Abstract
Free full text

Regulation of Tumor Metastasis by Myeloid-derived Suppressor Cells
Abstract
Accumulation of pathologically activated immature myeloid cells with potent immune-suppressive activity is one of the major immunological hallmarks of cancer. In recent years it became clear that in addition to their immune-suppressive activity, MDSC influence tumor progression in a variety of ways. They are directly implicated in the promotion of tumor metastases by participating in the formation of pre-metastatic niche, promoting angiogenesis and tumor cell invasion. In this review we discuss recent data describing various roles of MDSC in formation of tumor metastases.
INTRODUCTION
Myeloid cells are one of the largest groups of hematopoietic cells. They are comprised of mature terminally differentiated cells: polymorphonuclear neutrophils (PMN) and other granulocytes, macrophages, and dendritic cells (DCs), as well as relatively immature cells: monocytes and granulocytic precursors. During the last decade it became clear that this hierarchical system is not functional in cancer. Abnormal differentiation of myeloid compartment is now considered one of the major immunological hallmarks of cancer. As a result, tumor-bearing mice and cancer patients accumulate immunosuppressive macrophages with enhanced ability to promote angiogenesis and tumor cell invasion, as well as ineffective antigen presenting DCs in some cases able to directly inhibit immune responses. However, the most prominent changes in the myeloid compartment in cancer is the expansion of pathologically activated immature myeloid cells with the potent ability to suppress immune responses (1). Although these cells were observed in tumor-bearing hosts since the 1970s, their true biological role became appreciated only 15 years ago. These cells are now termed myeloid-derived suppressor cells (MDSC) to reflect their origin and major function (2). It has become clear that MDSC are not only an important element of negative regulation of immune responses in many pathologic conditions, but also contribute greatly to other aspects of tumor growth. In recent years, MDSC were directly implicated in the promotion of tumor metastasis. In this review we will briefly discuss the main features of MDSC, and in more details recent data describing important roles of these cells in tumor metastasis.
MAIN CHARACTERISTICS OF MDSC
Markers and subsets of mouse MDSC
MDSC represent a heterogeneous population of myeloid cells at different stages of differentiation (3). In mice, MDSC are generally characterized by co-expression of myeloid lineage differentiation markers, Gr-1 and CD11b (4). It is now established that MDSC consist of two major groups of cells with mononuclear and polymorphonuclear morphology. These cells can be identified with a combination of specific markers. Polymorphonuclear MDSC (PMNMDSC) are defined as CD11b+Ly6ClowLy6G+ cells and mononuclear MDSC (M-MDSC) as CD11b+Ly6ChighLy6G− cells (5, 6). PMN-MDSC are the largest population of MDSC in tumor-bearing mice, representing more than 80% of all MDSC.
PMN in tumor-free mice and PMN-MDSC in tumor-bearing mice have similar morphology and phenotype (4, 7). However, they have many distinctive features. In contrast to PMN, PMNMDSC inhibited antigen-specific T cell responses and a substantial proportion of PMN-MDSC expressed CD244 and M-CSFR. PMN had significantly higher phagocytic activity, expression of lysosomal proteins, and TNF-α production than PMN-MDSC. In contrast, PMN-MDSC had higher activity of arginase 1 (arg-1), myeloperoxidase and ROS production than PMN. Within 24 hours, in culture with GM-CSF, PMN-MDSC acquired all characteristics of PMN, and these cells became phenotypically and functionally undistinguishable (8).
M-MDSC share the phenotype and morphology with CD11b+Ly6ChighLy6G− inflammatory monocytes. In contrast to PMN-MDSC, M-MDSC are proliferative cells (9). In tumor site they preferentially differentiate into immunosuppressive macrophages (10). The distinctive feature of M-MDSC is their immune-suppressive activity. A substantial proportion of M-MDSC, in contrast to monocytes, differentiates into PMN-MDSC (9). This effect appears to be controlled by epigenetic silencing of retinoblastoma (rb1) tumor suppressor genes. Tumor explant supernatants can induce the differentiation of monocytes into PMN type of cells in vitro, suggesting that monocytes can be reprogrammed by tumor-derived factors rather than representing a separate developmental pathway (9).
