Abstract
Free full text

Thrombospondin-1 regulates bone homeostasis through effects on bone matrix integrity and nitric oxide signaling in osteoclasts
Associated Data
Abstract
Thrombospondin-1 (TSP1), an endogenous antiangiogenic, is a widely expressed secreted ligand with roles in migration, adhesion and proliferation and is a target for new therapeutics. While TSP1 is present in the bone matrix and several TSP1 receptors play roles in bone biology, the role of TSP1 in bone remodeling has not been fully elucidated. Bone turnover is characterized by coordinated activity of bone-forming osteoblasts (OB) and bone-resorbing osteoclasts (OC). TSP1−/− mice had increased bone mass and increased cortical bone size and thickness compared to wild type (WT). However, despite increased size, TSP1−/− femurs showed less resistance to bending than expected, indicative of diminished bone quality and a bone material defect. Additionally, we found that TSP1 deficiency resulted in decreased OC activity in vivo and reduced OC differentiation. TSP1 was critical during early osteoclastogenesis, and TSP1 deficiency resulted in a substantial overexpression of inducible nitric oxide synthase (iNOS). Importantly, administration of a NOS inhibitor rescued the OC function defects of TSP1−/− mice in vivo. To investigate the role of bone-derived TSP1 in osteoclastogenesis, we found that wild type (WT) pre-OCs had defective iNOS expression when cultured on TSP1−/− bone compared to WT bone, suggesting that TSP1 in bone plays a critical role in iNOS signaling during OC development. These data implicate a new role for TSP1 in bone homeostasis with roles in maintaining bone matrix integrity and regulating OC formation. It will be critical to monitor bone health of patients administered TSP1-pathway directed therapeutics in clinical use and under development.
INTRODUCTION
Bone is a dynamic tissue, characterized by balanced bone resorption and formation. This bone remodeling is necessary to preserve the structural integrity of the skeleton and to maintain calcium and phosphate homeostasis(1,2). Bone is a matrix composed of two compartments: osteoid (primarily collagen type I fibers) and mineral (calcium-phosphate hydroxyapatite crystals). The bone matrix also contains numerous noncollagenous proteins including growth factors, proteoglycans, and adhesion molecules. These molecules contribute to maintenance of homeostatic bone remodeling, promoting cell attachment, differentiation, and growth of bone remodeling cells. In addition, some molecules aid in osteoid and mineral matrix organization, directly influencing bone strength.
Mesenchymal osteoblasts (OBs) form bone by depositing collagen and other molecules to form osteoid and subsequently calcify this matrix to form rigid bone. Osteoclasts (OCs), polarized multi-nucleated hematopoietic cells formed from the fusion of macrophages, resorb bone through protease- and acid-dependent processes. The formation and activity of OCs and OBs is a tightly coupled process. OCs are activated by OB-derived cytokines such as M-CSF and RANK-L. In turn, OCs promote osteoblastic bone formation by releasing stored growth factors including bone morphogenic proteins, TGFβ, and insulin growth factor from the bone matrix. Misregulation of healthy bone remodeling, as in cancer metastasis, arthritis, or osteoporosis, leads to decreased bone integrity, pathologic fracture, nerve-compression syndromes, and hypercalcemia(3). While many factors that individually regulate OC and OB formation and activity have been identified, our understanding of the role of OB-deposited bone matrix-sequestered proteins in homeostatic OC activity remains incomplete.
Thrombospondin-1 (TSP1) is a large secreted protein that binds at least 11 distinct receptors, including β3 integrin, CD47, and CD36, thereby influencing downstream pathways. By regulating migration, adhesion and proliferation, TSP1 participates in diverse processes including angiogenesis, inflammation, and wound healing. TSP1 was the first identified endogenous antiangiogenic molecule, and TSP1 mimetics have entered clinical trials as cancer therapeutics(4). Antibodies against the TSP1 receptor CD47, used to induce macrophage phagocytosis of cancer cells, have completed pre-clinical studies(5,6), and a humanized antibody is under clinical development. In addition, nitric oxide (NO) signaling, a pathway regulated by TSP1, is being therapeutically targeted for vasodilation (NO donors) or hypotension (NO synthase inhibitors)(7). As these TSP1 pathway-targeted therapies enter broad clinical use, it is important to consider the action of TSP1 in other systems, including the skeleton, where TSP1 receptors CD47, β3 integrin, and CD36(8–13) are involved in OC and OB signaling. Bone-related off-target effects will be especially crucial to consider in advanced cancer patients at risk for skeletal metastasis and post-menopausal women who are at risk for osteoporosis.
TSP1 deficient mice are reported to have mild spine deformation(14) and mild growth plate cartilage disorganization(15) in addition to enhanced angiogenesis(16) but detailed analysis of bone has not been reported. TSP1 is expressed by OBs and is present in osteoid and mineralized bone matrix(17–19) where it is directly deposited by bone-forming OBs(19,20). TSP1 is involved in OB differentiation in part through activation of latent TGF-β(21,22), and TSP1 receptors CD47 and CD36 also regulate OB(9,10). TSP1 is a regulator of nitric oxide (NO) in endothelial cells, platelets, and vascular smooth muscle cells(23,24), but only receptors CD36 and CD47 have been implicated in NO regulation(24–26). TSP1 has been implicated in OC activity, but its mechanism of action remains unknown(27). TSP1-CD47 interactions play a role in the formation of multiple myeloma-dendritic cell fusions with bone-resorbing capacity(28), and TSP1-CD36 ligation can promote osteoclastic resorption on dentine slices(29). CD47−/− mice have a bone resorption defect(8,10,30), which we have shown is due to increased iNOS in OCs(8). Together these data led us to hypothesize that OB-secreted TSP1 sequestered in bone matrix will modulate OC function thereby coupling OB activity to OC formation.
