of the LRRK1 T1287 site, none of the sites in LRRK2 were conserved in LRRK1 and vice-versa (Figure S1B). Furthermore, none of the LRRK1 phosphosites represent predicted 14-3-3 binding motifs (assessed by Eurkaryotic Linear motif analysis: http://elm.eu.org/, (Yaffe et al., 1997)) suggesting that at least for 14-3-3 binding the differential protein interactions are likely related to differential phosphorylation sites in LRRK1 and LRRK2.
The LRRK2-IN-1 kinase inhibitor induces dephosphorylation of LRRK2 but not LRRK1
Given that LRRK2 is dephosphorylated in cells by LRRK2 kinase inhibitors (Dzamko et al., 2010; Vancraenenbroeck et al., 2014), we tested whether cellular treatment with LRRK2-IN-1 (Deng et al., 2011) affects the overall phosphorylation of LRRK1 via metabolic labeling. As shown in Figure 3A, both LRRK1 and LRRK2 are labeled with 32P. As expected, treatment of cell lines expressing 3xFlag-LRRK2 with 1 μM LRRK2-IN1 for 90 minutes resulted in a 90% reduction in LRRK2 phosphorylation, bur had no effect on LRRK1 phosphorylation (Figure 3B).
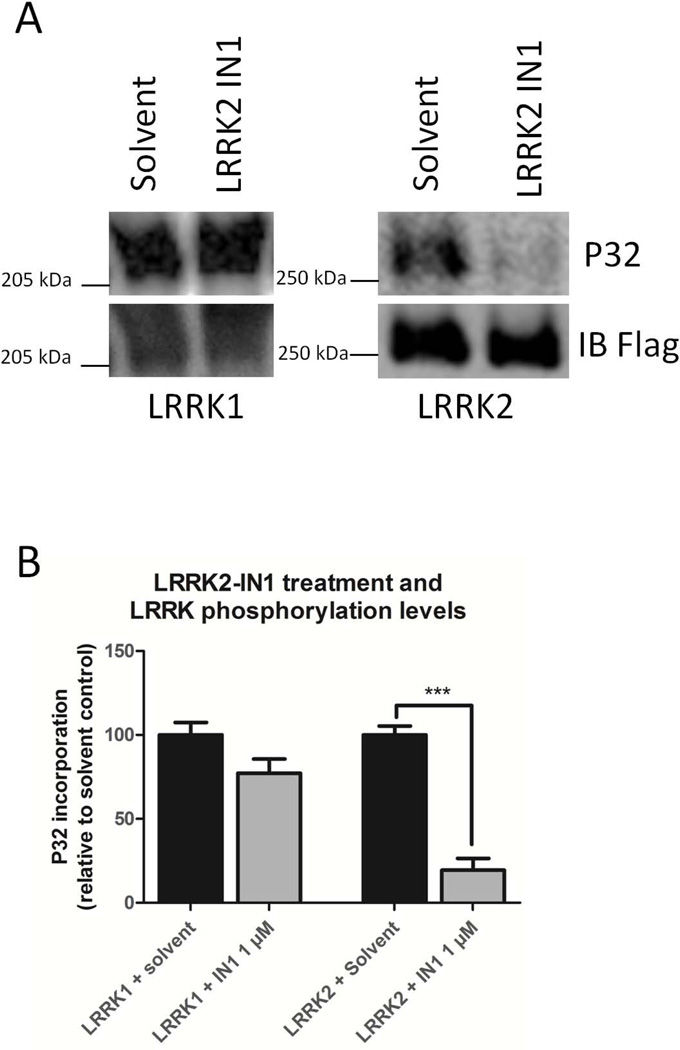
SH-SY5Y cells expressing 3xFlag-LRRK1 or 3xFlag-LRRK2 were metabolically labeled with 32P. Cells were treated with LRRK2-IN1 (1 μM, 90 minutes) and LRRK1 and LRRK2 phosphorylation levels were determined. Depicted are representative autoradiograms and immunoblots of the metabolically labeled samples after treatment with LRRK2-IN1 (A). Quantification of the incorporated 32P levels in each experimental group is shown in (B) (Significance was assessed by an unpaired t-test, n = 3-4, ***: P<0,001).
Epidermal growth factor treatment induces translocation of LRRK1 to endosomes independent of LRRK2
LRRK1 has previously been shown to translocate to endosomes upon treatment of HeLa cells with EGF (Hanafusa et al., 2011). This occurs through activation of the endogenous EGF-R receptor, which is expressed in SH-SY5Y cells (Michaelis et al., 2008), and is therefore an observable cellular phenotype of the LRRK1:EGF-R interaction.
Treatment of SH-SY5Y cells stably expressing eGFP-LRRK1 with rhodamine labeled EGF (EGF-Rh), caused LRRK1 recruitment to EGF-Rh positive endosomes (Figure 4A, S2 and S4), as expected. In our hands, approximately 50-60% of the cells responded between 15 and 30 minutes after stimulation with EGF (Figure 4C). However, EGF-Rh stimulation of SH-SY5Y-eGFP-LRRK2 stable lines did not recruit LRRK2 to endosomes (Figure 4A). Importantly, LRRK2 knockdown (Figure 4B) did not affect LRRK1 recruitment (Figure 4A and C), demonstrating that the signaling of EGFR to LRRK1 is independent of LRRK2. Conversely, LRRK1 knockdown did not influence the lack of EGF-induced recruitment LRRK2 to endosomes, suggesting that LRRK2 does not take over LRRK1 functions in EGF-positive endosomes after LRRK1 depletion. Equivalent observations were made using non-labeled EGF (Figure S3).