MDSC in cancer patients: the phenotype and clinical relevance
During the last decade, accumulation of MDSC has been reported in a large number of cancers (11). Historically, human MDSC were defined as lineage markers (CD3, CD14, CD19, CD56) negative, HLA-DR negative, and common myeloid marker CD33 positive cells co-purified with mononuclear cells on ficoll gradient (2). More recently, the existence of two subsets of cells (similar to murine models) has been reported in cancer patients, and PMN-MDSC are commonly characterized as CD11b+CD14− cells expressing a granulocytic marker: CD15 or CD66b (12, 13). M-MDSC are defined by two combinations of markers: CD11b+CD14−CD15− (or CD66b−); or CD11b+CD14+HLA-DRlo (14, 15). It is important to point out that similar to mouse models, PMN-MDSC represent the majority of MDSC in most types of human cancer.
Despite the fact that accumulation of MDSC in cancer patients is widely appreciated (16), the clinical relevance of MDSC accumulation remains a work in progress. In recent years, a substantial number of studies have shown a correlation between the level of MDSC and stage, overall survival, and response to therapy. Accumulation of circulating MDSC correlated with the stage in patients with solid tumors (mainly breast cancer) (17), gastric cancer (18) and colorectal cancer (19, 20). MDSC accumulation in tumor sites (both primary and metastatic) has also been shown to correlate with the overall survival as well as the disease-free survival in patients with ovarian cancer (21). Increased level of PMN-MDSC was detected in patients with pancreatic cancer (22) and renal cell carcinoma (23). Furthermore, in patients with small-cell lung cancer, circulating MDSC negatively correlated with the immune response to cancer vaccine (24). Targeting MDSC in these patients substantially improved antigen-specific immune responses to vaccination (25). More recently, the clinical relevance of M-MDSC accumulation in cancer patients has been reported. The presence of circulating M-MDSC was reported to correlate with the stage of hepatocellular carcinoma (26). The accumulation of M-MDSC has also been reported to correlate with the progression-free survival and the response to chemotherapy, as well as metastatic burden in melanoma and non-small cell lung cancer patient (27-29).
MECHANISMS OF MDSC EXPANSION AND IMMUNE SUPPRESSION
MDSC expansion
The mechanisms of regulating MDSC expansion were covered in other reviews (4, 30) and will not be discussed in details here. It is important to point out that expansion of MDSC in cancer is largely driven by soluble tumor-derived factors. These factors include prostaglandins, granulocyte-macrophage colony-stimulating factor (GM-CSF), macrophage colony-stimulating factor (M-CSF), IL-1β, IL-6, VEGF, TGFβ, IL-10, IL-12, IL-13, and others (4). Most of these factors activate signaling cascades involving Janus tyrosine kinase (JAK) protein family members, and signal transducer and activator of transcription 3 (STAT3) (31). Several downstream targets include S100A8 and S100A9 proteins (32) and CCAAT-enhancer-binding protein beta (C/EBPβ) (33). Other mechanisms include interferon regulatory factor-8 (IRF-8) (34), myeloid differentiation primary response gene 88 (MyD88) and NF-κB (35, 36), prostaglandin E2 (PGE2) (37, 38), TNF (39) and others.
MDSC mediated immune suppression
Numerous studies have established the potent immune-suppressive mechanisms of MDSC (4). Historically, first mechanisms of MDSC suppression included reactive oxygen species (ROS), arg-1, and nitric oxide (NO). More recently, it has been shown that peroxynitrite (PNT), the product of interaction of superoxide and NO could cause nitration of T cell receptor-CD8 complex, which reduced its binding to the peptide MHC class I (pMHC) complex, and rendered T cells unresponsive to antigen-specific stimulation (40). PNT also hampered the recognition of cancer cells by cytotoxic T lymphocytes (41). Accelerated depletion of L-arginine and cysteine in the tumor microenvironment caused by MDSC resulted in decreased CD3ζ chain expression, diminished production of IL-2 and IFN-γ, and inhibited T cell proliferation (42-44).