In this study, we describe the role of bone-sequestered TSP1 in the maintenance of bone homeostasis, both as a structural protein contributing to bone strength and as a signaling ligand promoting OC formation. Here we have used mice genetically deficient in TSP1 and neutralizing anti-TSP1 antibody to investigate the role of TSP1 in bone homeostasis. Together, these data implicate a novel role for TSP1 in bone homeostasis, both in mediating bone matrix integrity and by regulating OC formation as a matrix-derived paracrine signaling molecule. Our findings provide an important rationale to monitor the effects on bone health of TSP1 pathway-directed therapeutics, currently in clinical use and under development, especially in patients affected by bone pathology such as osteoporosis and skeletal metastasis.
MATERIALS AND METHODS
Animals
TSP1−/− mice were originally obtained from Jack Lawler(31) and backcrossed over 20 times to C57BL/6J. TSP1−/− and TSP1+/+ mice were housed in shared pathogen-free conditions according to the guidelines of the Division of Comparative Medicine, Washington University School of Medicine. The animal ethics committee approved all experiments.
Reagents
Anti-TSP1 function blocking antibody, clone A4.1 (IgM, mouse anti-human)(32) was purchased from Thermo Scientific (Waltham, MA). TSP1 is highly conserved among species and clone A4.1 has cross-species reactivity with mouse and bovine TSP1. IgM istoype control was purchased from eBioscience (San Diego, CA).
Micro-computed tomography
Tibiae and femurs from TSP1−/− and WT sex-matched mice were suspended in agarose and tibial metaphyses and femoral mid-diaphysis were scanned by micro-computed tomography (uCT-40; Scanco Medical) as described previously(8,33). Because there were no statistical differences between sexes, pooled values for both male and female mice are presented at each time point. For image acquisition, the long bones were placed in a 13-mm holder and scanned. The cancellous region was selected using contours inside the cortical shell on each 2d image. The growth plate was used as a marker to determine a consistent location to start analysis and 40 slices were analyzed. A 3-dimentional cubical voxel model of bone was built and calculations made: relative trabecular bone volume over total volume, trabecular number, and trabecular spacing. The cortical region was selected using contours outside the cortical shell at the mid-diaphysis and 50 slices were analyzed for total mineral density. The average cross-sectional geometry measurements were calculated for cortical thickness and total cross-sectional area. The bending moment of inertia was determined using standard engineering parallel-axis theory as previously described(34,35).
Bone biomechanics
Immediately following dissection, WT and TSP1−/− female mouse femurs were frozen at −20°C for a minimum of 12 hours. Femurs were thawed in phosphate-buffered saline for 1 h prior to use, and testing was carried out at room temperature using a servohydraulic testing machine (8841 Dynamite, Instron, Norwood, MA). Femurs were positioned on two supports 7 mm apart, and the central loading point was the mid-diaphysis. Displacement was applied transverse to the long axis of the bone at a rate of 0.03 mm/s until failure. Force-displacement data were recorded at 60 Hz and analyzed to determine stiffness (a measure of elastic resistance to bending), yield force (a measure of the force required to permanently deform the bone), ultimate force (the highest force attained prior to failure), post-yield displacement (a measure of ductility) and energy-to-fracture (a measure of overall resistance to fracture)(34,35). Estimated material properties (elastic modulus, yield stress, ultimate stress) were calculated using standard engineering beam theory that normalizes for bone size.
Reference point indentation
WT and TSP1−/− female mouse femurs were subjected to reference point indentation as previously described(36). A 25 μm testing probe was placed on the cortical bone surface and a preload force of 0.1N and a waveform of 10 cycles of 2N force was applied as a frequency of 2 Hz (Biodent, Active Life Sciences, Santa Barbara, CA). Each bone was subjected to 3 indents 1mm apart. Measurements were made for each indent: initial indentation distance and indentation distance increase (cycle 1 – cycle 10).
Serum CTX assay
Carboxy-terminal telopeptide collagen crosslinks (CTX) was measured in the serum of overnight fasted mice by ELISA (RatLaps; Immunodiagnostic Systems, Scottsdale, AZ) according to the manufacturer’s instructions.
In vivo calcein labeling
Calcein labeling was completed as previously described(37). Briefly, mice were injected 8 and 2 days prior to sacrifice with 20 mg/kg calcein (Sigma-Aldrich, St. Louis, MO) in a 2% sodium bicarbonate solution i.p. Calveria were fixed in 70% ethanol and embedded in methylmethacrylate and sectioned.
L-NAME administration
L-NAME (Sigma Aldrich, St. Louis, MO) was administered in the drinking water of 8-week old mice for 6 weeks, changed every 2 days (40 mg/kg/day).
In vitro osteoblast differentiation
To generate bone marrow stromal cells (BMSCs), whole bone marrow cells were harvested from 8–10 week old male and female mice were cultured in ascorbic-acid free αMEM, 10% FBS, 1% penicillin-streptomycin for 7 days. For each experiment WT and TSP1−/− cells were age-matched and sex-matched. For OB differentiation, 2x105 BMSC/ml were cultured in αMEM, 10% FBS, 1% penicillin-streptomycin, 10 mM β-glycerophosphate, 50 ug/ml ascorbic acid for 14 days, refed every 7 days. Cultures were fixed in 10% neutral buffered formalin and stained with Alkaline Phosphatase (Fast Blue RR salt, Sigma Aldrich, St. Louis, MO) and 0.4% Alizarin Red. Alkaline phosphatase activity (phosphatase substrate, Sigma Aldrich, St. Louis, MO) and Alizarin red levels (osteogenesis quantification kit, Millipore, Billerica, MA) were performed according to the manufacturer’s instructions.