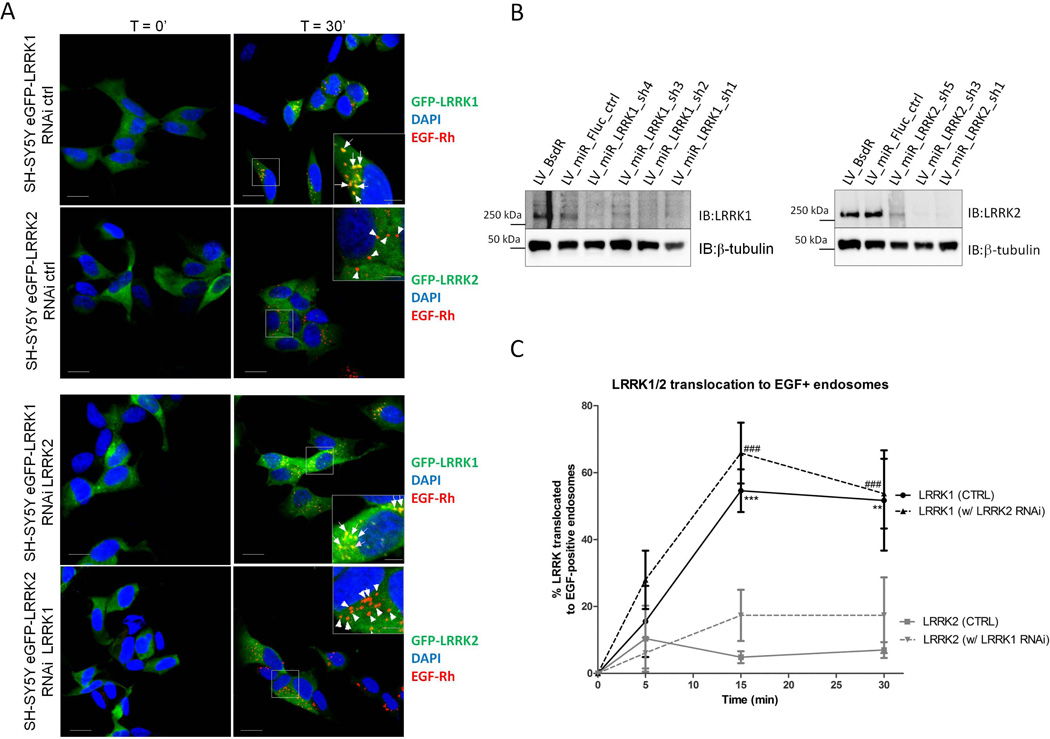
(A) Western blot showing LRRK1 and LRRK2 expression levels in control and knockdown cell lines. (B-C) Cell lines were treated with EGF-rhodamine (EGF-Rh) and endosomal EGF-Rh was imaged together with eGFP-LRRK1 or eGFP-LRRK2 in order to quantify translocation of LRRK1 or LRRK2 to EGF-positive endosomes. Depicted in panel (B) are representative confocal images with merged channels of DAPI, EGF-Rh and eGFP-LRRK in basal conditions as well as 30 minutes after EGF-Rh treatment, both for the control cell lines (RNAi ctrl, top 2 rows of images) as well as for the knockdown cell lines: eGFP-LRRK1 with LRRK2 knockdown (sh3 construct, third row of images) and eGFP-LRRK2 with LRRK1 knockdown (sh1 construct, bottom row of images). Arrows point to LRRK-positive, EGF-Rh-positive endosomes, triangles indicate LRRK-negative, EGF-Rh-positive endosomes. Translocation of eGFP-LRRK1 by non-labeled EGF is given in supplemental Figure S3. Scale bar, 10 μm for overview pictures and 5 μm for magnifications. (C) Quantification of the translocation LRRK1 or LRRK2 to EGF positive endosomes in the basal state, as well as after 5, 15 and 30 minutes of stimulation with EGF for the 4 different combinations tested was quantified as the % positive cells. Significance was assessed by 2-way ANOVA using experimental group (LRRK1 or LRRK2 cells, with control or knockdown of the partner LRRK) and time as factors, n=3 (statistically significant results after the Bonferroni post-hoc test comparing groups to the LRRK2 group are found in the LRRK1 control group: **: P<0,01, ***: P<0,001 as well as in the LRRK1 with LRRK2 RNAi group (###, P<0,001)).
LRRK2-IN1 treatment induces translocation of LRRK2 to filamentous structures independent of LRRK1
To ask whether LRRK1 was important for LRRK2 function, we used the relocalization of LRRK2 to filamentous structures upon treatment with LRRK2 kinase inhibitor (IN1) as a readout (Dzamko et al., 2010; Deng et al., 2011; Hermanson et al., 2012). The selected dose of 1 μM LRRK2-IN1 is a minimal dose at which a full cellular effect is obtained, as measured by LRRK2-IN1 induced dephosphorylation of LRRK2. In our cell lines, the cellular IC50 of LRRK2-IN1 is ~0,3-0,4 μM and 1 μM corresponds to the IC90 (Vancraenenbroeck et al., 2014). As expected, LRRK2-IN1 caused redistribution of LRRK2 in our stable cell lines, but did not affect LRRK1 localization (Figure 5). More importantly, genetic depletion of LRRK1 did not affect the LRRK2-IN1 induced translocation of LRRK2 (Figure 5B-C). These results show that LRRK2 interaction with 14-3-3 and functional effects in cells are independent of LRRK1.