The two populations of MDSC employ different mechanisms of immune suppression. PMNMDSC produce high levels of ROS and an undetectable amount of NO; whereas M-MDSC have high levels of NO, but undetectable ROS (5). Both populations use arg-1 for their suppressive activity.
Several studies showed the ability of MDSC to induce differentiation and/or proliferation of Foxp3+ Tregs using various mechanisms including TGF-β (45, 46), or CD40 (47). However, one other study showed that MDSC-mediated Treg induction was TGF-β independent but required arg-1 (48). MDSC also have the ability to recruit Tregs to the tumor site in a CCR5 dependent manner (49). Interestingly, this Tregs induction ability seems to be restricted to the M-MDSC subset (50). In contrast, PMN-MDSC did not promote Treg differentiation, but actually have the ability to impair TGF-β induced Treg generation or proliferation (51).
IL-17 could be involved in the immune-suppressive function of MDSC in mammary carcinoma model. IL-17 increased the immune-suppressive function of MDSC through the up-regulation of arg-1, MMP-9, indoleamine 2,3-dioxygenase (IDO), and COX-2 (52). Transmembrane but not secreted TNF-α enhanced suppressive activity of MDSC by up-regulating arg-1 and inducible NO synthase (iNOS), promoting secretion of NO, ROS, IL-10, and TGF-β (53).
The nature of immune suppression by MDSC can be defined by the local microenvironment. MDSC from tumor tissues suppressed both antigen-specific and non-specific T cell activity, whereas on the periphery, antigen-specific suppression was more prevalent (10). Exposure of splenic MDSC to hypoxia resulted in the conversion of these cells to non-specific suppressors, and hypoxia-inducible factor (HIF-1α) was found to be primarily involved in the observed effects (10).
MDSC ROLE IN TUMOR METASTASIS
In order to metastasize, tumors need to invade the surrounding tissue, enter the circulation, seed and proliferate in a distant permissive niche. There is increasing evidence that MDSC play an important role in all steps leading to metastasis. Although the immune-suppressive activity of MDSC is critically important for the formation of metastatic niche, these cells employ a number of other mechanisms promoting metastases. Clinical data support possible role of MDSC in metastasis. In non-small cell lung cancer (NSCLC) patients, circulating CD14+HLA-DRlow MMDSC correlated with extra-thoracic metastases (28). Increase in IDO-expressing CD45+CD33+CD14−CD15− MDSC in breast cancer tissue also correlated with increased lymph node metastasis in breast cancer patients (54). In patients with melanoma, the development of metastases and poor survival was associated with increase in both circulating CD11b+CD14-CD15+ PMN-MDSC (55) and M-MDSC (56).
MDSC migration to the tumor site or pre-metastatic niche
Several chemokines and chemokine receptors are involved in the recruitment of MDSC to the tumor site or to the pre-metastatic niche (Table 1). Chemokines CXCL1, CXCL2, and CXCL5 have been shown to recruit MDSC to the tumor site (57) or to the pre-metastatic niche (58-60).
Table 1
Mechanisms of MDSC migration to the tumor site
These chemokines bind to the same receptor CXCR2. CXCR2 deficiency has been shown to decrease tumorigenesis and tumor growth due to a strongly reduced accumulation of MDSC (61). All these CXCR2 ligands are well known for their ability to recruit neutrophils suggesting that they could mainly be responsible for the recruitment of PMN-MDSC. However, this specificity remains to be confirmed.
CXCL12, which binds the CXCR4 receptor, has also been suggested to cause accumulation of MDSC in tumors of patients with ovarian cancer (62). CCL2 and macrophage migration inhibitory factor (MIF), two chemotactic factors for monocytes, have been shown to specifically recruit M-MDSC to tumors in mice and cancer patients (63-65). Interestingly, MDSC via PNT release can nitrate CCL2, which prevent the chemokine to recruit CTL, but does not affect its ability to recruit MDSC (66). MIF can promote tumor growth, associated with an increased accumulation of M-MDSC inside the tumor (67). Accordingly, tumors deficient of MIF had lower levels of M-MDSC (68). Pro-inflammatory proteins S100A8 and S100A9 are potent chemoattractants for MDSC and have been implicated in the promotion of tumor growth and metastases by MDSC (69-71). Further study demonstrated that serum amyloid A (SAA) 3 induced by S100A8/A9 directly attracted MDSC to pre-metastatic lungs, stimulated NF-κB signaling in a TLR4-dependent manner and facilitated metastasis (72). Thus, it appears that MDSC recruitment to tumor sites may represent a vicious circle when MDSC initially recruited to the tumor site by tumor-derived chemokines can facilitate the recruitment of other MDSC via release of S100A8/A9 proteins (58).