In vitro osteoclast differentiation
To generate macrophages, whole bone marrow cells from 8–10 week old male and female mice were cultured in aMEM, 10% FBS, 1% penicillin-streptomycin, 100 ng/ml MCSF for 3 days as previously described(38). For each experiment WT and TSP1−/− cells were age-matched and sex-matched. To generate OCs, 9x104/ml were plated on plastic or bone powder as indicated and cultured in αMEM, 10% FBS (on plastic), 1% penicillin-streptomycin, 50 ng/ml MCSF, 50 ng/ml RANKL, refed every 48 hours. Where indicated, macrophages were cultured with 5 ug/ml antibody. TRAP staining was performed according to the manufacturer’s instructions (Sigma-Aldrich, St. Louis, MO).
Quantitative reverse-transcription PCR
RNA was extracted using RNeasy Mini kit (Qiagen, Venlo, Netherlands) and cDNA generated using iScript (Bio-Rad, Hercules, CA). Quantitative PCR was completed using SsoFast EVA Green Supermix (Bio-Rad, Hercules, CA). Primer sequences are in supplemental methods.
Bone powder coated plates
Plates were coated with bone powder as previously described(39) with modifications. Bone marrow was flushed from the long bones of both male and female 8–10 week old mice (WT and TSP1−/− mice were age- and sex-matched), and the bones were frozen at −20°C. Frozen bones were crushed to powder using a Bullet Blender Bead Lysis Kit (Midsci, St. Louis, MO). The powder was pooled for each genotype and washed 3 times in 70% ethanol. Plates were coated with 1:20 bone powder: PBS + 4 ug/ml poly-l-lysine for 4 hours at 68°C. Plates were sterilized under UV light and experiments completed as described.
Statistical analysis
All experiments were analyzed using Student’s t-test. In calculating two-tailed significance levels for equality of means, equal variances were assumed for both populations. Results were considered to reach significance at p<0.05. *p<0.05; **p<0.01; ***p<0.001.
RESULTS
TSP1−/− mice had increased cancellous and cortical bone mass
We have previously shown that mice deficient in the TSP1-receptor CD47 have modestly increased bone mass(8,10,30). To determine if TSP1−/− mice would similarly display increased bone volume, we performed microCT analysis on WT and TSP1−/− mice to evaluate cancellous and cortical bone. We observed a significant increase in trabecular bone volume in 8 week-old (+32.6%), 16 week-old (+112.1%), and 32 week old (+158.8%) TSP1−/− mice (Fig. 1A–B). Coordinately, trabecular number was increased and trabecular spacing was decreased in TSP1−/− mice (Fig. 1C–D). This dramatic increase in trabecular bone volume is in contrast to the modest bone volume phenotypes of TSP1-receptor deficient mice (CD47, CD36, β3 integrin−/−(8–10,12,40)), and is consistent with TSP1 binding to multiple receptors. TSP1−/− mice also had significantly increased cortical bone thickness at 8-, 16-, and 32-weeks of age and increased total cortical area at 32 weeks (Supplementary Table 1), indicating a geometrically larger bone and increased periosteal expansion in TSP1−/− compared to WT mice. Therefore, TSP1−/− mice had both increased size and increased relative bone mass compared to WT mice.
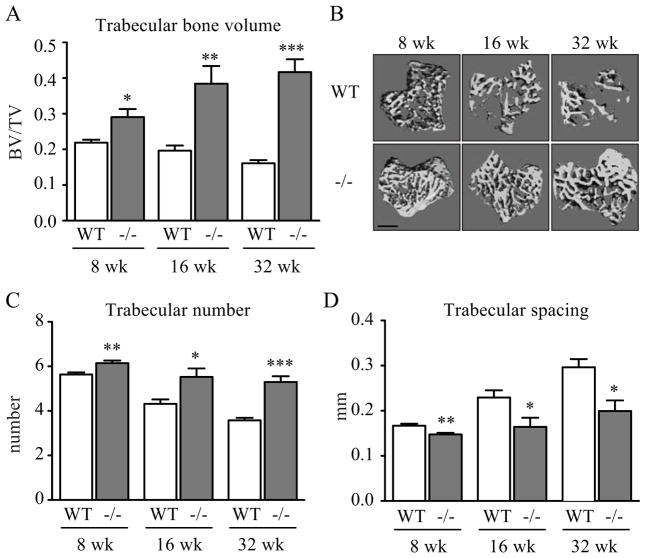
TSP1−/− mice had increased cancellous bone mass. MicroCT analysis of WT and TSP1−/− mice tibiae for calculation of bone morphological parameters (8- and 32-week n=6/group; 16-week n=5/group): (A) trabecular bone volume, (C) trabecular number, and (D) trabecular spacing. (B) Representative images of three-dimensional microCT reconstruction (scale bar = 500 μm)
TSP1 improved bone strength through effects on the bone material properties
Because TSP1 null mice showed enhanced cortical thickness and cross-sectional area (Supplementary Table 1), and because TSP1 is a known component of the bone matrix(19,20), we hypothesized that TSP1 deletion may influence bone mechanical properties. Bone mechanical properties are influenced by many factors including overall structural morphology and the material properties (quality) of the bone matrix. We directly assessed bone strength in 12-week old WT and TSP1−/− mice. As anticipated, 12-week old TSP1−/− mice had increased cortical bone thickness and total cortical area (Fig. 2A–C).