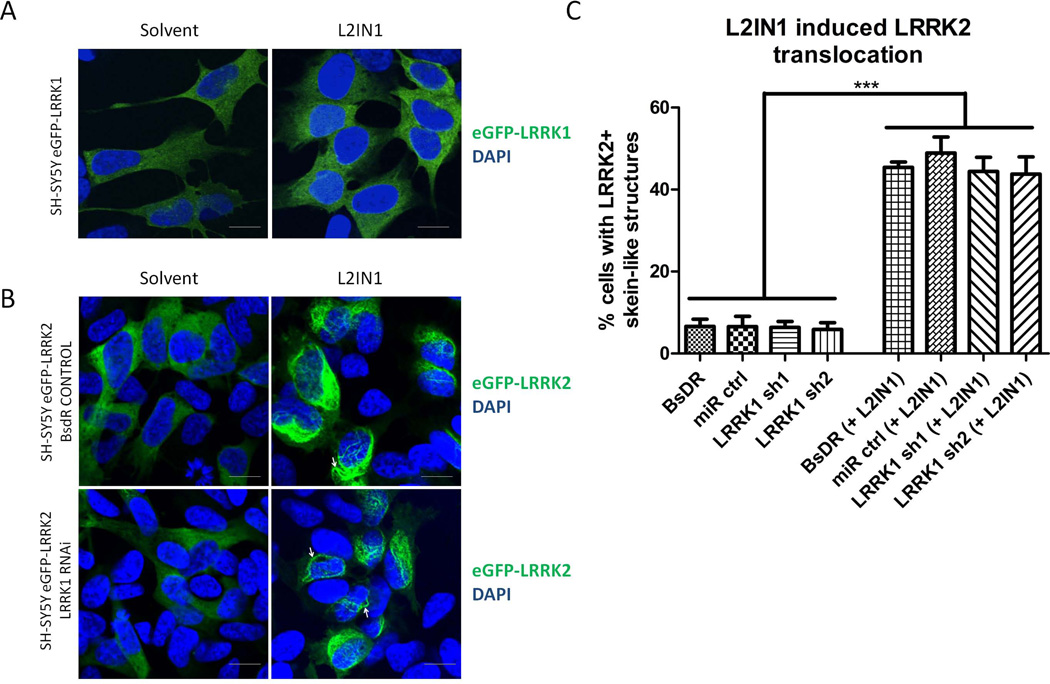
LRRK2-IN1 induced translocation of LRRK1 or LRRK2 to skein-like structures was assessed in SH-SY5Y cells stably expressing eGFP-LRRK1 or eGFP-LRRK2 and together with control shRNA sequences or sequences for shRNA mediated knockdown of LRRK1 or LRRK2. (A) Confocal images of eGFP-LRRK1 in SH-SY5Y cells after treatment with solvent or LRRK2-IN1 (1 μM, 90 minutes) showing diffuse cytoplasmic distribution of eGFP-LRRK1, unaltered by LRRK2-IN1. (B) Confocal images of eGFP-LRRK2 in SH-SY5Y cells after treatment with solvent or LRRK2-IN1 (1 μM, 90 minutes) showing diffuse cytoplasmic distribution of eGFP-LRRK2 in cells treated with solvent and translocation of eGFP-LRRK2 to skein-like structures by LRRK2-IN1 treatment, both in control cells (shown here are cells with empty vector containing selection marker, BsdR) as well as in cells with LRRK1 knockdown (sh1 construct). Scale bar, 10 μm applies to all photomicrographs. (C) Quantification of cells with LRRK2-positive skein-like structures in 2 control cell lines (cells with empty vector containing selection marker, BsdR, and expressing a control short hairpin, miR ctrl) as well as 2 cell lines with LRRK1 knockdown (sh1 construct and sh2 construct). Significance was assessed by One-way ANOVA, Dunnett post-hoc test, n=3, ***: P<0,001)
Discussion
In this study, we compared the cellular functions of LRRK1 and LRRK2 by identifying their interaction partners using two different proteomic screening approaches, protein microarray and AP-MS (Figure 1). Given that the nature of the analyzed complexes is different in the two approaches, the protein microarray interactions are assessed in vitro while AP-MS complexes are isolated from living cells, the combination of both approaches in parallel allows us to obtain a more complete picture of the proteins’ interactomes.
It should be noted though that isolated complexes are strongly dependent on the cell type or tissue that is used and this should also be taken into account for the interpretation of the results. Also, as for any screening methodology, results will include true hits as well as a certain proportion of false-positives and false-negatives. Therefore, results obtained with these screening experiments should be carefully interpreted and thoroughly validated. As a first validation step, we overexpressed LRRK1 or LRRK2 in HEK293T cell lines and tested their interaction (either on endogenous protein or after overexpression depending on the endogenous protein levels of the interactor tested) by CoIP. Our results confirm the LRRK1:EGF-R, LRRK2-14-3-3 interactions which were specific for each LRRK and Hsc70, BAG5 and Hsp90 as common interactors. Since the previously reported interactions between LRRK1:EGF-R and LRRK2:14-3-3 (Dzamko et al., 2010; Nichols et al., 2010; Titz et al., 2010; Hanafusa et al., 2011; Li et al., 2011) were the only ones identified with the two screening methodologies, we decided to focus on these two and study the potential crosstalk between LRRK1 and LRRK2 for these interactions.
Previous studies have reported that the phosphorylation status of LRRK2 is an important regulatory mechanism. LRRK2 is basally phosphorylated in cells (Greggio et al., 2007; Ito et al., 2007; Berger et al., 2010; Taymans et al., 2011; 2013) with several phosphosites clustering in a region between the ankyrin and leucine-rich repeat domains (S910, S935, S955 and S973) (Gloeckner et al., 2010; Nichols et al., 2010). These sites have generated much interest as their phosphorylation is reduced for several disease mutant forms of LRRK2 (Dzamko et al., 2010; Nichols et al., 2010; Li et al., 2011). Since the LRRK2:14-3-3 interaction is dependent on phosphorylation of LRRK2 at S910 and S935 (Nichols et al., 2010) we mapped LRRK1 and LRRK2 phosphosites in parallel and found that these map to distinct regions of the respective proteins: i.e. in the ANK, COR or kinase domains for LRRK1 and in the region linking the ankyrin repeat domain with the leucine-rich repeat domain for LRRK2 (Figure S1B). In particular, LRRK1 is not phosphorylated in the region between the ANK and LRR domains that mediates 14-3-3 binding in LRRK2, consistent with the lack of LRRK1 binding to 14-3-3. A further indication that LRRK1 and LRRK2 phosphosites are differentially regulated is their sensitivity to the LRRK2 IN-1 inhibitor (Deng et al., 2011). Treatment of cells in vitro with LRRK2-IN1 results in dephosphorylation of LRRK2 at the 14-3-3 binding sites of S910 and S935 (Dzamko et al., 2010; Deng et al., 2011). Here we show via metabolic labeling that LRRK1 phosphorylation is not significantly influenced by LRRK2-IN1 treatment, while LRRK2 was dephosphorylated under the same conditions (Figure 3). These observed differences between LRRK1 and LRRK2 suggest low LRRK1 off-target risks of LRRK2 inhibition. Inhibition of the kinase function of LRRK2 is considered to have therapeutic potential (reviewed in (Greggio and Singleton, 2007; Vancraenenbroeck et al., 2011)), and the fact that dephosphorylation following LRRK2 kinase inhibition occurs for LRRK2 but not LRRK1 might suggest that targeting LRRK2 by kinase inhibitors does not interfere with the normal function of LRRK1.