MDSC effect on angiogenesis
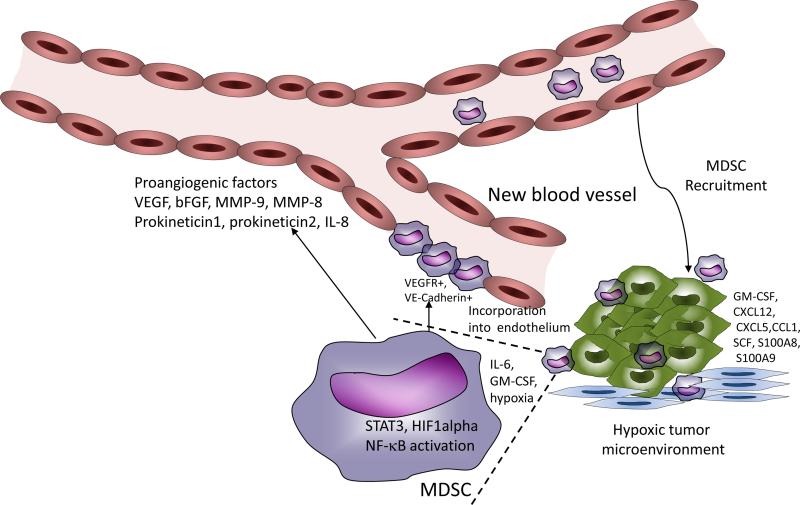
Rapid growth of solid tumors results in hypoxia, and hypoxia-induced up-regulation of proangiogenic factors such as VEGF, PDGF, b-FGF, angiopoietins, etc. (73). Hypoxia can enhance MDSC migration to the tumor site via HIF-1α mediated production of chemokines (58, 74). Inhibition of MDSC infiltration of the tumor sites results in the inhibition of tumor angiogenesis (75). Another important pro-angiogenic factor secreted by MDSC in the tumor site is bombina variegate peptide 8 (Bv8), which is up-regulated by STAT3 (76). STAT3 also directly induced the secretion of VEGF and bFGF by MDSC (77). Bv8 production by PMN-MDSC has also been shown to promote lung metastasis (78). Blockade of Bv8 in combination with VEGF antibody showed an additive effect in inhibiting angiogenesis and tumor growth (79). Another important mechanism by which MDSC can promote tumor neovascularization is by secreting matrix metalloproteinase 9 (MMP-9). MMP-9 promotes bioavailability of VEGF in the tumor microenvironment (Fig. 1). There was a report that MDSC could be directly incorporated into the vascular endothelium by differentiating into endothelial-like cells expressing VE-cadherin and VEGF-R2 (80). However, this observation was not directly confirmed by other studies. Although VEGF antibody-mediated therapy has some success in clinics, tumors eventually become refractory to this treatment. Recruitment of MDSC has been shown to be a key mechanism that mediates resistance to anti-VEGF therapy. MDSC were able to promote new vessel growth even in the presence of VEGF antibody (81, 82). MDSC have also been shown to mediate the resistance to anti-angiogenic agent tyrosine kinase inhibitor sunitinib in preclinical models of renal cell carcinoma (83). In patients with renal cell cancer, the clinical response to sunitinib inversely correlated with the presence of circulating PMN-MDSC. These cells had increased levels of MMP9, MMP8 and IL-8, suggesting that MDSC in sunitinib resistant tumors could still promote angiogenesis through alternative mechanisms (83). The exact nature of those mechanisms needs to be elucidated.