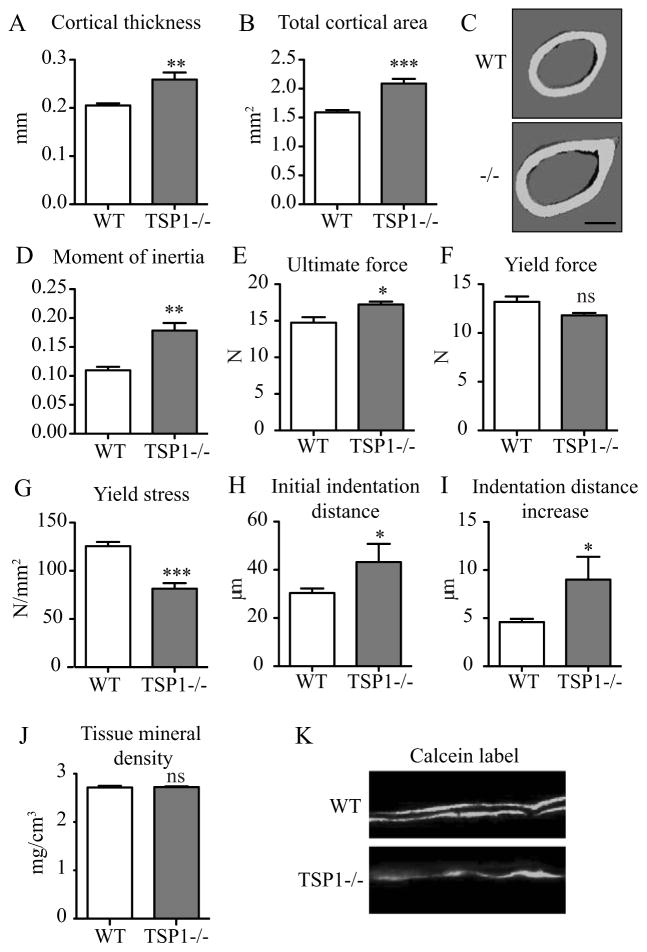
TSP1 improved bone strength through effects on bone material properties. 12-week old WT and TSP1−/− femurs were evaluated for structural and material strength. (A) MicroCT analysis of WT (n=10) and TSP1−/− (n=8) mice femurs for (A) cortical thickness, (B) total cortical area, (D) minimum moment of inertia, and (J) tissue mineral density. (C) Representative images of three-dimential microCT reconstruction (scale bar = 500 μm). Femurs were subjected to three-point bending to evaluate structural mechanical parameters (WT n=5; TSP1−/− n=4): (E) ultimate force and (F) yield force (ns, p=0.0727). (G) Yield stress was calculated to normalize for differences in cortical bone morphology. Reference point indentation was completed to evaluate local material properties: (H) initial indentation distance, (I) indentation distance increase. (n=3 tests/femur; WT n=5 femurs; TSP1−/− n = 3 femurs). (K) Representative images of double calcein labeling of 16-week old WT and TSP1−/− mice (n = 5/group).
MicroCT analysis of the femoral mid-diaphysis showed a striking increase in moment of inertia in TSP1−/− mice in 12-, 16-, and 32-week old mice (+62.8% at 12-weeks), predicting increased strength in TSP1−/− mice based on morphology alone (Fig. 2D, Supplementary Table 1). Surprisingly, however, direct measurement of mechanical strength by 3-point bending did not recapitulate these results. Bone is permanently deformed upon reaching its yield force and eventually fractures after reaching its highest (ultimate) force. TSP1−/− femurs showed only a modest increase ultimate force (+16.7%) and no difference compared to WT in yield force (Fig. 2E–F). Post-yield displacement and energy to fracture were also modestly increased in TSP1−/− mice, indicating that the TSP1−/− femurs plastically deformed more than WT prior to fracture (Supplementary Table 2). Notably, yield stress, a measure of yield force that normalizes for differences in morphology, is significantly decreased in TSP1−/− femurs (Fig. 2G). Together, these data suggests a bone material defect in TSP1−/− mice that offsets the morphological size advantage.
To directly evaluate the local material properties of the bone matrix, we performed reference point indentation by applying a repetitive force on the cortical bone surface through a small probe. TSP1−/− femurs had increased initial indentation distance and a larger increase in indentation at the end of 10 cycles (Fig. 2H–I). These data indicate that TSP1−/− bone material had less resistance to microfracture compared to WT control. MicroCT analysis showed that cortical tissue mineral density was unchanged between WT and TSP1−/− mice at all ages queried, indicating no gross differences in mineral density between genotypes (Fig. 2J, Supplementary Table 1). Dynamic histomorphometric analysis to quantitate bone formation rate and mineral apposition rates demonstrated diffuse and inconsistent bone mineral labeling in the TSP1−/− mice (Fig. 2K), consistent with a bone matrix defect. Taken together, the mechanical and material properties in TSP1−/− bones demonstrate that TSP1 plays a role in maintaining the matrix quality necessary for normal bone strength.