Formation of both LRRK1:EGF-R and LRRK2:14-3-3 complexes result in LRRK cellular translocation: LRRK1 traffics together with EGF-R to endosomes upon stimulation of the receptor with EGF (Hanafusa et al., 2011) while LRRK2 translocates to filamentous structures upon inhibitor-induced dephosphorylation and loss of 14-3-3 binding (Dzamko et al., 2010; Deng et al., 2011; Lobbestael et al., 2013). We exploited these cellular translocation properties to assess potential crosstalk between LRRK1 and LRRK2 cellular functioning. We first tested for overlap in the LRRK1 and LRRK2 translocation phenotypes after treatment with LRRK2-IN1 or EGF. We saw that only LRRK2, and not LRRK1, translocated after LRRK2-IN1 treatment. Similarly, treatment with EGF did not affect the localization of LRRK2 while it was sufficient to translocate LRRK1 to endosomes. Furthermore, to exclude that the partner LRRK co-regulates the translocation phenotype or that one LRRK may functionally take over the role of the partnering LRRK upon its depletion from the cell, we tested LRRK1 and LRRK2 translocation after silencing of the partnering LRRK by RNAi. Here also, LRRK1 and LRRK2 translocation patterns remained unchanged, allowing us to conclude that the LRRK1:EGF-R and LRRK2:14-3-3 interactions mediate distinct cellular processes for each LRRK.
Despite the absence of crosstalk between LRRK1 and LRRK2 for these interactions, our proteomic screenings revealed also a number of shared interactors (Figure 1C, Tables 1C and 1F). Out of these, we found several chaperone proteins such as Hsc70, Hsp90 (identified via AP-MS) and BAG proteins (identified using protein microarray) suggesting that the mechanisms regulating folding and stability of LRRK1 and LRRK2 are similar. Furthermore, several studies have shown the importance of proper folding for ROCO proteins, which engage in intramolecular interactions with domains with distinct folds (Greggio et al., 2008; Marín et al., 2008; Daniëls et al., 2011). Hsp90 is reported to assist up to 10% of all cellular proteins and is heavily involved in conformational regulation of signaling proteins, including kinases such as Raf1, Akt, cyclin dependent kinase 4 (Cdk4) (Pearl and Prodromou, 2006; Li et al., 2012) and LRRK2 (Wang et al., 2008).
Other shared interactors include proteins related to cytoskeleton such as tubulins (AP/MS) or to vesicular physiology such as the cyclin G activated kinase (GAK), the secretory protein chromogranin B or neurosecretory protein VGF. Both LRRKs have already been functionally linked to vesicular physiology however so far these appear to be in different vesicular processes, such as receptor trafficking in endosomes for LRRK1 and autophagy or Golgi-related functions for LRRK2 (Beilina et al., 2014). Therefore, although these data indicate that there is no link between LRRK2 and endosomal trafficking of EGFR, LRRK2 may be involved in other vesicular processes as it was shown to co-localize with synaptic vesicles in cell culture and animal models, interact with Rab5, an early endosomal marker, and functionally associate with the synaptic vesicle endocytosis protein Endophilin A (Moore, 2008; Shin et al., 2008; Higashi et al., 2009; Dodson et al., 2012; Matta et al., 2012). We also show that LRRK2 does not respond to EGF treatment. However, LRRK2 is reported to play a role in mitogen-activated protein kinase (MAPK) signaling cascades (Gloeckner et al., 2009; Hsu, Chan, Greggio, et al., 2010; Hsu, Chan, and Wolozin, 2010) which are downstream of growth factor receptors. Further work will be required to confirm these other interactions reported here and explore any functional links between LRRK1 and LRRK2 through these interactions.
In conclusion, our comparative cellular study shows that, although LRRK1 and LRRK2 have a number of common interactors, there is no evidence of signaling crosstalk between the LRRK2 proteins at the level of two LRRK-specific cellular interactions: LRRK1:EGF-R and LRRK2:14-3-3. Our work should stimulate further studies aiming at identifying similarities and differences in the cellular processes mediated by LRRK proteins, as these will provide further clues on the molecular mechanisms by which LRRK2 causes Parkinson’s disease and LRRK1 does not.
Acknowledgements
This study was supported by the Michael J. Fox Foundation. We thank the FWO-Vlaanderen (Research Foundation – Flanders)-project G.0666.09, research credit KAN2012 1.5.216.12 and fellowships to L.R., E.L. and J.-M.T., the IWT (Agency for Innovation by Science and Technology) SBO/100042, the Belspo IAP 7/24 grant and the KU Leuven [OT/08/052A and GOA 12/016] for their support.
M.G.D.G. was supported by P.O.R. SARDEGNA F.S.E. 2007-2013 - Obiettivo competitività regionale e occupazione, Asse IV Capitale umano, Linea di Attività l.3.1.
This research was also supported in part by the Intramural Research Program of the National Institutes of Health, National Institute on Aging and by the Fund Druwé-Eerdekens managed by the King Baudouin Foundation.
The financial support of Telethon – Italy (GGP12237) is gratefully acknowledged.
We thank Professor Johan Hofkens and Charlotte David (Molecular Imaging and Photonics, KU Leuven) for the use of the confocal laser scanning microscope. We also acknowledge the technical assistance of Fangye Gao and the Leuven Viral Vector Core (http://www.kuleuven.be/molmed/lvvc/vectorproduction.html) for the production of LV vectors.