Mechanisms of MDSC effect on tumor metastasis
MDSC role in promotion of metastases was extensively investigated in the mouse models of breast cancer and melanoma. In the 4T1 model of breast cancer, accumulation of PMN-MDSC correlated with increased bone metastasis, and co-injection of MDSC and 4T1 cells led to increased lung metastasis. MDSC in 4T1 tumors up-regulated the expression of several MMPs, which was critical in mediating invasiveness of 4T1 cells in vitro and in vivo (84). MDSC also down-regulated protease inhibitors such as the neutrophilic granule protein, an inhibitor of tumor invasiveness and metastasis (85). Each subset of MDSC might contribute differently to tumor metastasis promotion (Table 2).
Table 2
Mechanisms of regulation of tumor metastases by subsets of MDSC
| MDSC subset | Mechanism of action | Reference |
|---|---|---|
| Total MDSC (subset is not defined yet) | Metalloproteinase | 83,84,85 |
| Total MDSC (subset is not defined yet) | Induction of stemness of cancer cells in human ovarian tumor cells via microRNA101 | 21 |
| PMN-MDSC | EMT via production of HGF and TGF-β | 91, 60 |
| PMN-MDSC | Production of bv8 | 77, 78 |
| PMN-MDSC | Production of MCP-1 | 90 |
| PMN-MDSC | TGF-β - TGF-β receptor II interaction | 87 |
| M-MDSC | MET via versican release | 93 |
| M-MDSC | Local immune suppression | 70, 92 |
| M-MDSC | Production of IL-6 | 88 |
| M-MDSC | Differentiation osteoclasts - bone metastases | 94 |
TGF-β is involved in the regulation of mammary carcinoma metastasis by MDSC. However, its precise role remains controversial. Deletion of TGF-β receptor II (Tgfbr2) in mammary carcinoma cells resulted in increased MDSC infiltration into tumors mediated by SDF-1 and CXCL5. These MDSC were observed at the leading invasive tumor edge and produced MMPs that contributed to breast tumor cell invasion (84). Inhibition of TGF-β signaling in SMAD4-deficient mouse colon carcinoma also induced MDSC recruitment and tumor invasion, which was dependent on CCL9 (86). In contrast, a recent study demonstrated that the specific deletion of Tgfbr2 in myeloid cells significantly inhibited tumor metastasis (which could be reverted by transfer of wild-type PMN-MDSC). Tgfbr2 deficiency in myeloid cells decreased arg-1 activity and NO production, which promoted IFN-γ production and improved systemic immunity (87). MIF was implicated in the promotion of metastases by inducing MDSC accumulation in mouse breast cancer model (67). MDSC in the primary tumor and metastatic sites produce IL-6, which conferred invasive potential of breast cancer cells and stimulated distant metastases through persistent activation of STAT3 in cancer cells. Blocking of IL-6 signaling successfully reduced primary tumor growth and lung metastasis (88). MDSC recruited to pre-metastatic lungs stimulated the migration of tumor cells by secreting TNFα, CXCL2 and TGFβ (70). In a mouse mammary tumor model, HIF-1α-dependent kit ligand expression by hypoxic tumor cells mobilizes c-Kit+ CD11b+Ly6Ghigh PMN-MDSC to the primary tumor and promotes metastasis (89). PMN-MDSC recruitment to pre-metastatic niche was dependent on hypoxic tumor cell– derived monocyte chemotactic protein-1 (MCP-1) (90).