TSP1−/− mice had decreased bone resorption and TSP1 is necessary for OC formation
While we identified differences in long bone size and bone material properties that contribute to reduced bone quality, the underlying mechanism leading to the increased bone mass in TSP1−/− mice remained unclear. The progressive increase in trabecular bone volume with age in TSP1 null animals (Fig. 1A–B) is consistent with a failure in bone resorption. To test this, we measured serum markers of bone turnover, which include CTX (carboxy-terminal telopeptide collagen crosslinks), a measure of osteoclastic bone resorption and P1NP (pro-collagen type I N-terminal telopeptide) and osteocalcin, measures for OB function. There were no statistically significant differences in serum P1NP at 8, 16, or 32 weeks of age or serum osteocalcin in 8-week old mice (Supplementary Fig. 1 A–B). To directly assess OB formation and mineralization, we cultured primary OB from WT and TSP1−/− mice (Fig. 3A). We assessed OB cultures at multiple time points, and did not observe any differences in alkaline phosphatase or alizarin red quantitative assays (Fig. 3B–C) or in OB differentiation markers via RT-qPCR (Runx2, osterix, osteopontin, osteocalcin) at any time point (Fig. 3D–E). We did observe significant decreases in serum CTX levels in TSP1−/− mice compared to WT controls at 8 weeks and 16 weeks of age (Fig. 4A) consistent with a defect in OC resorption. Histomorphometric analysis of mouse tibae showed that TSP1−/− mice did not have significantly different OC number/bone surface at 8 or 32 weeks of age (Supplementary Fig. 2 A–C). To investigate the role of TSP1 in osteoclastogenesis and function, we differentiated primary OCs from bone marrow macrophages in vitro. TSP1 is secreted by activated platelets, leading to high levels of the protein in blood serum. Fetal bovine serum (FBS), necessary in OC culture media, contains approximately 100 ng/ml TSP1 (data not shown). To neutralize FBS-derived TSP1, we treated cultures with TSP1 neutralizing antibody (clone A4.1). In contrast, OC differentiation in the presence of anti-TSP1 antibody was dramatically reduced, while cells treated with the IgM control antibody developed large multinucleated TRAP+ OCs (Fig. 3B, left panels). Notably, ex vivo cultured TSP1−/− OC were indistinguishable from WT OCs, indicating that exogenous, FBS-derived TSP1 was sufficient to maintain normal OC formation (Supplementary Fig. 3). Interestingly, in anti-TSP1 antibody treated cultures, while there was no change in NFATc1, a marker of OC lineage commitment, RT-qPCR analysis showed decreased expression of OC differentiation markers (DC-STAMP, TRAP, β3 integrin, Cathepsin K), consistent with reduced osteoclastogenesis (Fig. 4C).
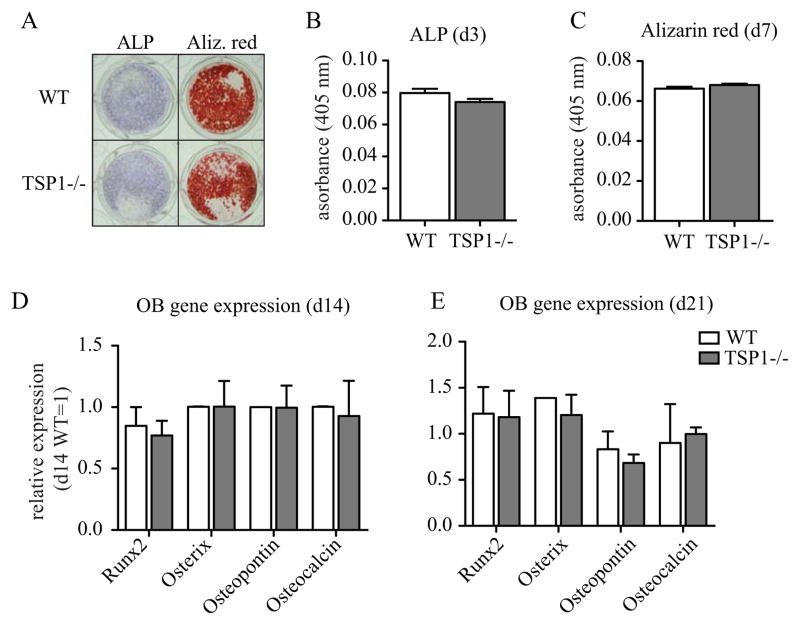
(A) WT and TSP1−/− OB after 21 days of culture in osteogenic media were stained with alkaline phosphatase (ALP) or alizarin red (Aliz. red) (representative images of n>3 biological replicates). (B) ALP activity by chromogenic assay of day 3 cultures. (C) Osteogenesis by alizarin red quantification of day 7 cultures. qRT-PCR gene expression analysis for (D) day 14 and (E) day 21 OB cultures (n=3 biological replicates).