References
- Beilina A, Rudenko IN, Alice K, Civiero L, Chau H, Kalia SK, Kalia LV, Lobbestael E, Chia R, Ndukwe K, Ding J, Nalls MA, International Parkinson's Disease Genomics Consortium, North American Brain Expression Consortium. Olszewski M, Hauser DN, Kumaran R, Lozano AM, Baekelandt V, Greene LE, Taymans J-M, Greggio E, Cookson MR. Unbiased screen for interactors of leucine-rich repeat kinase 2 supports a common pathway for sporadic and familial Parkinson disease. Proceedings of the National Academy of Sciences. 2014 [Europe PMC free article] [Abstract] [Google Scholar]
- Berger Z, Smith KA, LaVoie MJ. Membrane Localization of LRRK2 Is Associated with Increased Formation of the Highly Active LRRK2 Dimer and Changes in Its Phosphorylation. Biochemistry. 2010;49:5511–5523. [Europe PMC free article] [Abstract] [Google Scholar]
- Civiero L, Vancraenenbroeck R, Belluzzi E, Beilina A, Lobbestael E, Reyniers L, Fangye G, Micetic I, De Maeyer M, Bubacco L, Baekelandt V, Cookson MR, Greggio E, Taymans J-M. Biochemical characterization of highly purified leucine-rich repeat kinases 1 and 2 demonstrates formation of homodimers. PLoS ONE. 2012;7:e43472. [Europe PMC free article] [Abstract] [Google Scholar]
- Daniëls V, Baekelandt V, Taymans J-M. On the Road to Leucine-Rich Repeat Kinase 2 Signalling: Evidence from Cellular and in vivo Studies. Neurosignals. 2011;19:1–15. [Abstract] [Google Scholar]
- Daniëls V, Vancraenenbroeck R, Law BMH, Greggio E, Lobbestael E, Fangye G, De Maeyer M, Cookson MR, Harvey K, Baekelandt V, Taymans J-M. Insight into the mode of action of the LRRK2 Y1699C pathogenic mutant. Journal of Neurochemistry. 2010;116:304–315. [Europe PMC free article] [Abstract] [Google Scholar]
- Davies P, Hinkle KM, Sukar NN, Sepulveda B, Mesias R, Serrano G, Alessi DR, Beach TG, Benson DL, White CL, Cowell RM, Das SS, West AB, Melrose HL. Comprehensive characterization and optimization of anti-LRRK2 (leucine-rich repeat kinase 2) monoclonal antibodies. Biochem. J. 2013;453:101–113. [Europe PMC free article] [Abstract] [Google Scholar]
- Dächsel JC, Nishioka K, Vilariño-Güell C, Lincoln SJ, Soto-Ortolaza AI, Kachergus J, Hinkle KM, Heckman MG, Jasinska-Myga B, Taylor JP, Dickson DW, Gibson RA, Hentati F, Ross OA, Farrer MJ. Heterodimerization of Lrrk1–Lrrk2: Implications for LRRK2-associated Parkinson disease. Mechanisms of Ageing and Development. 2010;131:210–214. [Europe PMC free article] [Abstract] [Google Scholar]
- Deng X, Dzamko N, Prescott A, Davies P, Liu Q, Yang Q, Lee J-D, Patricelli MP, Nomanbhoy TK, Alessi DR, Gray NS. Characterization of a selective inhibitor of the Parkinson's disease kinase LRRK2. Nature Chemical Biology. 2011;7:203–205. [Europe PMC free article] [Abstract] [Google Scholar]
- Dodson MW, Zhang T, Jiang C, Chen S, Guo M. Roles of the Drosophila LRRK2 homolog in Rab7-dependent lysosomal positioning. Human Molecular Genetics. 2012;21:1350–1363. [Europe PMC free article] [Abstract] [Google Scholar]
- Dzamko N, Deak M, Hentati F, Reith AD, Prescott AR, Alessi DR, Nichols RJ. Inhibition of LRRK2 kinase activity leads to dephosphorylation of Ser 910/Ser 935, disruption of 14-3-3 binding and altered cytoplasmic localization. Biochem. J. 2010;430:405–413. [Europe PMC free article] [Abstract] [Google Scholar]
- Gajadhar A, Guha A. A proximity ligation assay using transiently transfected, epitope-tagged proteins: application for in situ detection of dimerized 30 receptor tyrosine kinases. BioTechniques. 2010;48:145–152. [Abstract] [Google Scholar]
- Gloeckner CJ, Boldt K, Zweydorf, von F, Helm S, Wiesent L, Sarioglu H, Ueffing M. Phosphopeptide Analysis Reveals Two Discrete Clusters of Phosphorylation in the N-Terminus and the Roc Domain of the Parkinson-Disease Associated Protein Kinase LRRK2. J. Proteome Res. 2010;9:1738–1745. [Abstract] [Google Scholar]
- Gloeckner CJ, Schumacher A, Boldt K, Ueffing M. The Parkinson disease-associated protein kinase LRRK2 exhibits MAPKKK activity and phosphorylates MKK3/6 and MKK4/7, in vitro. Journal of Neurochemistry. 2009;109:959–968. [Abstract] [Google Scholar]
- Greggio E, Lewis PA, van der Brug MP, Ahmad R, Alice K, Ding J, Beilina A, Baker AK, Cookson MR. Mutations in LRRK2/dardarin associated with Parkinson disease are more toxic than equivalent mutations in the homologous kinase LRRK1. Journal of Neurochemistry. 2007;102:93–102. [Abstract] [Google Scholar]
- Greggio E, Singleton A. Kinase signaling pathways as potential targets in the treatment of Parkinson's disease. Expert Rev Proteomics. 2007;4:783–792. [Abstract] [Google Scholar]
- Greggio E, Zambrano I, Alice K, Beilina A, Taymans J-M, Daniëls V, Lewis P, Jain S, Ding J, Syed A, Thomas KJ, Baekelandt V, Cookson MR. The Parkinson disease-associated leucine-rich repeat kinase 2 (LRRK2) is a dimer that undergoes intramolecular autophosphorylation. Journal of Biological Chemistry. 2008;283:16906–16914. [Europe PMC free article] [Abstract] [Google Scholar]
- Hanafusa H, Ishikawa K, Kedashiro S, Saigo T, Iemura S-I, Natsume T, Komada M, Shibuya H, Nara A, Matsumoto K. Leucine-rich repeat kinase LRRK1 regulates endosomal trafficking of the EGF receptor. Nat Comms. 2011;2:158. [Europe PMC free article] [Abstract] [Google Scholar]