Recently, several studies have shown the role of MDSC in epithelial-mesenchymal transition (EMT). To disseminate, invade tissues and metastasize, some tumor cells undergo EMT, a process where polarized epithelial cells lose epithelial markers, and differentiate to cells with mesenchymal features (91). Abastado et al. have shown that PMN-MDSC were recruited to the tumor site in the RET transgenic mouse model of spontaneous melanoma. Once in the tumor site, PMN-MDSC produced HGF and TGF-β and induced EMT of primary melanoma cells. The depletion of PMN-MDSC led to decreased EMT and fewer metastatic lesions in mice (60). MDSC are also able to promote cancer metastasis by inducing stemness of cancer cells or by expanding the cancer stem cell population. In ovarian cancer patients, accumulation of Lin− CD45+ CD33+ MDSC correlated with poor survival in metastatic and non-metastatic disease. MDSC directly interacted with ovarian tumor cells and induced their stemness. This effect was mediated by up-regulation of microRNA-101 in ovarian cancer cells, which in turn targeted CtBP2, a co-repressor of stem cell genes. Further, culture of human ovarian tumor cells with MDSC, before inoculation into immunodeficient mice, led to increased engraftment and number of metastatic lesions in lung and liver (21). In a mouse model of pancreatic cancer, M-MDSC directly induced expansion of aldehyde dehydrogenase-1+ (ALDH1) pancreatic cancer stem cells. Similar effect was observed with human CD14+ HLA-DR− M-MDSC (92).
The current concept suggests that MDSC arrive to the pre-metastatic site before the tumor cells. Once in the site, MDSC condition it to promote tumor seeding. This process involves creating an immunosuppressive microenvironment and secretion of b-FGF, IGF-1, IL-10, IL-4, MMP9, and S100A8/A9 (70, 93) (Fig. 2). Since most of the metastases are represented by epithelial cells, similar in morphology to the primary tumor, but not mesenchymal cells, it is suggested that EMT is a temporary event, and after arriving to a metastatic site, tumor cells undergo reverse transition from mesenchymal to epithelial phenotype in order to colonize the niche. This process is known as mesenchymal-epithelial transition (MET). In one model, MDSC were implicated in MET transition. Mittal et al. showed that MDSC (mainly M-MDSC), accumulated in the premetastatic lung of MMTV-PyMT spontaneous breast tumor-bearing mice, secrete versican, an extracellular matrix proteoglycan. Versican contributed to MET and the formation of macrometastasis in the lungs (94). MDSC isolated from the bone marrow of tumor-bearing mice could differentiate into functional osteoclasts which are closely linked with bone metastasis. NO was crucial for the differentiation of MDSC into osteoclasts (95).
Despite a body of literature demonstrating the pro-metastatic role of MDSC, one recent study suggested that MDSC had functional plasticity, and in some cases, could actually inhibit metastasis. Metastatic and non-metastatic prostate and breast tumors equally induced accumulation of MDSC in the lung pre-metastatic site (96). MDSC from the non-metastatic tumors produced large amounts of TSP-1, a potent anti-angiogenic matrix protein, and inhibited metastasis. Non-metastatic tumors secreted prosaposin, a potent inducer of TSP-1, and a prosaposin 5 amino-acid peptide mimetic was sufficient to cause up-regulation of TSP-1 in MDSC in vivo and inhibit tumor metastasis (96). This is an interesting new mechanism challenging MDSC metastasis-promoting functions. However, more studies confirming the role of TSP-1 and MDSC in metastasis inhibition in other tumor models is required.
CONCLUSIONS
MDSC were originally described as cells that potently suppress T cell immune responses in cancer. It is clear now that the effect of MDSC is much broader. Their important role was established not only in cancer, but also in chronic infectious diseases and inflammation, autoimmune diseases, trauma, sepsis, etc. At the same time, it became apparent that MDSC contribution to tumor progression extend far beyond immune-suppression and include regulation of tumor development, progression, and metastasis. MDSC utilize a variety of different mechanisms not involving immune-suppression. One of the most intriguing roles attributed to MDSC is their contribution to the formation of pre-metastatic niche. This may open new therapeutic opportunities in blocking metastases by targeting MDSC. However, the mechanisms responsible for MDSC seeding of the tissues and specific regulation of tumor cell seeding in metastatic sites by MDSC remain rather poorly understood. Understanding the molecular mechanisms, which govern the relationship between MDSC and tumor cells in pre-metastatic niche, will provide novel opportunities for targeting metastases.