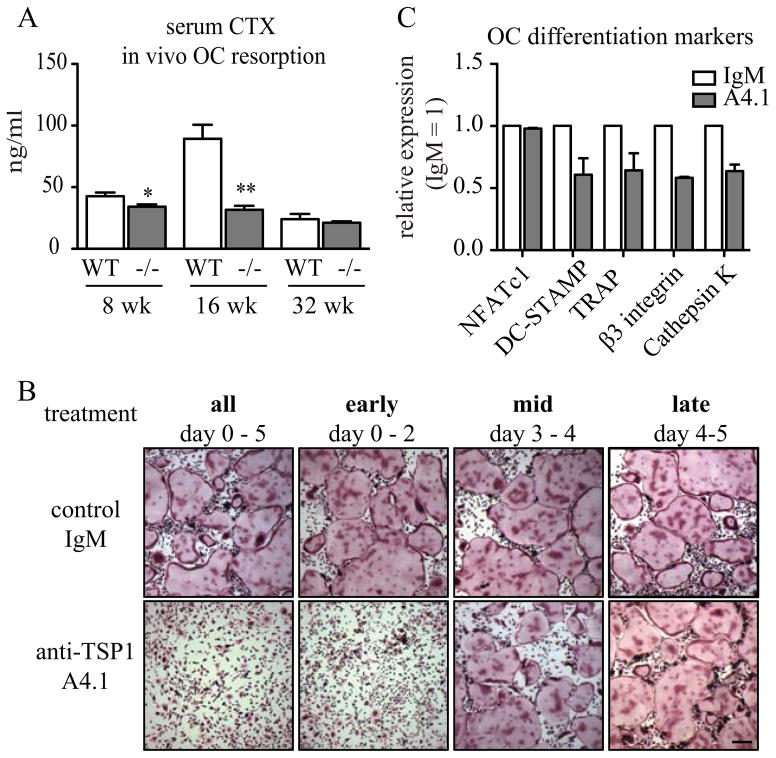
(A) Collagen breakdown products (CTX) was measured in the serum of fasted WT and TSP1−/− mice (8-week n=5/group; 16-week n=4/group; 32-week n=7/group). (B) WT bone marrow macrophages differentiated into OC in the presence of anti-TSP1 A4.1 antibody or IgM isotype control antibody at indicated days. TRAP staining for multinucleated OC at day 5 shown (representative images of n>3 biological replicates, scale bar = 0.25 mm). (C) qRT-PCR analysis for day 3 OC cultured with anti-TSP1 A4.1 or IgM control (n=3 biological replicates).
To define the temporal requirement of TSP1 in OC formation, we treated early (d0–2), mid (d3–4), or late (d4–5) stage OC cultures with A4.1 to neutralize TSP1 at different times during OC formation. We found that neutralizing TSP1 in early OC precursors (d0–2) was sufficient to decrease OC differentiation, while mid- or late-stage TSP1 neutralization had no effect (Fig. 4B). These data indicate that exogenous TSP1 promotes early-stage OC formation while it is dispensable for later maturation.
Inhibition of NOS rescued TSP1−/− bone resorption phenotype
TSP1 is an established mediator of NO signaling in endothelium and inflammatory macrophages(23,24), and we have previously shown that TSP1-receptor CD47 null mice show reduced OC formation due to increased levels of iNOS transcription(8). In primary OC cultures treated with TSP1 neutralizing antibody, we saw a dramatic elevation of iNOS expression compared to isotype control (Fig. 5A). Neither nNOS nor eNOS were elevated in anti-TSP1 antibody treated cultures (data not shown). To test whether the bone resorption defect in TSP1−/− mice was attributable to elevated NO signaling, we inhibited NO production in vivo by administration of the pan-NOS inhibitor L-NAME in the drinking water of TSP1−/− and WT mice (40 mg/kg/day) for six weeks. L-NAME-treatment increased the serum CTX level of TSP1−/− mice to that of their WT counterparts, demonstrating that osteoclastic bone resorption was restored (Fig. 5B). Together, these data indicate that aberrant NO signaling in TSP1−/− mice was in part responsible for decreased OC function in vitro and in vivo.
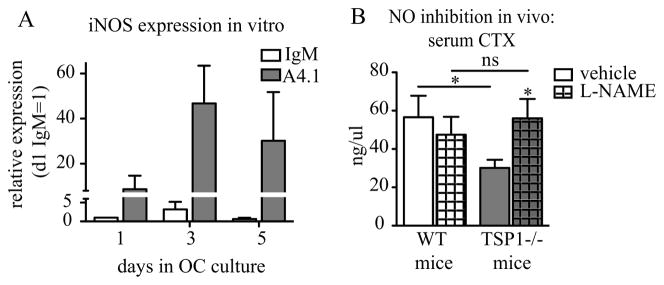
(A) qRT-PCR of iNOS from WT OC cultures in the presence of TSP1 neutralizing antibody (A4.1) or istoype control (IgM). (n=3 biological replicates). (B) WT and TSP1−/− mice were administered pan-NOS inhibitor L-NAME, 40 mg/kg/day for 6 weeks, and fasted serum CTX was measured (WT n=7/group; TSP1−/− n=8/vehicle, n=10/L-NAME).
TSP1 in bone was sufficient to inhibit OC iNOS
It is well established that TSP1 is secreted by OBs and is present in the bone matrix(19,20). We found that TSP1 contributes to maintaining bone strength (Fig. 2), but its role as matricellular signaling molecule in the bone remained unknown.
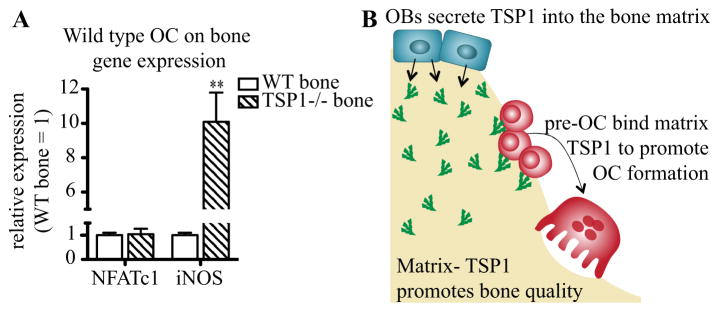
We hypothesized that bone-sequestered TSP1 signals to immature OCs to inhibit RANKL-induced iNOS. We therefore differentiated WT bone marrow macrophages into OC on plates coated with bone powder derived from WT or TSP1−/− mice. The osteoclastogenic culture medium was serum free to ensure that the bone was the only source of TSP1. OCs plated on TSP1−/− bone powder had increased iNOS expression compared to the same cells plated on WT bone after 24 hours of differentiation (Fig. 6A). Notably, the lineage marker NFATc1 was unchanged between cells plated on WT and TSP1−/− bone, indicating that osteoclastogenic signaling was initiated equally (Fig. 6A). Together, these data indicate that bone-sequestered TSP1 plays a critical role in iNOS signaling during early osteoclastogenesis.