- Haugarvoll K, Rademakers R, Kachergus JM, Nuytemans K, Ross OA, Gibson JM, Tan EK, Gaig C, Tolosa E, Goldwurm S, Guidi M, Riboldazzi G, Brown L, Walter U, Benecke R, Berg D, Gasser T, Theuns J, Pals P, Cras P, De Deyn PP, Engelborghs S, Pickut B, Uitti RJ, Foroud T, Nichols WC, Hagenah J, Klein C, Samii A, Zabetian CP, Bonifati V, Van Broeckhoven C, Farrer MJ, Wszolek ZK. Lrrk2 R1441C parkinsonism is clinically similar to sporadic Parkinson disease. Neurology. 2008;70:1456–1460. [Europe PMC free article] [Abstract] [Google Scholar]
- Haugarvoll K, Toft M, Ross OA, White LR, Aasly JO, Farrer MJ. Variants in the LRRK1 gene and susceptibility to Parkinson's disease in Norway. Neuroscience Letters. 2007;416:299–301. [Abstract] [Google Scholar]
- Healy DG, Falchi M, O'Sullivan SS, Bonifati V, Durr A, Bressman S, Brice A, Aasly J, Zabetian CP, Goldwurm S, Ferreira JJ, Tolosa E, Kay DM, Klein C, Williams DR, Marras C, Lang AE, Wszolek ZK, Berciano J, Schapira AH, Lynch T, Bhatia KP, Gasser T, Lees AJ, Wood NW. Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson's disease: a case-control study. The Lancet Neurology. 2008;7:583–590. [Europe PMC free article] [Abstract] [Google Scholar]
- Hermanson SB, Carlson CB, Riddle SM, Zhao J. Screening for novel LRRK2 inhibitors using a high-throughput TR-FRET cellular assay for LRRK2 Ser935 phosphorylation. PLoS ONE. 2012;7 [Europe PMC free article] [Abstract] [Google Scholar]
- Higashi S, Moore DJ, Yamamoto R, Minegishi M, Sato K, Togo T, Katsuse O, Uchikado H, Furukawa Y, Hino H, Kosaka K, Emson PC, Wada K, Dawson VL, Dawson TM, Arai H, Iseki E. Abnormal localization of leucine-rich repeat kinase 2 to the endosomal-lysosomal compartment in lewy body disease. J. Neuropathol. Exp. Neurol. 2009;68:994–1005. [Europe PMC free article] [Abstract] [Google Scholar]
- Hsu CH, Chan D, Greggio E, Saha S, Guillily MD, Ferree A, Raghavan K, Shen GC, Segal L, Ryu H, Cookson MR, Wolozin B. MKK6 binds and regulates expression of Parkinson’s disease-related protein LRRK2. Journal of Neurochemistry. 2010;112:1593–1604. [Europe PMC free article] [Abstract] [Google Scholar]
- Hsu CH, Chan D, Wolozin B. LRRK2 and the Stress Response: Interaction with MKKs and JNK-Interacting Proteins. Neurodegenerative Dis. 2010;7:68–75. [Europe PMC free article] [Abstract] [Google Scholar]
- Ito G, Okai T, Fujino G, Takeda K, Ichijo H, Katada T, Iwatsubo T. GTP Binding Is Essential to the Protein Kinase Activity of LRRK2, a Causative Gene Product for Familial Parkinson's Disease. Biochemistry. 2007;46:1380–1388. [Abstract] [Google Scholar]
- Klein CL, Rovelli G, Springer W, Schall C, Gasser T, Kahle PJ. Homo- and heterodimerization of ROCO kinases: LRRK2 kinase inhibition by the LRRK2 ROCO fragment. Journal of Neurochemistry. 2009;111:703–715. [Abstract] [Google Scholar]
- Law BMH, Spain VA, Leinster VHL, Chia R, Beilina A, Cho HJ, Taymans J-M, Urban MK, Sancho RM, Ramírez MB, Biskup S, Baekelandt V, Cai H, Cookson MR, Berwick DC, Harvey K. A Direct Interaction between Leucine-rich Repeat Kinase 2 and Specific β-Tubulin Isoforms Regulates Tubulin Acetylation. J. Biol. Chem. 2014;289:895–908. [Europe PMC free article] [Abstract] [Google Scholar]
- Lewis PA. The function of ROCO proteins in health and disease. Biology of the Cell. 2009;101:183–191. [Abstract] [Google Scholar]
- Li J, Soroka J, Buchner J. The Hsp90 chaperone machinery: Conformational dynamics and regulation by co-chaperones. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2012;1823:624–635. [Abstract] [Google Scholar]
- Li X, Wang QJ, Pan N, Lee S, Zhao Y, Chait BT, Yue Z. Phosphorylation-Dependent 14-3-3 Binding to LRRK2 Is Impaired by Common Mutations of Familial Parkinson's Disease. PLoS ONE. 2011;6:e17153. [Europe PMC free article] [Abstract] [Google Scholar]
- Lobbestael E, Zhao J, Rudenko IN, Beylina A, Fangye G, Wetter J, Beullens M, Bollen M, Cookson MR, Baekelandt V, Nichols RJ, Taymans J-M. Identification of protein phosphatase 1 as a regulator of the LRRK2 phosphorylation cycle. Biochem. J. 2013;456:119–128. [Europe PMC free article] [Abstract] [Google Scholar]
- Marín I. The Parkinson Disease Gene LRRK2: Evolutionary and Structural Insights. Molecular Biology and Evolution. 2006;23:2423–2433. [Abstract] [Google Scholar]
- Marín I. Ancient Origin of the Parkinson Disease Gene LRRK2. J Mol Evol. 2008;67:41–50. [Abstract] [Google Scholar]
- Marín I, van Egmond WN, Van Haastert PJM. The Roco protein family: a functional perspective. 2008 [Abstract] [Google Scholar]
- Matta S, Van Kolen K, da Cunha R, van den Bogaart G, Mandemakers W, Miskiewicz K, De Bock P-J, Morais VA, Vilain S, Haddad D, Delbroek L, Swerts J, Chávez-Gutiérrez L, Esposito G, Daneels G, Karran E, Holt M, Gevaert K, Moechars DW, De Strooper B, Verstreken P. LRRK2 controls an EndoA phosphorylation cycle in synaptic endocytosis. Neuron. 2012;75:1008–1021. [Abstract] [Google Scholar]