Acronyms and definition
| MDSC | myeloid-derived suppressor cells |
| PMN-MDSC | polymorphonuclear MDSC |
| M-MDSC | mononuclear MDSC |
| EMT | epithelial-mesenchymal transition |
| MET | mesenchymal-epithelial transition |
| MMP | metalloproteinase |
References
[This report suggests that MDSC can regulate stemness of tumor cells.] [Europe PMC free article] [Abstract] [Google Scholar]
[Reports describing the value of MDSC as prognostic markers in patients treated with cancer vaccines.] [Abstract] [Google Scholar]
[Reports describing the value of MDSC as prognostic markers in patients treated with cancer vaccines.] [Abstract] [Google Scholar]
[First report describing the effect of MDSC targeting in cancer patients on immue response to vaccines.] [Europe PMC free article] [Abstract] [Google Scholar]
[This study using genetically modified MDSC (lacking CXCR2) demonstrated a direct role of these cells in colitis induced carcinogenesis.] [Europe PMC free article] [Abstract] [Google Scholar]
[This study demontrated a direct role of myeloid-derived TGF-beta in the promotion of tumor metastases.] [Europe PMC free article] [Abstract] [Google Scholar]
[MDSC implicated in MET.] [Europe PMC free article] [Abstract] [Google Scholar]
[Interesting report indicating anti-metastatic role of MDSC.] [Europe PMC free article] [Abstract] [Google Scholar]
Full text links
Read article at publisher's site: https://doi.org/10.1146/annurev-med-051013-052304
Read article for free, from open access legal sources, via Unpaywall:
https://www.annualreviews.org/doi/pdf/10.1146/annurev-med-051013-052304
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1146/annurev-med-051013-052304
Article citations
Disease progression associated cytokines in COVID-19 patients with deteriorating and recovering health conditions.
Sci Rep, 14(1):24712, 21 Oct 2024
Cited by: 0 articles | PMID: 39433797 | PMCID: PMC11494080
Locoregional therapies combined with immune checkpoint inhibitors for liver metastases.
Cancer Cell Int, 24(1):302, 31 Aug 2024
Cited by: 0 articles | PMID: 39217341 | PMCID: PMC11365172
Review Free full text in Europe PMC
Metabolic reprogramming and immune evasion: the interplay in the tumor microenvironment.
Biomark Res, 12(1):96, 03 Sep 2024
Cited by: 1 article | PMID: 39227970 | PMCID: PMC11373140
Review Free full text in Europe PMC
Strategies for overcoming tumour resistance to immunotherapy: harnessing the power of radiation therapy.
Br J Radiol, 97(1160):1378-1390, 01 Aug 2024
Cited by: 0 articles | PMID: 38833685 | PMCID: PMC11256940
Review Free full text in Europe PMC
Myeloid‑derived suppressor cells: Key immunosuppressive regulators and therapeutic targets in colorectal cancer (Review).
Int J Oncol, 65(3):85, 26 Jul 2024
Cited by: 0 articles | PMID: 39054950 | PMCID: PMC11299769
Review Free full text in Europe PMC
Go to all (308) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Transcriptional regulation of myeloid-derived suppressor cells.
J Leukoc Biol, 98(6):913-922, 03 Sep 2015
Cited by: 211 articles | PMID: 26337512 | PMCID: PMC4661041
Review Free full text in Europe PMC
Contribution of myeloid-derived suppressor cells to tumor-induced immune suppression, angiogenesis, invasion and metastasis.
J Genet Genomics, 37(7):423-430, 01 Jul 2010
Cited by: 44 articles | PMID: 20659706
In Brief: Myeloid-derived suppressor cells in cancer.
J Pathol, 242(1):7-9, 21 Mar 2017
Cited by: 18 articles | PMID: 28097660 | PMCID: PMC5413806
Myeloid-Derived Suppressor Cells: Critical Cells Driving Immune Suppression in the Tumor Microenvironment.
Adv Cancer Res, 128:95-139, 12 May 2015
Cited by: 310 articles | PMID: 26216631 | PMCID: PMC4662416
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NCI NIH HHS (6)
Grant ID: CA 100062
Grant ID: P30 CA010815
Grant ID: CA 84488
Grant ID: R01 CA084488
Grant ID: R01 CA100062
Grant ID: R01 CA141438