DISCUSSION
We demonstrate here that TSP1 is critical for the maintenance of bone homeostasis, contributing both to matrix material integrity and regulation of OC formation (Fig. 6B). TSP1−/− mice were protected from age-associated bone loss and had increased cross-sectional area, but failed to show proportional resistance to bending. Instead, we found that TSP1 null mice had a bone material properties defect, resulting in decreased bone material quality and reduced strength. The increased trabecular bone mass in TSP1−/− mice was due to decreased osteoclastic resorption that was rescued upon in vivo pharmacologic inhibition of NO signaling. We demonstrated that TSP1 inhibition of iNOS was necessary for early OC formation, but was dispensable for later OC maturation. Finally, we showed that bone-sequestered TSP1 modulated OC iNOS levels, indicating TSP1 as a novel paracrine signaling molecule, coupling OB function to OC formation.
The bone matrix is composed of two compartments: mineral of calcium-phosphate hydroxyapatite crystals and osteoid of collagen (90%) and other non-collagenous proteins (10%, NCP). Many diseases including osteogenesis imperfecta and rickets impact bone quality through modification of the osteoid tissue or mineralized matrix leading to increased risk of fragility fracture. Notably, TSP1 binds many components of the bone matrix, including collagen and hydroxyapatite, as well as numerous NCPs including osteonectin, fibronectin, osteopontin, and proteoglycans, among others (reviewed in(41)). These NCPs are critical for bone matrix organization, both for collagen organization and hydroxyapatite deposition(42). In osteogenesis imperfecta patient OBs, secretion of TSP1 and its NCP binding partners are misregulated, suggesting that TSP1 participates with collagen and other NCPs for osteoid organization(43). Our data indicate that TSP1 is critical for maintaining the material properties of bone necessary for high bone quality. We postulate that TSP1 mediates the proper physical organization and protein stoichiometry of the bone matrix through its interactions with other bone matrix components. Further work is necessary to understand the role of the matrix proteins that interact with TSP1 to mediate bone quality.
The TSP1−/− bones had both cancellous and cortical bone phenotypes. The increased periosteal expansion of the TSP1−/− bones suggests an effect of TSP1 loss on periosteal bone formation. Notably, however, total mineral density was unchanged in TSP1−/− mice, indicating that there were no gross alterations levels of mineral content. Assessment of bone formation rate and mineral apposition rates by dynamic histomorphometry in the TSP1−/− mice were hampered by the lack of double labels and diffuse single labels (Fig. 2K). These labeling abnormalities are consistent with a bone matrix defect. Ex vivo osteoblast formation assays were not abnormal in the TSP1−/− cells or in WT cells in the presence of TSP1 neutralizing antibody (data not shown). It is possible that there was a compensatory increase in bone mineral formation due to defects in bone quality or that there are effects of TSP1 on osteocyte and OB formation and function that may be present early in development or during the process of aging. Experiments are underway to explore the role of TSP1 in OBs in both young and aged mice.
In addition to its role in maintaining bone matrix material integrity, we also found that TSP1 binding to early OC precursors promotes differentiation. TSP1−/− mice had overall low bone turnover, with young and mid-aged bone resorption at levels comparable to aged 32-week old WT mice. While our data supports the hypothesis that bone-resident TSP1 regulates iNOS in early OC, it is also possible that TSP1 modulation of the matrix, including stoichiometry of NCPs and protein presentation, may indirectly influence NO signaling. As ongoing research better characterizes the protein interactions in the bone matrix, the impact of matricellular TSP1 on cell signaling will be elucidated. In addition, it is possible that there are multiple sources of TSP1 in addition to the bone matrix that promote osteoclastogenesis, such as autocrine signaling by OC or paracrine signaling by other bone marrow cells such as macrophages, endothelial cells, or platelets. Our data shows that exogenous TSP1 promotes osteoclastogenesis through modulation of NO signaling in OC precursors, similar to its function in endothelial cells, platelets, and vascular smooth muscle cells through CD36 and CD47(23–26). The dramatic OC phenotype of TSP1 deficiency, in contrast with the modest effects reported for receptors CD47 and CD36(8–10,29,30), suggests that TSP1 binds multiple receptors to influence OC formation. We have previously reported that CD47 promotes OC formation through inhibition of iNOS(8). In the setting of bone homeostasis, OCs sense increased NO produced by iNOS early in differentiation as negative feedback to regulate the extent of osteoclastic bone resorption(44). We demonstrated in vitro that TSP1 inhibits iNOS expression in OC precursors, thereby permitting OC formation. Notably, inhibition of NO synthesis in vivo rescued the TSP1−/− bone resorption defect, consistent with TSP1’s influence on OC through modulation of NO signaling.
We present data to support novel roles of TSP1 that place it as a critical member of the bone microenvironment. We propose the following model: TSP1 is directionally secreted by OB into the matrix during bone formation(19,20). In the bone, TSP1 promotes matrix material integrity through interactions with other essential bone matrix proteins. Moreover, the matrix-derived TSP1 acts as a paracrine signaling molecule to promote OC formation via inhibition of iNOS, thus linking OB function to OC formation via the bone matrix (Fig. 6B).