- Mellacheruvu D, Wright Z, Couzens AL, Lambert JP. The CRAPome: a contaminant repository for affinity purification-mass spectrometry data. Nature. 2013 [Europe PMC free article] [Abstract] [Google Scholar]
- Michaelis M, Bliss J, Arnold SC, Hinsch N, Rothweiler F, Deubzer HE, Witt O, Langer K, Doerr HW, Wels WS, Cinatl J. Cisplatin-Resistant Neuroblastoma Cells Express Enhanced Levels of Epidermal Growth Factor Receptor (EGFR) and Are Sensitive to Treatment with EGFR-Specific Toxins. Clinical Cancer Research. 2008;14:6531–6537. [Abstract] [Google Scholar]
- Moore DJ. The biology and pathobiology of LRRK2: implications for Parkinson's disease. Parkinsonism & Related Disorders. 2008;14(Suppl 2):S92–S98. [Abstract] [Google Scholar]
- Nichols RJ, Dzamko N, Morrice NA, Campbell DG, Deak M, Ordureau A, Macartney T, Tong Y, Shen J, Prescott AR, Alessi DR. 14-3-3 binding to LRRK2 is disrupted by multiple Parkinson's disease-associated mutations and regulates cytoplasmic localization. Biochem. J. 2010;430:393–404. [Europe PMC free article] [Abstract] [Google Scholar]
- Osório L, Gijsbers R, Oliveras-Salvá M, Michiels A, Debyser Z, Van den Haute C, Baekelandt V. Viral vectors expressing a single microRNA-based short-hairpin RNA result in potent gene silencing in vitro and in vivo. Journal of Biotechnology. 2013;169:71–81. [Abstract] [Google Scholar]
- Paisan-Ruiz C, Jain S, Evans EW, Gilks WP, Simón J, van der Brug M, López de Munain A, Aparicio S, Gil AM, Khan N, Johnson J, Martinez JR, Nicholl D, Carrera IM, Pena AS, de Silva R, Lees A, Marti-Masso J-F, Pérez-Tur J, Wood NW, Singleton AB. Cloning of the gene containing mutations that cause PARK8-linked Parkinson's disease. Neuron. 2004;44:595–600. [Abstract] [Google Scholar]
- Parisiadou L, Xie C, Cho HJ, Lin X, Gu X-L, Long C-X, Lobbestael E, Baekelandt V, Taymans J-M, Sun L, Cai H. Phosphorylation of ezrin/radixin/moesin proteins by LRRK2 promotes the rearrangement of actin cytoskeleton in neuronal morphogenesis. Journal of Neuroscience. 2009;29:13971–13980. [Europe PMC free article] [Abstract] [Google Scholar]
- Pearl LH, Prodromou C. Structure and mechanism of the Hsp90 molecular chaperone machinery. Annu. Rev. Biochem. 2006;75:271–294. [Abstract] [Google Scholar]
- Ross OA, Soto-Ortolaza AI, Heckman MG, Aasly JO, Abahuni N, Annesi G, Bacon JA, Bardien S, Bozi M, Brice A, Brighina L, Van Broeckhoven C, Carr J, Chartier-Harlin M-C, Dardiotis E, Dickson DW, Diehl NN, Elbaz A, Ferrarese C, Ferraris A, Fiske B, Gibson JM, Gibson R, Hadjigeorgiou GM, Hattori N, Ioannidis JPA, Jasinska-Myga B, Jeon BS, Kim YJ, Klein C, Krüger R, Kyratzi E, Lesage S, Lin C-H, Lynch T, Maraganore DM, Mellick GD, Mutez E, Nilsson C, Opala G, Park SS, Puschmann A, Quattrone A, Sharma M, Silburn PA, Sohn YH, Stefanis L, Tadic V, Theuns J, Tomiyama H, Uitti RJ, Valente EM, van de Loo S, Vassilatis DK, Vilariño-Güell C, White LR, Wirdefeldt K, Wszolek ZK, Wu R-M, Farrer MJ Genetic Epidemiology Of Parkinson's Disease (GEO-PD) Consortium. Association of LRRK2 exonic variants with susceptibility to Parkinson's disease: a case-control study. Lancet Neurol. 2011;10:898–908. [Europe PMC free article] [Abstract] [Google Scholar]
- Satake W, Nakabayashi Y, Mizuta I, Hirota Y, Ito C, Kubo M, Kawaguchi T, Tsunoda T, Watanabe M, Takeda A, Tomiyama H, Nakashima K, Hasegawa K, Obata F, Yoshikawa T, Kawakami H, Sakoda S, Yamamoto M, Hattori N, Murata M, Nakamura Y, Toda T. Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson's disease. Nat Genet. 2009;41:1303–1307. [Abstract] [Google Scholar]
- Shin N, Jeong H, Kwon J, Heo HY, Kwon JJ, Yun HJ, Kim C-H, Han BS, Tong Y, Shen J, Hatano T, Hattori N, Kim K-S, Chang S, Seol W. LRRK2 regulates synaptic vesicle endocytosis. Experimental Cell Research. 2008;314:2055–2065. [Abstract] [Google Scholar]
- Simón-Sánchez J, Schulte C, Bras JM, Sharma M, Gibbs JR, Berg D, Paisan-Ruiz C, Lichtner P, Scholz SW, Hernandez DG, Krüger R, Federoff M, Klein C, Goate A, Perlmutter J, Bonin M, Nalls MA, Illig T, Gieger C, Houlden H, Steffens M, Okun MS, Racette BA, Cookson MR, Foote KD, Fernandez HH, Traynor BJ, Schreiber S, Arepalli S, Zonozi R, Gwinn K, van der Brug M, Lopez G, Chanock SJ, Schatzkin A, Park Y, Hollenbeck A, Gao J, Huang X, Wood NW, Lorenz D, Deuschl G, Chen H, Riess O, Hardy JA, Singleton AB, Gasser T. Genome-wide association study reveals genetic risk underlying Parkinson's disease. Nat Genet. 2009;41:1308–1312. [Europe PMC free article] [Abstract] [Google Scholar]
- Taylor JP, Hulihan MM, Kachergus JM, Melrose HL, Lincoln SJ, Hinkle KM, Stone JT, Ross OA, Hauser R, Aasly J, Gasser T, Payami H, Wszolek ZK, Farrer MJ. Leucine-rich repeat kinase 1: a paralog of LRRK2 and a candidate gene for Parkinson’s disease. Neurogenetics. 2007;8:95–102. [Abstract] [Google Scholar]
- Taymans J-M, Fangye G, Baekelandt V. Metabolic Labeling of Leucine Rich Repeat Kinases 1 and 2 with Radioactive Phosphate. Journal of Visualized Experiments (JoVE) 2013 [Europe PMC free article] [Abstract] [Google Scholar]
- Taymans J-M, Vancraenenbroeck R, Ollikainen P, Beilina A, Lobbestael E, De Maeyer M, Baekelandt V, Cookson MR. LRRK2 Kinase Activity Is Dependent on LRRK2 GTP Binding Capacity but Independent of LRRK2 GTP Binding. PLoS ONE. 2011;6:e23207. [Europe PMC free article] [Abstract] [Google Scholar]