Due to its pleiotropic nature, with roles in inflammation, angiogenesis, and bone health, the TSP1-signaling axis represents a promising therapeutic target for many disease states. NOS inhibitors, including the compound L-NAME used in our study, are under clinical investigation to modulate blood pressure. Their converse, nitric oxide donors, have been in clinical use for decades to relieve angina and hypertension. Given our data of elevated OC activity upon administration of L-NAME to TSP1−/− mice, it will be important to monitor the bone health of patients administered these NO-targeted drugs long-term, especially in an elderly patient population with common bone pathological comorbidities such as osteoporosis and osteoarthritis.
Bone pathologies such as osteoporosis and bone metastasis are characterized by a disruption of healthy bone turnover leading to pathologic fracture, hypercalcemia, and nerve compression. Modulation of OCs and OBs represent an attractive point for therapeutic intervention with agents such as bisphosphonates and anti-RANKL antibody (denosumab) in clinical use(3). Our findings provide further rationale to target the TSP1-pathway, especially in comorbidities such as osteoporosis and bone metastasis. Importantly, we showed that TSP1−/− mice are protected from age-associated bone loss, but the increased bone mass was accompanied by a loss in bone quality. This provides important rationale to monitor bone health, both bone density and bone quality, as part of studies investigating TSP1-pathway directed therapeutics for bone-related and other indications. As targeted drugs, including anti-CD47 antibodies, TSP1 mimetics, and NOS inhibitors, enter development and clinical use, it will be important to further investigate the role of TSP1, its receptors, and its downstream signaling molecules in bone health, to identify and monitor both harmful and positive bone effects.
Supplementary Material
Supp FigureLegends
Supp FigureS1-S3
Supp MaterialS1
Supp TableS1-S2
Acknowledgments
The authors thank Drs. Roberta Faccio, Michael Tomasson, Steven Teitelbaum, and Roberto Civitelli for their suggestions and comments. The authors also thank Xinming Su, Alison Esser, Jingyu Xiang, Rong Zeng, and Julie Dimitry for help and experimental assistance. Grant support: 2R01 CA097250 (K.N.W., M.H., S.R.A., O.U.), NIH research fellowship 1F31CA174096-01A1 (S.R.A.), St. Louis Men’s Group Against Cancer, Barnes-Jewish Foundation, Washington University Musculoskeletal Research Center (NIH P30 AR057235).
Footnotes
Supplemental data have been included in this submission.
DISCLOSURES
The authors state that they have no conflicts of interest.
Authors’ roles: S.R.A., O.U., M.H., and K.N.W. designed the research; S.R.A., O.U., M.H., and D.L. performed experiments; S.R.A., O.U., M.H., D.L., D.V.N., M.S., W.F., and K.N.W. analyzed data; and S.R.A., O.U., M.H., D.L., D.V.N., M.S., W.F., and K.N.W. wrote the paper.
References
Full text links
Read article at publisher's site: https://doi.org/10.1002/jbmr.2308
Read article for free, from open access legal sources, via Unpaywall:
https://asbmr.onlinelibrary.wiley.com/doi/pdfdirect/10.1002/jbmr.2308
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Matricellular proteins in immunometabolism and tissue homeostasis.
BMB Rep, 57(9):400-416, 01 Sep 2024
Cited by: 0 articles | PMID: 38919018 | PMCID: PMC11444987
Review Free full text in Europe PMC
Exploring the impact of naltrexone on the THBS1/eNOS/NO pathway in osteoporotic bile duct-ligated rats.
Sci Rep, 14(1):48, 02 Jan 2024
Cited by: 0 articles | PMID: 38167957 | PMCID: PMC10761994
Unique Spatial Transcriptomic Profiling of the Murine Femoral Fracture Callus: A Preliminary Report.
Cells, 13(6):522, 16 Mar 2024
Cited by: 0 articles | PMID: 38534368 | PMCID: PMC10969736
Thrombospondins modulate cell function and tissue structure in the skeleton.
Semin Cell Dev Biol, 155(pt b):58-65, 07 Jul 2023
Cited by: 2 articles | PMID: 37423854
Review
The impact of aging and physical training on angiogenesis in the musculoskeletal system.
PeerJ, 10:e14228, 03 Nov 2022
Cited by: 1 article | PMID: 36348663 | PMCID: PMC9637352
Go to all (34) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Expression of thrombospondin-1 modulates the angioinflammatory phenotype of choroidal endothelial cells.
PLoS One, 9(12):e116423, 30 Dec 2014
Cited by: 23 articles | PMID: 25548916 | PMCID: PMC4280221
TSP1 ameliorates age-related macular degeneration by regulating the STAT3-iNOS signaling pathway.
Exp Cell Res, 388(1):111811, 30 Dec 2019
Cited by: 8 articles | PMID: 31899207
The contribution of cross-talk between the cell-surface proteins CD36 and CD47-TSP-1 in osteoclast formation and function.
J Biol Chem, 293(39):15055-15069, 06 Aug 2018
Cited by: 23 articles | PMID: 30082316 | PMCID: PMC6166722
Matricellular protein thrombospondin-1 in pulmonary hypertension: multiple pathways to disease.
Cardiovasc Res, 113(8):858-868, 01 Jul 2017
Cited by: 19 articles | PMID: 28472457 | PMCID: PMC5852507
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NCI NIH HHS (4)
Grant ID: F31 CA174096
Grant ID: R01 CA097250
Grant ID: 2R01 CA097250
Grant ID: 1F31CA174096-01A1
NIAMS NIH HHS (2)
Grant ID: P30 AR057235
Grant ID: R01 AR047867
NIH (2)
Grant ID: 1F31CA174096-01A1
Grant ID: P30 AR057235