- Titz B, Low T, Komisopoulou E, Chen SS, Rubbi L, Graeber TG. The proximal signaling network of the BCR-ABL1 oncogene shows a modular organization. Oncogene. 2010;29:5895–5910. [Europe PMC free article] [Abstract] [Google Scholar]
- Vancraenenbroeck R, De Raeymaecker J, Lobbestael E, Fangye G, De Maeyer M, Voet A, Baekelandt V, Taymans J-M. In silico, in vitro and cellular analysis with a kinome-wide inhibitor panel correlates cellular LRRK2 dephosphorylation to inhibitor activity on LRRK2. Front. Mol. Neurosci. 2014;7 [Europe PMC free article] [Abstract] [Google Scholar]
- Vancraenenbroeck R, Lobbestael E, Maeyer MD, Baekelandt V, Taymans J-M. Kinases as targets for Parkinson's disease: from genetics to therapy. CNS Neurol Disord Drug Targets. 2011;10:724–740. [Abstract] [Google Scholar]
- Vancraenenbroeck R, Lobbestael E, Weeks SD, Strelkov SV, Baekelandt V, Taymans J-M, De Maeyer M. Expression, purification and preliminary biochemical and structural characterization of the leucine rich repeat namesake domain of leucine rich repeat kinase 2. Biochim. Biophys. Acta. 2012;1824:450–460. [Abstract] [Google Scholar]
- Wang L, Xie C, Greggio E, Parisiadou L, Shim H, Sun L, Chandran J, Lin X, Lai C, Yang WJ, Moore DJ, Dawson TM, Dawson VL, Chiosis G, Cookson MR, Cai H. The Chaperone Activity of Heat Shock Protein 90 Is Critical for Maintaining the Stability of Leucine-Rich Repeat Kinase 2. Journal of Neuroscience. 2008;28:3384–3391. [Europe PMC free article] [Abstract] [Google Scholar]
- Yaffe MB, Rittinger K, Volinia S, Caron PR, Aitken A, Leffers H, Gamblin SJ, Smerdon SJ, Cantley LC. The Structural Basis for 14-3-3:Phosphopeptide Binding Specificity. Cell. 1997;91:961–971. [Abstract] [Google Scholar]
- Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S, Kachergus J, Hulihan M, Uitti RJ, Calne DB, Stoessl AJ, Pfeiffer RF, Patenge N, Carbajal IC, Vieregge P, Asmus F, Müller-Myhsok B, Dickson DW, Meitinger T, Strom TM, Wszolek ZK, Gasser T. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–607. [Abstract] [Google Scholar]
Full text links
Read article at publisher's site: https://doi.org/10.1111/jnc.12798
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc4272680?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Protein Translation in the Pathogenesis of Parkinson's Disease.
Int J Mol Sci, 25(4):2393, 18 Feb 2024
Cited by: 1 article | PMID: 38397070 | PMCID: PMC10888601
Review Free full text in Europe PMC
Network Analysis Performed on Transcriptomes of Parkinson's Disease Patients Reveals Dysfunction in Protein Translation.
Int J Mol Sci, 25(2):1299, 21 Jan 2024
Cited by: 2 articles | PMID: 38279299 | PMCID: PMC10816150
PAK6-mediated phosphorylation of PPP2R2C regulates LRRK2-PP2A complex formation.
Front Mol Neurosci, 16:1269387, 18 Dec 2023
Cited by: 1 article | PMID: 38169846 | PMCID: PMC10759229
Structure and regulation of full-length human leucine-rich repeat kinase 1.
Nat Commun, 14(1):4797, 09 Aug 2023
Cited by: 0 articles | PMID: 37558661 | PMCID: PMC10412621
PKC isoforms activate LRRK1 kinase by phosphorylating conserved residues (Ser1064, Ser1074 and Thr1075) within the CORB GTPase domain.
Biochem J, 479(18):1941-1965, 01 Sep 2022
Cited by: 4 articles | PMID: 36040231 | PMCID: PMC9555798
Go to all (35) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
RefSeq - NCBI Reference Sequence Database (2)
- (1 citation) RefSeq - NM_198578.3
- (1 citation) RefSeq - NM_024652
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Deciphering the LRRK code: LRRK1 and LRRK2 phosphorylate distinct Rab proteins and are regulated by diverse mechanisms.
Biochem J, 478(3):553-578, 01 Feb 2021
Cited by: 22 articles | PMID: 33459343 | PMCID: PMC7886321
Biochemical characterization of highly purified leucine-rich repeat kinases 1 and 2 demonstrates formation of homodimers.
PLoS One, 7(8):e43472, 29 Aug 2012
Cited by: 64 articles | PMID: 22952686 | PMCID: PMC3430690
Human leucine-rich repeat kinase 1 and 2: intersecting or unrelated functions?
Biochem Soc Trans, 40(5):1095-1101, 01 Oct 2012
Cited by: 11 articles | PMID: 22988872
Review
Cryo-EM analysis of homodimeric full-length LRRK2 and LRRK1 protein complexes.
Sci Rep, 7(1):8667, 17 Aug 2017
Cited by: 26 articles | PMID: 28819229 | PMCID: PMC5561129
Funding
Funders who supported this work.
Belspo (1)
Grant ID: IAP 7/24
CARIPLO foundation
FWO-Vlaanderen (2)
Grant ID: G.0666.09
Grant ID: KAN2012 1.5.216.12
IWT (1)
Grant ID: SBO/100042
Intramural NIH HHS (1)
Grant ID: Z01 AG000948-01
KU Leuven (2)
Grant ID: OT/08/052A
Grant ID: GOA 12/016
King Baudouin Foundation
MIUR
Michael J. Fox Foundation
National Institute on Aging
National Institutes of Health
P.O.R. SARDEGNA (1)
Grant ID: F.S.E. 2007-2013
Telethon (1)
Function and dysfunction of the Parkinson's disease kinase LRRK2 at the pre-synaptic site
Prof.ssa Elisa Greggio, Università di Padova, Dipartimento di Biologia - Università di Padova
Grant ID: GGP12237
Telethon - Italy (1)
Grant ID: GGP12237





